Polyhedral perspectives on the capacity limit of cathode compounds for lithium-ion batteries: a case study for Li6CoO4†
Abstract
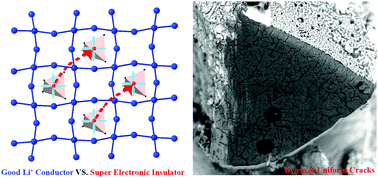
Anionic redox revealed reversibility in Li-rich layered oxides Li2MO3, which was strongly dependent on transition metal element M and geometrical structures. This sheds new light on high energy lithium ion batteries, and also inspires the question whether super high capacity is achievable in lithium compounds with stoichiometry close to Li2O. The tetrahedron structured Li6CoO4 is one kind of oxides with extremely high Li stoichiometry. In this study, DFT calculations combined with ex situ experimental stoichiometry detection are performed to investigate the delithiation mechanism during its full range. It reveals that Li6CoO4 undergoes two distinct delithiation reactions. The first process is a topotactic delithiation with conventional oxidation of Co2+ to Co3+ then continuing to Co4+; and the successive one is suggested to be decomposition reaction to Li2O and cobalt oxides. Surprisingly, very dense and uniform cracks are present over the cross-section of the micron-sized particles even at the early stage of charging with a capacity of 320 mA h g−1, the EDS of which suggests that the delithiated phase is homogeneous Li4CoO4. This phenomenon may be attributed to the unusually large discrepancy between ionic and electronic conductivity. CI-NEB calculations show a barrier of ca. 0.31 eV for the two dimensional Li ion migration network, corresponding to an ionic conductivity in the order of 10−6 S cm−1. On the other hand, there is lack of an effective path for electron hopping, because CoO4 tetrahedra are isolated from each other, pointing to electronic conductivity lower than 10−14 S cm−1. This study proposes a strategy to achieve super high capacity by invoking a reversible anionic redox to replace the decomposition reaction in tetrahedron structured lithium compounds. It is also worth pointing out that the geometrical connectivity of MO4 is crucial in the design of a new generation of cathode materials.


 Please wait while we load your content...
Please wait while we load your content...