Self-assembly and biological activities of ionic liquid crystals derived from aromatic amino acids†
Abstract
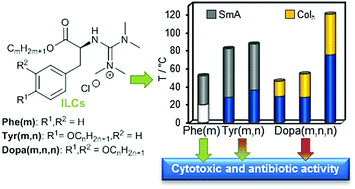
The self-assembly of amino acid-derived ionic liquid crystals (ILCs) into lamellar or micellar-like aggregates suggests that they might interact with biological membranes. To get some insight, guanidinium chlorides derived from the natural L-amino acids phenylalanine (Phe), tyrosine (Tyr) and 3,4-dihydroxyphenylalanine (DOPA) were synthesized and their mesomorphic properties were investigated via polarizing optical microscopy (POM), differential scanning calorimetry (DSC) and X-ray diffraction (SAXS, WAXS). Mesophase types depended on the number of alkoxy side chains. Phe- and Tyr-based ILCs with one and two side chains, respectively, self-assembled into smectic A bilayers (SmA2), while Dopa-derived ILCs with three side chains formed columnar (Colh) mesophases. The mesophase ranges for Phe ILCs increased steadily with side chain length, for Tyr- and Dopa-based ILCs, however, size matching effects were observed. To clarify whether the mesomorphic behaviour has an impact on biological properties, cytotoxic and antibacterial activities of the ILCs were studied. Phe and Tyr ILCs exhibited much higher cytotoxicities (against the L-929 mouse fibroblast cell line) and/or antibacterial activities (against Staphylococcus aureus) than Dopa ILCs, which were mostly inactive. Furthermore, within each series, the side chain length largely influenced the biological activity. Thus, the bulk mesophase behaviour appeared to correlate with the biological properties, in particular, the interactions with membranes, as shown by measuring the intracellular Ca2+ concentration in human monocytic U937 cells after treatment with the amino acid-based ILCs.
- This article is part of the themed collection: Bunsentagung 2017: Physical Chemistry for Life Sciences


 Please wait while we load your content...
Please wait while we load your content...