 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Rate constants, processivity, and productive binding ratio of chitinase A revealed by single-molecule analysis
Akihiko
Nakamura
ab,
Tomoyuki
Tasaki
c,
Yasuko
Okuni
a,
Chihong
Song
d,
Kazuyoshi
Murata
d,
Toshiya
Kozai
e,
Mayu
Hara
c,
Hayuki
Sugimoto
f,
Kazushi
Suzuki
f,
Takeshi
Watanabe
f,
Takayuki
Uchihashi
g,
Hiroyuki
Noji
c and
Ryota
Iino
 *abh
*abh
aOkazaki Institute for Integrative Bioscience, Institute for Molecular Science, National Institutes of Natural Sciences, Aichi 444-8787, Japan. E-mail: iino@ims.ac.jp; Tel: +81-564-59-5230
bDepartment of Functional Molecular Science, School of Physical Sciences, SOKENDAI, Kanagawa 240-0193, Japan
cDepartment of Applied Chemistry, Graduate School of Engineering, University of Tokyo, Tokyo 113-8656, Japan
dNational Institute for Physiological Sciences, National Institutes of Natural Sciences, Aichi 444-8787, Japan
eDepartment of Physics, Kanazawa University, Kanazawa 920-1192, Japan
fDepartment of Applied Biological Chemistry, Faculty of Agriculture, Niigata University, 8050 Ikarashi-2, Nishi-ku, Niigata 950-2181, Japan
gDepartment of Physics, Nagoya University, Aichi 464-8602, Japan
hInstitute for Molecular Science, National Institutes of Natural Sciences, Aichi 444-8787, Japan
First published on 23rd January 2018
Abstract
Correction for ‘Rate constants, processivity, and productive binding ratio of chitinase A revealed by single-molecule analysis’ by Akihiko Nakamura et al., Phys. Chem. Chem. Phys., 2018, DOI: 10.1039/c7cp04606e.
The authors would like to correct a mistake in Fig. 1 of this manuscript. The amino acid residue numbers in Fig. 1 were incorrect in the originally published version. A corrected version of the figure appears below. This correction does not change the results, discussion and conclusions of the paper.
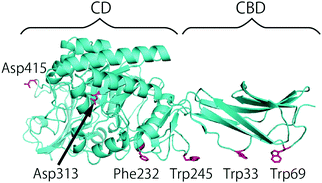 | ||
| Fig. 1 Crystal structure of SmChiA (pdb ID: 1CTN) and amino acid residues mutated in this study. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Owner Societies 2018 |
