Key factor governing the physicochemical properties and extent of proton transfer in protic ionic liquids: ΔpKa or chemical structure?†
Muhammed Shah
Miran‡§
a,
Mahfuzul
Hoque§
 a,
Tomohiro
Yasuda
a,
Seiji
Tsuzuki
a,
Tomohiro
Yasuda
a,
Seiji
Tsuzuki
 b,
Kazuhide
Ueno
b,
Kazuhide
Ueno
 a and
Masayoshi
Watanabe
a and
Masayoshi
Watanabe
 *a
*a
aDepartment of Chemistry and Biotechnology, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. E-mail: mwatanab@ynu.ac.jp
bResearch Centre for Computational Design of Advanced Functional Materials (CD-FMat), National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Umezono, Tsukuba, Ibaraki 305-8568, Japan
First published on 4th December 2018
Abstract
In order to identify the key factor governing the transport properties and extent of proton transfer in protic ionic liquids (PILs), a series of PILs were prepared by simple neutralisation of a super-strong acid, bis(trifluoromethanesulfonyl)amide acid (H[NTf2]), with a range of amines comprising diverse structures, including secondary and tertiary amines. The bulk physicochemical properties of the resulting PILs with a wide variation in the ΔpKa values of the constituent acid and protonated bases were compared to those of previously reported PILs derived from a super-strong base, 1,8-diazabicyclo[5.4.0]-7-undecene (DBU), and different acids (M. S. Miran, H. Kinoshita, T. Yasuda, M. A. B. H. Susan and M. Watanabe, Phys. Chem. Chem. Phys., 2012, 14, 5178–5186) with emphasis on the thermal stability, vibrational spectroscopy, density, viscosity, conductivity, and ionicity characteristics. The thermal stability of all PILs, as analysed by thermogravimetric analysis (TGA), was found to linearly increase with the ΔpKa values. However, isothermal TGA measurements for an extended duration indicated that [NTf2]-based PILs exhibit higher thermal stability than [DBU]-based PILs at low ΔpKa values (<15). PILs with secondary amines showed higher viscosity and lower ionic conductivity than those with tertiary amines because of the formation of multiple hydrogen bonds. The evaluated ionicity suggested that all [NTf2]-based PILs are “good PILs”, irrespective of the basicity of the amines, and have higher ionicity than [DBU]-based PILs particularly at low ΔpKa values (<15).
Introduction
Protic ionic liquids (PILs) are an important subclass of ionic liquids (ILs) that, by suitable molecular design, can be endowed with unique physicochemical properties similar to those of their aprotic counterparts, such as high ionic conductivity, low-vapour pressure, high thermal stability, and low flammability.1–3 PILs have shown competence in versatile applications, such as media for sustainable cotton dyeing,4 natural product extraction,5 biomass processing,6 protein7 and virus stabilisation,8 biocatalysis,9 catalysis,10 CO2 capture,11 nanostructure formation,12 pharmaceutical applications,13 fuel processing,14 and as capacitor electrolytes.15 However, the most relevant example is their possible use as fuel cell electrolytes16–21 due to the presence of labile protons in their cationic structure. However, correlation of the physicochemical properties of PILs with suitable parameters underpinning their key properties remains a challenge. Thus, systematic studies on said physicochemical properties are an emerging need in current research.Recent developments in PIL research have revealed that the most significant parameter to control the physicochemical properties of PILs is the ΔpKa value resulting from the pKa values of the constituent acid and protonated base in water.22–24 Using pKa data, a proton energy diagram can be constructed by analysis of the thermodynamics of proton transfer.25 However, discrepancies exist on the conditions determining the extent of proton transfer in terms of the ΔpKa value.22,23,26 It has been reported that ΔpKa > 10 is a requisite to ensure satisfactory proton transfer to produce highly ionised PILs23 and that even ΔpKa ≤ 4 is enough for complete proton transfer using primary amines.26 Recently, we have determined the ΔpKa threshold to obtain desirable physicochemical properties for a series of PILs based on an organic super-strong base, 1,8-diazabicyclo[5.4.0]-7-undecene (DBU), with various Brønsted acids.22 For instance, PILs with ΔpKa > 15 display high thermal stability and ionicity, comparable to those of typical aprotic ionic liquids (AILs). Recently, it has been shown that the inclusion of a hydroxyl functional group on a PIL cation significantly increases its ionic nature as compared to that of a di-amino or a non-functionalised cation despite having the same ΔpKa value.27 Primary and secondary ammonium carboxylate-based PILs exhibit significantly high apparent ionicity even at low ΔpKa values.26 We observed remarkable disparity in the ionic nature of two PILs based on water with a super-strong acid and a super-strong base.28 High proton dissociation was also found when the PIL was used as a solvent.29 It has also been shown that the dissociation constants of Brønsted acids in water and in the PIL, ethylammonium nitrate, barely vary.29 Thus, determining the dominant factors governing the proton transfer in PILs based on various acids and bases is of great interest and importance, since the establishment of an equilibrium between ionic and non-ionic species is a critical issue for PILs. Particularly, the presence of free acids and bases is not desirable for applications under open atmosphere and elevated temperatures.30,31 For instance, neutral species seriously affect the electrochemical activity of fuel cell electrolytes owing to their corrosive nature.31–33 Our previous study was simply based on PILs obtained from an organic super-strong base with various acids (8 < ΔpKa < 24).22 Thus, using a similar ΔpKa range, a systematic study with super-strong acid-based PILs is quintessential to answer critical questions on whether the ΔpKa value or the proton accepting/donating ability of the base/acid in water governs the thermal, transport, and ionic properties of PILs, which has not been clarified to date.
In this study, we prepared a novel series of PILs by neutralisation of a super-strong acid, bis(trifluoromethanesulfonyl)amide acid (H[NTf2]), with different amines as the base (8 < ΔpKa < 24),34–41 including tertiary amines DBU, pyrazine (Pyra), pyridine (Pyri), 1-ethylimidazole (EIm), diethylmethylamine (dema), 5-methylisoxazole (MIox); and secondary amines pyrrolidine (Pyrr), morpholine (Morp), and 1,2,4-triazole (TZL); and investigated the factors governing the properties of PILs (Fig. 1) by comparison with those previously obtained for [DBU]-based PILs with different acids (8 < ΔpKa < 24).22 The primary focus was on the thermal and transport properties, ionicity, and extent of the proton transfer for the PILs based on this super-strong acid and different bases so as to determine whether the ΔpKa value or the structure of the acid/bases dominates the key characteristics of these promising materials.
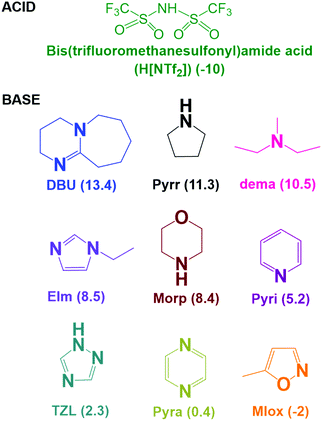 | ||
| Fig. 1 Chemical structure of the acid and bases used in this work. The values in parentheses indicate the pKa values in aqueous medium.34–41 | ||
Experimental
Reagents
For this study, DBU (98%), Pyrr (99%), EIm (98%), dema (98%), Morp (99%), Pyri (99%), TZL (98%), Pyra (98%), and MIox (95%) were purchased from Tokyo Chemical Industries. In addition, (CF3SO2)2NH (99%) was obtained from Kanto Chemical. All chemicals were utilised as received without further purification.Preparation of PILs
The PILs were prepared through simple, solvent-less neutralisation reactions at stoichiometric ratios of the acid and base in accordance with a previous report.22 The synthesised PILs, as shown in Table 1, were kept and handled in an inert atmosphere glovebox (VAC, [O2] < 1 ppm, [H2O] < 1 ppm). The moisture content of the PILs was below 100 ppm, as determined via Karl-Fischer titration.| PILs | ΔpKa |
|---|---|
| [MIox][NTf2] | 8.0 |
| [Pyra][NTf2] | 10.4 |
| [TZL][NTf2] | 12.3 |
| [Pyri][NTf2] | 15.2 |
| [Morp][NTf2] | 18.4 |
| [EIm][NTf2] | 18.5 |
| [dema][NTf2] | 20.5 |
| [Pyrr][NTf2] | 21.3 |
| [DBU][NTf2] | 23.4 |
Measurement of bulk properties
The bulk physicochemical properties, such as the density, viscosity, ionic conductivity, as well as the thermal properties and vibrational spectra, of the prepared PILs were evaluated in this study as described below. The corresponding data for [DBU]-based PILs were taken from our previous report.22The thermal properties, such as melting, crystallisation, and glass transition temperatures, were determined using a Seiko Instruments differential scanning calorimeter (DSC 6220C) with tightly sealed aluminium pans handled under Ar atmosphere. The samples were then heated to 150 °C, after which they were cooled to −150 °C and then heated again to 150 °C at heating and cooling rates of 10 °C min−1. The DSC data were recorded during the reheating scans. For thermogravimetric analysis (TGA), a Seiko Instruments thermogravimetry-differential thermal analyser (TG-DTA 6200) was operated from 30 to 550 °C at a heating rate of 10 °C min−1 under N2 atmosphere in open aluminium pans. The temperature at which the mass loss began was considered the decomposition temperature (Td). Isothermal TGA was conducted at 130 °C for 2 h under N2 atmosphere in order to compare with our previous data on DBU-based PILs.22 Certain electrochemical devices (i.e. fuel cells) require electrolytes with extended thermal stability, hence ITG experiments for PILs were recorded at 130 °C.2
Fourier transform infrared (FT-IR) spectra were recorded on an Avatar 360 FT-IR spectrometer in the 1000–4000 cm−1 range using CaF2 cells and sampling inside an inert atmosphere glovebox. For solid samples at 25 °C, an aliquot of the solvent (CD3Cl) was added to prepare dispersions. After placing a small drop of the sample on the surface of a CaF2 cell, it was left at room temperature for evaporation of the solvent.
Ab initio molecular orbital calculations were performed with the Gaussian 09 program20 using its implemented basis sets. The interaction energies between the cations and anion (Eint) were calculated at the MP2/6-311G** level for geometry optimisation of the ion pair at the HF/6-311G** level.21–23 The basis set superposition error (BSSE)24 was corrected by the counterpoise method.25 The stabilisation energy derived from the formation of a complex from isolated species (Eform) was calculated as the sum of the interaction energy (Eint) and the deformation energy (Edef), where Edef is the sum of the increases in the energies of the monomers due to the deformation associated with the formation of complexes.26 The Edef values were calculated at the MP2/6-311G** level.
A rheometer (Physica MCR 301, Anton Paar) was used for viscosity estimation in the range of 30–150 °C under dry air conditions. A steady pre-shear was applied at a shear rate of 1 s−1 for 60 s, followed by a 120 s rest period before each measurement to eliminate any previous shear history.
The ionic conductivity was measured using a locally designed dip cell probe consisting of two platinum wires sheathed in glass. The cell constant was determined with a solution of 0.1 M KCl at 25 °C. The complex impedance spectra in the frequency range of 10 MHz to 0.01 Hz were used for the determination of conductivities on a potentiostat (Autolab, PGSTAT30). The conductance was estimated from the real axis intercept in the Nyquist plots. The temperature was controlled at 20 °C intervals in the range of 30–150 °C via thermal equilibration at each temperature for at least 1 h using a constant temperature oven (DKN 611).
A JEOL ECX400 nuclear magnetic resonance (NMR) spectrometer with a 9.4 T narrow bore superconducting magnet equipped with a JEOL pulse field gradient probe and current amplifier were used to perform pulsed-gradient spin-echo nuclear magnetic resonance (PGSE-NMR) measurements to study the self-diffusion coefficients of each component in the [NTf2]-based PILs (1H, 399.7 MHz) and fluorinated anion (19F, 376.1 MHz). The detailed experimental procedure for the PGSE-NMR analysis has been previously reported.22,34
Density measurements were performed using a thermo-regulated density/specific gravity meter DA-100 (Kyoto Electronics Manufacturing Co. Ltd) in the range of 15 to 40 °C. The density of PILs exhibiting high melting points was measured with a pycnometer.
Results and discussion
Thermal properties
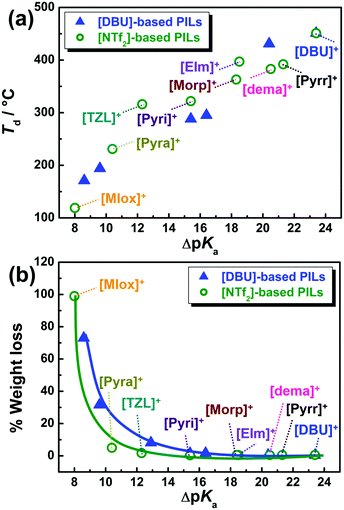
Thermo-analytical techniques such as TGA and DSC were employed to investigate the thermal properties of the PILs. From the TGA, the thermal stability was assessed under both non-isothermal and isothermal conditions. First, the decomposition temperature, Td, was determined from the onset of the weight loss in the TGA curves, as presented in Fig. S1 (ESI†). The Td value appears to linearly increase at first with the increasing ΔpKa value (Fig. 2a). However, the Td values for the [NTf2]-based PILs follow the order: [DBU]+ > [EIm]+ > [Pyrr]+ > [dema]+ > [Morp]+ > [TZL]+ ≈ [Pyri]+ > [Pyra]+ > [MIox]+, showing good correlation with the N–H stretching frequencies (vide infra) and coinciding with our previous results for a series of DBU-based PILs.22 Similar findings were obtained for another series of PILs through correlation of the boiling point with the ΔpKa values.23 However, non-isothermal TGA is a technique in which the temperature is ramped at a fast rate, thus affording an overestimation of the thermal stability of PILs.42 Therefore, harsher isothermal TGA were conducted to distinguish differences in the thermal stability of the [NTf2] and [DBU]-derived PILs. Fig. 2b shows the correlation between the weight losses during isothermal TG measurements at 130 °C for 2 h with the ΔpKa values. The [NTf2]-based PILs exhibit no weight losses at ΔpKa > 15, similarly to the case of [DBU]-based PILs. Surprisingly, [Pyra][NTf2] and [TZL][NTf2] also showed high weight retention even though their ΔpKa values fall within 15 > ΔpKa > 10. Note that, in a previous study,43 high intermolecular energy densities were also observed in [TZL]-based AILs. Apparently, these TG measurements indicate that PILs with high Td are those with low weight loss in the isothermal measurement. However, difference in weight loss in the low ΔpKa regime becomes more apparent by the isothermal measurement, as seen Fig. 2b. One can see the chemical structure effect for [Pyra][NTf2] and [TZL][NTf2] against the [DBU][CH3CO2] and [DBU][CF3CO2], respectively, despite having similar ΔpKa.Then, DSC measurements were performed to gain insight into the phase transition behaviour of the [NTf2]-based PILs. The DSC thermograms under heating exhibited glass transition temperatures (Tg), cold crystallisation temperatures (Tcc) above the Tg, and subsequent melting of the crystallised samples at the melting temperature (Tm) (Fig. 3). Cold crystallisation peaks were mainly observed for [TZL][NTf2] and [Morp][NTf2] during the heating step, indicating that these PILs have relatively slow crystallisation kinetics under the same cooling conditions. Thermal heating provided the necessary energy to exert the conformational changes required for crystallisation, indicating strong intermolecular interactions43,44 in the above PILs. These results support the earlier observations made by Nikolakis45 and Krishnan et al.46 for [TfO] and [NTf2]-based PILs, respectively. The high glass transition temperatures also suggest the presence of high cohesive energies in these PILs.46 The endothermic peaks corresponding to the melting points, Tm, were found at temperatures below 100 °C for all the samples. Note that [Pyra][NTf2] (ΔpKa = 10.4) exhibits the highest Tm value (70 °C), while [EIm][NTf2] (ΔpKa = 18.5) shows the lowest Tm (12 °C). It is well-known that H-bonding is prevalent in PILs compared to conventional AILs. In primary alkylamine-derived PILs with [NTf2]−, dominance of hydrogen bonding contributed to the higher melting points of the PILs in contrast with their secondary and tertiary counterparts.47 But, ion-pairing is not dominated by the cations only. For example, in a recent report on AILs, weaker effect of ion-pairing was clearly demonstrated for [EMIM][NTf2], in contrast with [EMIM][OAc], where [OAc]− stands for acetate anion by using glucose as the disruptive agents.48 So, the depolarized anionic charge in [NTf2]− in comparison with [OAc]−, reduces the strength of ion-pairing owing to the weaker hydrogen bonding network.48 So, the extent of H-bonding can strongly influence the ion-pair binding energy (IPBE) for PILs depending on the structure of the cations and anions, which in turn affects their melting points as evidenced in the phase behaviour of the [NTf2]-based PILs.
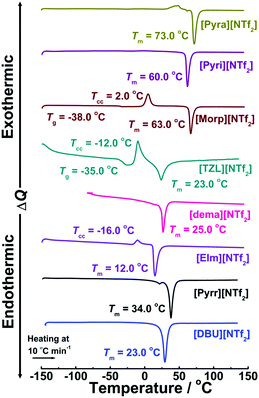 | ||
| Fig. 3 DSC thermograms of [NTf2]-based PILs. Tm, Tcc, and Tg correspond to the melting, cold crystallisation, and glass-transition temperatures, respectively. | ||
FT-IR spectra
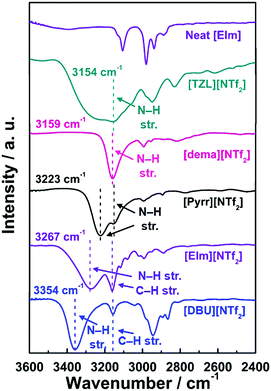
Inter and intra-ionic interactions are easily identified by IR (vibrational) spectroscopy as information on the specific cation–anion bonding interactions in ILs. Fig. 4 displays the FT-IR spectra for [NTf2]-based PILs at ambient temperature. The N–H bands were assigned in the spectra according to a previous report.22 N–H stretching was observed at 3154–3354 cm−1 for the [NTf2]-based PILs depending on the cationic structure. A closer look at the FT-IR spectra revealed that the N–H stretching frequency follows the order: [DBU]+ > [EIm]+ > [Pyrr]+ > [dema]+ > [TZL]+, in agreement with the order of the thermal decomposition temperature shown in Fig. 2a. However, the trend considering only the ΔpKa values22,41 could be expected to follow the order: [DBU]+ > [Pyrr]+ > [dema]+ > [EIm]+ > [TZL]+. Stronger H-bonding interactions, indicated by lower N–H stretching frequencies, result in easy dissociation into the constituent acid and base, which in turn leads to lower thermal stability.Here, it is important to consider the special “anionic-structure effect” of the [NTf2]− in terms of H-bonding interactions. Triflate anion, [TfO]−, is a suitable candidate to compare with [NTf2]− as they are derived from super strong monoprotic acids. Hence, both are quite distinct in terms of H-bond basicity and conformational flexibility of their structures. [NTf2]− is presumably a weaker proton acceptor than [TfO]−, as the negative charge in [NTf2]− is more distributed than in [TfO]−. However, the conformational variability45 of [NTf2]− and multiple H-bond acceptor sites (N and O atoms) affords a configurational entropic contribution and thus, H-bonding may also be established in [NTf2]-based PILs, as previously reported by Umebayashi and co-workers.49 For example, ab initio calculations provided twice the number of stable geometries (local minima) for [DBU][NTf2] with very similar and smaller ion-pair stabilisation energies (Eform) than for [DBU][TfO], indicating the variable spatial accessibility of [NTf2]− in PILs (Fig. S2, ESI†).
Moreover, the FT-IR results were further supported by ab initio molecular orbital calculations, demonstrating the decreasing N–H bond length in the order: [TZL]+ > [Pyrr]+ > [dema]+ > [EIm]+ > [DBU]+ (Fig. 5a). The Eform parameter follows the same trend, as shown in Fig. 5b–d. Once we switched from [NTf2]-based PILs to [DBU]-based PILs, the N–H bond length and Eform were exclusively dictated by the ΔpKa values (Fig. 5a–d). Thus, the extra stabilisation energy gain seems to stem from the unique structural features of the cations in [NTf2]-based PILs. Multiple strong H-bonding is possible in [Pyrr][NTf2] as the cation derives from a secondary amine. It is already well-known that ion-pair interactions are intrinsically stronger in PILs than AILs because of the presence of H-bonding,50 which further increases from tertiary amine to primary amine-based PILs.48 Next, the similarity in the stabilisation energy values (Fig. 5b) for [Pyrr][NTf2] and [TZL][NTf2] could be correlated with their similar H-bond density despite the large difference in their ΔpKa values. Finally, [EIm][NTf2] also exhibits rather weaker H-bonding compared to [Pyrr][NTf2] and [dema][NTf2], as displayed in Fig. 4. In [EIm][NTf2], the probable protonated site (N-1) presents delocalisation of the positive-charge density owing to the sp2-configuration of N-1 in [EIm]+, whereas it is localised in [Pyr]+ and [dema]+.
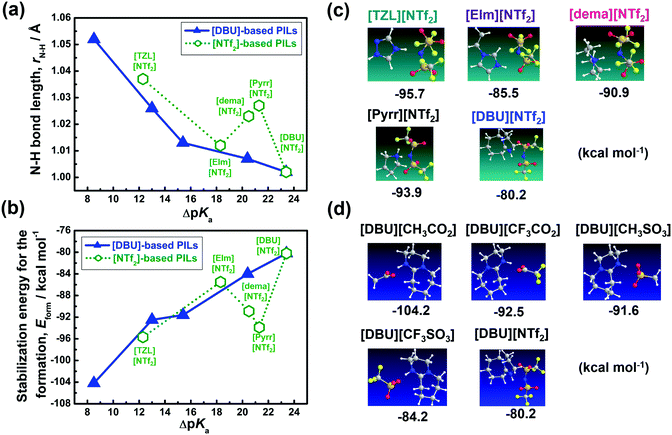 | ||
| Fig. 5 (a and b) Variation in the N–H bond length and stabilisation energy, and (c and d) most stable geometries for [DBU] and [NTf2]-based PILs as a function of ΔpKa. | ||
Thus, the fluctuation frequency of N–H bond becomes higher, and blue-shifting (toward higher wavenumbers) is observed together with band broadening.51,52 Moreover, a recent study by Nikolakis and colleagues45 showed that amongst various imidazole (Im)-derived PILs, [HCnIm][NTf2] (where n represents the length of the alkyl chain at N-3) parallel π–π stacking facilitates H-bonding, which presents the lowest probability in the case of [HC2Im][NTf2]. Thus, it can be envisaged that, in our case, the density and strength of H-bonding in [EIm][NTf2] (where n = 2) are considerably lower and weaker than those in [Pyrr][NTf2] and [dema][NTf2].
Viscosity
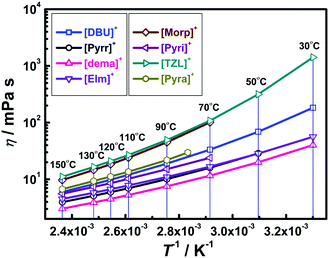
Fig. 6 displays the temperature dependence of the viscosity (η) for the [NTf2]-based PILs after fitting with the Vogel–Tamman–Fulcher (VTF) equation53–55 (see, ESI† for details). The best fit parameters are listed in Table S1 (ESI†).From Fig. 6, the viscosity values at 130 °C follow the order based on the cations: [TZL]+ > [Morp]+ > [Pyra]+ > [DBU]+ > [Pyri]+ > [EIm]+ ∼ [Pyrr]+ > [dema]+. Ignatiev et al.56 reported that other than the influence of electronic, inter, and intra-molecular forces, the size of the ions, i.e. their mass, also dictates the viscosity of ILs. The high viscosities of [Morp][NTf2] and [TZL][NTf2] are indicative of strong H-bonding, which has also been observed in [Morp][F] (F = formate anion) and [TZL]-based AILs.57
Conductivity
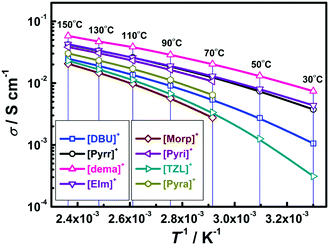
The temperature dependence of the ionic conductivity (σ) for [NTf2]-based PILs is shown in Fig. 7 after fitting with the VTF equation53–55 (see, ESI† for details and Table S2).All PILs exhibit very high ionic conductivities comparable to those of other previously reported highly conductive PILs.59 Especially, [dema][NTf2] exhibits the highest ionic conductivity at every temperature of the studied range. At 130 °C, the ionic conductivity values follow the order: [dema]+ > [Pyrr]+ ∼ [EIm]+ > [Pyri]+ > [Pyra]+ > [DBU]+ > [TZL]+ > [Morp]+, which is almost the opposite of the viscosity order (vide supra). From the viscosity data analysis (Fig. 6), [dema][NTf2] was found to be the most fluidic PIL and thus the most conductive (Fig. 7). Conversely, ions in H-bonding-dominated fluids are less mobile, as observed by the low ionic conductivity values of [TZL][NTf2] and [Morp][NTf2]. Therefore, the Ea of the ionic conductivity is quite high (i.e. a large barrier) in these PILs.
Ionicity and extent of proton transfer
We now turn our focus onto the ionicity and extent of proton transfer, which is a challenging aspect of PILs. AILs entirely consist of ions whereas PILs exhibit dynamic acid–base equilibria. Therefore, the meaning of ionicity in PILs is rather more complex than that in AILs. As such, it is essential to differentiate PILs from acid–base mixtures to understand better the ionic nature of PILs, which may be governed by the extent of proton transfer, i.e. ΔpKa.Among many approaches,22,60–64 Walden plots65 are the easiest way to roughly distinguish qualitatively between good/poor ILs and non-ionic liquids, as proposed by Angell et al.60Fig. 8 presents the Walden plots of the [NTf2]-based PILs, whereby all the [NTf2]-based PILs in this study can be considered “good PILs”. In general, the data points against the ideal line show significant negative deviation depending on the ΔpKa values of the PILs. We have also estimated quantitatively the ionicity as the ratio of the molar conductivities measured by both impedance and PGSE-NMR analyses, (Λimp/ΛNMR),61 which can be effectively applied for all types of ILs such as AILs,61,62 solvate ILs,66 and PILs.22,26,48Λimp was calculated from the equation:
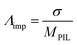 | (1) |
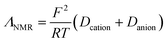 | (2) |
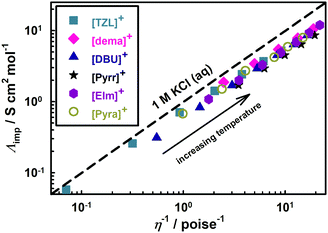 | ||
| Fig. 8 Walden plots for [NTf2]-based PILs. The dashed straight line corresponds to a 1 M KCl aqueous solution. | ||
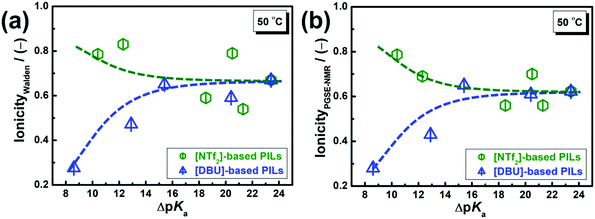 | ||
| Fig. 9 ΔpKa dependency of the ionicity for [DBU] and [NTf2]-based PILs at 50 °C. The ionicity was estimated from (a) Walden plots and (b) PGSE-NMR data. The lines are drawn as a guide to the eye. | ||
The ionicity values are less than unity for all the PILs considered herein. The Walden plot analysis provided more scattered ionicity values than the PGSE-NMR approach. In Coulombic fluids, the ionicity is mainly governed by Coulombic interactions. Yet, as reported in numerous studies,34,60–63 the subtle balance of Coulombic and dispersion forces and H-bonding ultimately determines the ionicity of AILs. Moreover, we have reported that the directionality of interaction in the ion pairs of PILs is generally larger than that of AILs from ab initio MO calculations.50,67 In the case of AILs, the ionicity tends to decrease with the increasing interaction energy of the ion pairs as well as with the increasing directionality of said interactions.68 As shown in these figures, the ionicity of the [DBU]-based PILs increased up to ΔpKa = 15 and then remained constant. Proton transfer is incomplete at ΔpKa < 15 for [DBU]-based PILs. The maximum ionicity value (∼0.6) for the [DBU]-based PILs (Fig. 9) is lower than that for high-ionicity AILs.69,70 Therefore, we concluded that stronger H-bonding in [DBU]-based PILs results in weaker ionic nature of the PILs as compared to that in AILs.22
On the other hand, the ionicity of [NTf2]-based PILs appeared to be higher at low ΔpKa values and then became independent of the ΔpKa values. Interestingly, [Pyra][NTf2] and [TZL][NTf2] (10 < ΔpKa < 15) exhibited higher ionicity than [DBU]-based PILs with similar ΔpKa values ([DBU][CH3CO2] and [DBU][CF3CO2]). Furthermore, it is fascinating that the ionicity of [Pyra][NTf2] (ΔpKa = 10.4) is comparable to that of [dema][NTf2] (ΔpKa = 20.5). Note that [NTf2]-based PILs with primary alkyl ammonium cations exhibit lower ionicity owing to the dominance of H-bonding.48 Therefore, by suitable choice of the cation, highly dissociative PILs at low ΔpKa values can be obtained by tuning the balance between Coulombic and intermolecular interactions. In the case of [TZL][NTf2], [TZL]+ possibly allows the rapid exchange of protons between the N-1 and N-3 sites,57 a phenomenon also observed in guanidine-derived PILs,71,72 which may weaken the directionality of the interactions. Also, in the case of [Pyra][NTf2], [Pyra]+ is not only an H-bond donor but also a weak H-bond acceptor;58 thus, the PIL displays a more dissociated nature. Such characteristics of [NTf2]-based PILs of their ionicity would originate from the multiple proton donating and accepting sites within the structure of the certain cations and [NTf2]− anion, respectively.
While the ΔpKa value is considered the key parameter determining the degree of proton transfer in PILs,22 the individual role of the proton donors and acceptors has not been considered in detail before.22–24 Complete proton transfer from a Brønsted acid to a base is assumed for PILs with high ΔpKa values owing to fast kinetics.73 However, in Brønsted acid–base mixtures, neutral acids and bases do exist at low ΔpKa values and, in such case, the proton transfer is incomplete. Such systems are not regarded as true PILs but pseudo-PILs.74
Furthermore, the kinetics of the proton transfer process is also affected by the thermodynamics of a given system, which in turn depends on several factors such as the exothermic nature of the reaction, electron delocalisation in the base, and intra-molecular H-bonding.75 Even at the same ΔpKa values, the structural flexibility of [NTf2]− may also accelerate the proton transfer for [NTf2]-based PILs, in contrast to the less flexible carboxylate anions such as [CH3CO2]− and [CF3CO2]− for [DBU]-based PILs. Therefore, the extent of proton transfer, which corresponds to the ionicity at low ΔpKa values, depends not only on ΔpKa values but also on the chemical structure of the acids and bases. At high ΔpKa values, the degree of proton transfer is very high owing to the sufficient proton donating and accepting properties of the reactant acid and base, respectively. However, at relatively low ΔpKa values, the effect of the chemical structure of bases/acids becomes significant. Quantitative analysis via potential energy surfaces75 would provide further insight to clarify this phenomenon, but this type of analysis was beyond the scope of this manuscript.
Conclusions
In this study, we reported the physicochemical properties of PILs based on a super-strong acid, H[NTf2], with a wide variety of amines in term of structures and basicities. In addition, we compared the properties of the prepared PILs with those of previously reported [DBU]-based PILs derived from a super-strong base and different acids. The thermal stability of PILs depended almost linearly with the ΔpKa values of the reactant acid–base pair. However, under harsher conditions, even at low ΔpKa values, [NTf2]-based PILs exhibited superior thermal stability. According to the Walden plots, the [NTf2]-based PILs are “good PILs”. Moreover, [NTf2]-based PILs with low ΔpKa values exhibited higher ionicity than [DBU]-based PILs. Such characteristics of [NTf2]-based PILs in terms of their thermal stability and ionicity seem to originate from the multiple proton donating and accepting sites within the structure of the cations and [NTf2]− anion, respectively, especially in low ΔpKa PILs. As a result, the threshold value of ΔpKa for obtaining thermally stable and high ionicity PILs can be lowered from 15 to 10 for [NTf2]-based PILs. To the best of our knowledge, this is the first demonstration about the effect of the chemical structure on the physicochemical features and extent of proton transfer in PILs with a systematic study via suitable selection of acids and bases.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Grants-in-Aid for Scientific Research (S/15H05758) and Grants-in-Aid for Specially Promoted Research on “Iontronics” from the Japan Society for the Promotion of Science.Notes and references
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2003 Search PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- P. Walden, Bull. Acad. Imp. Sci., 1914, 8, 405–422 Search PubMed.
- R. S. Andrade, D. Torres, F. R. Andrade, D. Torres, F. R. Ribeiro, B. G. C. Andreo, J. A. Oshiro Jr and M. Iglesias, ACS Sustainable Chem. Eng., 2017, 5, 8756–8765 CrossRef.
- M. W. Sanders, L. Wright, L. Tate, G. Fairless, L. Crowhurst, N. C. Bruce, A. J. Walker, G. A. Hembury and S. Shimizu, J. Phys. Chem. A, 2009, 113, 10143–10145 CrossRef CAS PubMed.
- C. Chiappe, A. Mezzetta, C. S. Pomelli, B. Masciocchi, A. Gentile and G. Iaquaniello, Green Chem., 2016, 18, 4982–4989 RSC.
- N. Byrne, L. M. Wang, J.-P. Belieres and C. A. Angell, Chem. Commun., 2007, 2714–2716 RSC.
- N. Byrne, B. Rodoni, F. Constable, S. Varghes and J. H. Davis Jr., Phys. Chem. Chem. Phys., 2012, 14, 10119–10121 RSC.
- R. A. Sheldon, Chem. – Eur. J., 2016, 22, 12984–12999 CrossRef CAS PubMed.
- Q. Zhang, H.-Y. Yuan, N. Fukaya, H. Yasuda and J.-C. Choi, Green Chem., 2017, 19, 5614–5624 RSC.
- K. A. Mumford, S. J. Pas, T. Linseisen, T. M. Statham, N. J. Nicholas, A. Lee, K. Kezia, R. Vijayraghavan, D. R. Macfarlane and G. W. Stevens, Int. J. Greenhouse Gas Control, 2015, 32, 129–134 CrossRef CAS.
- T. L. Greaves, S. T. Mudie and C. J. Drummond, Phys. Chem. Chem. Phys., 2011, 13, 20441–20452 RSC.
- J. Stoimenovski, P. M. Dean, E. I. Izgorodina and D. R. MacFarlane, Faraday Discuss., 2012, 154, 335–352 RSC.
- Z. Li, J. Xu, D. Li and C. Li, RSC Adv., 2015, 5, 15892–15897 RSC.
- P. L. Mayrand, S. Lin, D. Lazzerini and D. Rochefort, J. Power Sources, 2010, 195, 5114–5121 CrossRef.
- S.-Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9767–9773 Search PubMed.
- J. Snyder, T. Fujita, M. W. Chen and J. Erlebacher, Nat. Mater., 2010, 9, 904–907 CrossRef CAS PubMed.
- M. Watanabe, M. L. Thomas, S. Zhang, K. Ueno, T. Yasuda and K. Dokko, Chem. Rev., 2017, 117, 7190–7239 CrossRef CAS PubMed.
- A. Noda, M. A. B. H. Susan, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- D. R. Macfarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis Jr., M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232–250 RSC.
- A. Khan, C. A. Gunawan and C. Zhao, ACS Sustainable Chem. Eng., 2017, 5, 3698–3715 CrossRef CAS.
- M. S. Miran, H. Kinoshita, T. Yasuda, M. A. B. H. Susan and M. Watanabe, Phys. Chem. Chem. Phys., 2012, 14, 5178–5186 RSC.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS PubMed.
- H. Luo, G. A. Baker, J. S. Lee, R. M. Pagni and S. Dai, J. Phys. Chem. B, 2009, 113, 4181–4183 CrossRef CAS PubMed.
- J.-P. Belieres and C. A. Angell, J. Phys. Chem. B, 2007, 111, 4926–4937 CrossRef CAS PubMed.
- J. Stoimenovski, E. I. Izgorodina and D. R. MacFarlane, Phys. Chem. Chem. Phys., 2010, 12, 10341–10347 RSC.
- E. S. J. Reid, C. E. S. Bernardes, F. Martins, S. Shimizu, M. E. M. Piedade and A. J. Walker, Phys. Chem. Chem. Phys., 2017, 19, 28133–28138 RSC.
- M. S. Miran, T. Yasuda, R. Tatara, M. A. B. H. Susan and M. Watanabe, Faraday Discuss., 2018, 206, 353–364 RSC.
- J. R. Kanzaki, H. Kodamatani, T. Tomiyasu, H. Watanabe and Y. Umebayashi, Angew. Chem., Int. Ed., 2016, 55, 6266–6269 CrossRef PubMed.
- S. E. Goodwin, D. E. Smith, J. S. Gibson, R. G. Jones and D. A. Walsh, Langmuir, 2017, 33, 8436–8446 CrossRef CAS PubMed.
- C. A. Angell, Y. Ansari and Z. Zhao, Faraday Discuss., 2012, 154, 9–27 RSC.
- M. S. Miran, T. Yasuda, M. A. B. H. Susan, K. Dokko and M. Watanabe, RSC Adv., 2013, 3, 4141–4144 RSC.
- M. S. Miran, T. Yasuda, M. A. B. H. Susan, K. Dokko and M. Watanabe, J. Phys. Chem. C, 2014, 118, 27631–27639 CrossRef CAS.
- M. Hasani, J. L. Yarger and C. A. Angell, Chem. – Eur. J., 2016, 22, 13312–13319 CrossRef CAS PubMed.
- I. Hermecz, Adv. Heterocycl. Chem., 1987, 42, 83–202 CrossRef CAS.
- E. A. Braude, F. C. Nachod and W. D. Phillips, Determination of organic structures by physical methods, Academic Press, New York, 1971 Search PubMed.
- R. Linnell, J. Org. Chem., 1960, 25, 290 CrossRef CAS.
- B. Lenarcik, B. Barszcz and J. Kulig, Pol. J. Chem., 1979, 53, 963 CAS.
- K. Hall Jr., J. Am. Chem. Soc., 1957, 79, 5441–5444 CrossRef.
- S. Li, Z. Zhou, Y. Zhang and M. Liu, Chem. Mater., 2005, 17, 5884–5886 CrossRef CAS.
- Relative strength of acids and bases were maintained according to the results of FT-IR spectra of pure PILs.
- P. Navarro, M. Larriba, E. Rojo, J. García and F. Rodríguez, J. Chem. Eng. Data, 2013, 58, 2187–2193 CrossRef CAS.
- C. Cadena and E. J. Maginn, J. Phys. Chem. B, 2006, 110, 18026–18039 CrossRef CAS PubMed.
- L. V. N. R. Ganapatibhotla, J. Zheng, D. Roy and S. Krishnan, Chem. Mater., 2010, 22, 6347–6360 CrossRef CAS.
- A. M. Moschovi, V. Dracopoulos and V. Nikolakis, J. Phys. Chem. B, 2014, 118, 8673–8683 CrossRef CAS PubMed.
- J. L. Lebga-Nebane, S. E. Rock, J. Franclemont, D. Roy and S. Krishnan, Ind. Eng. Chem. Res., 2012, 51, 14084–14098 CrossRef CAS.
- M. Hoque, M. L. Thomas, M. S. Miran, M. Akiyama, M. Marium, K. Ueno, K. Dokko and M. Watanabe, RSC Adv., 2018, 8, 9790–9794 RSC.
- C. D’Agostino, M. D. Mantle, C. L. Mullan, C. Hardacre and L. F. Gladden, ChemPhysChem, 2018, 19, 1081–1088 CrossRef PubMed.
- H. Watanabe, H. Doi, S. Saito, M. Matsugami, K. Fujii, R. Kanzaki, Y. Kameda and Y. Umebayashi, J. Mol. Liq., 2016, 217, 35–42 CrossRef CAS.
- S. Tsuzuki, W. Shinoda, M. S. Miran, H. Kinoshita, T. Yasuda and M. Watanabe, J. Chem. Phys., 2013, 139, 174504 CrossRef PubMed.
- R. Janoschek, E. G. Weidemann, H. Pfeiffer and G. Zundel, J. Am. Chem. Soc., 1972, 94, 2387–2396 CrossRef CAS.
- P. A. Hunt, C. R. Ashworth and R. P. Matthews, Chem. Soc. Rev., 2015, 44, 1257–1288 RSC.
- H. Vogel, Phys. Time, 1921, 22, 645–646 CAS.
- G. Tammann and W. Hesse, J. Inorg. Gen. Chem., 1926, 156, 245–257 CAS.
- G. S. Fulcher, J. Am. Ceram. Soc., 1925, 8, 339–355 CrossRef CAS.
- A. Noda, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2001, 105, 4603–4610 CrossRef CAS.
- C. Brigouleix, M. Anouti, J. Jacquemin, M. Caillon-Caravanier, H. Galiano and D. Lemordant, J. Phys. Chem. B, 2010, 114, 1757–1766 CrossRef CAS PubMed; D. D. Zorn, J. A. Boatz and M. S. Gordon, J. Phys. Chem. B, 2006, 110, 11110–11119 CrossRef PubMed.
- M. Watanabe, D. Kodama, T. Makino and M. Kanakubo, J. Chem. Eng. Data, 2016, 61, 4215–4221 CrossRef CAS.
- H. Nakamoto and M. Watanabe, Chem. Commun., 2007, 2539–2541 RSC.
- W. Xu, E. I. Cooper and C. A. Angell, J. Phys. Chem. B, 2003, 107, 6170–6178 CrossRef CAS.
- H. Tokuda, K. Ishii, M. A. B. H. Susan, S. Tsuzuki, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2006, 110, 2833–2839 CrossRef CAS PubMed.
- K. Ueno, H. Tokuda and M. Watanabe, Phys. Chem. Chem. Phys., 2010, 12, 1649–1658 RSC.
- D. R. MacFarlane, M. Forsyth, E. I. Izgorodina, A. P. Abbott, G. Annat and K. Fraser, Phys. Chem. Chem. Phys., 2009, 11, 4962–4967 RSC.
- M. Shen, Y. Zhang, K. Chen, S. Che, J. Yao and H. Li, J. Phys. Chem. B, 2017, 121, 1372–1376 CrossRef CAS PubMed.
- P. Walden, J. Phys. Chem., 1906, 55, 207 CAS.
- K. Ueno, K. Yoshida, M. Tsuchiya, N. Tachikawa, K. Dokko and M. Watanabe, J. Phys. Chem. B, 2012, 116, 11323–11331 CrossRef CAS PubMed.
- T. Yasuda, H. Kinoshita, M. S. Miran, S. Tsuzuki and M. Watanabe, J. Chem. Eng. Data, 2013, 58, 2724–2732 CrossRef CAS.
- S. Tsuzuki, H. Tokuda, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2005, 109, 16474–16481 CrossRef CAS PubMed.
- M. S. Miran, H. Kinoshita, T. Yasuda, M. A. B. H. Susan and M. Watanabe, Chem. Commun., 2011, 47, 12676–12678 RSC.
- M. Watanabe, Electrochemistry, 2016, 84, 642–653 CrossRef CAS.
- M. Kuroha, H. Gotoh, M. S. Miran, T. Yasuda, M. Watanabe and K. Sakakibara, Chem. Lett., 2014, 43, 649–651 CrossRef CAS.
- Z. Zhao, K. Ueno and C. A. Angell, J. Phys. Chem. B, 2011, 115, 13467–13472 CrossRef CAS PubMed.
- A. J. Kresge, Acc. Chem. Res., 1975, 8, 354–360 CrossRef CAS.
- H. Doi, X. Song, B. Minofar, R. Kanzaki, T. Takamuku and Y. Umebayashi, Chem. – Eur. J., 2013, 19, 11522–11526 CrossRef CAS PubMed.
- I. V. Fedorova, M. A. Krestyaninov and L. P. Safonova, J. Phys. Chem. A, 2017, 121, 7675–7683 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: TG curves of [NTf2]-based PILs; optimised structures of [DBU][NTf2] and [DBU][TfO]; VTF fitting parameters for the viscosity, conductivity, and molar conductivity; fitting parameters for the density and molar conductivity at various temperatures for the [NTf2]-based PILs. See DOI: 10.1039/c8cp06973e |
| ‡ Present address: Department of Chemistry, University of Dhaka, Dhaka 1000, Bangladesh. |
| § These two authors equally contributed to this work. |
| This journal is © the Owner Societies 2019 |