Competition between hydrogen bonds and van der Waals forces in intermolecular structure formation of protonated branched-chain alcohol clusters†
Abstract
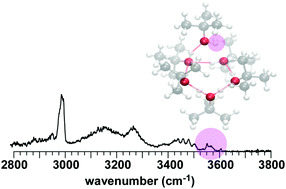
To investigate the influence of bulky alkyl groups on hydrogen-bonded (H-bonded) network structures of alcohols, infrared (IR) spectra of protonated clusters of 2-propanol (2-PrOH) and tert-butyl alcohol (t-BuOH) were observed in the OH and CH stretch regions. In addition, by varying the tag species, the temperature dependence profile of the isomer population of H+(t-BuOH)n was revealed. An extensive search for stable isomers was performed using dispersion-corrected density functional theory methods, and temperature-dependent IR spectral simulations were done on the basis of the harmonic superposition approximation. The computational results qualitatively agreed with the observed size and temperature dependence of the H-bonded network structures of these protonated bulky alcohol clusters. However, the difficulty in the quantitative evaluation of dispersion was also demonstrated. It was shown that H+(2-PrOH)n (n = 4–7) have essentially the same network structures as the protonated normal alcohol clusters studied so far. On the other hand, H+(t-BuOH)n (n = 4–8) showed a clear preference for the smaller-membered ring structures, that is very different from the preference of the protonated normal alcohol clusters. The origin of the different structure preferences was discussed in terms of the steric effect and dispersion.


 Please wait while we load your content...
Please wait while we load your content...