The activation of carbon dioxide by first row transition metals (Sc–Zn)†
Abstract
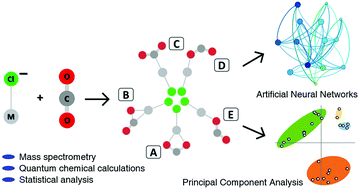
The activation of CO2 by chloride-tagged first-row transition metal anions [ClM]− (M = Sc–Zn), was examined by mass spectrometry, quantum chemical calculations, and statistical analysis. The direct formation of [ClM(CO2)]− complexes was demonstrated in the reaction between [ClM]− and neutral CO2. In addition, the reverse reaction was investigated by energy-variable collisionally induced dissociation (CID) of the corresponding [ClM(CO2)]− anions generated in-source. Five different mono- and bi-dentate binding motifs present in the ion/CO2 complexes were identified by quantum chemical calculations and the relative stability of each of these isomers was established and analyzed for all first-row transition metals based on the experimental and theoretical ion/molecule binding energies. It was found that the early first row transition metals form strong covalent bonds with the neutral CO2 molecule, while the late ones and in particular copper and zinc are weakly bonded. Using simple valence bond Lewis diagrams, the different binding motifs and their relative stabilities across the first row were described using multi-configurational self consistent field (MSCSCF) wavefunctions in a quantitative manner based on the electronic structure of the individual metals. This analysis provides an explanation for the change of the most favorite bonding motif of the transition metals with CO2 along the 1st transition metal row. The nature of the activated CO2 complex and the relationship between its stability and other structural and spectral properties was also analyzed by Principal Component Analysis (PCA) and artificial neural networks.


 Please wait while we load your content...
Please wait while we load your content...