Intermolecular charge fluxes and far-infrared spectral intensities of liquid formamide
Abstract
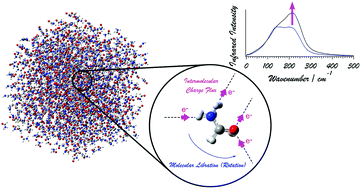
The intensity generation mechanisms of the far-infrared (far-IR) [or terahertz (THz)] spectrum of liquid formamide, particularly with regard to the behavior of electrons induced by modulations of the hydrogen-bonding conditions of the molecules, are examined theoretically. The theoretical analysis is done in the following two steps. First, density functional theory (DFT) calculations are carried out for the dimers and larger clusters of formamide to analyze the change in the dipole derivative (the square of which is proportional to the IR intensity) induced by hydrogen-bond formation. Then, by using the information derived in the first step, molecular dynamics-based spectral simulations are carried out. It is shown that, upon formation of a hydrogen bond, a change in the dipole derivative is induced along the direction of the hydrogen bond, and is reasonably modeled by the intermolecular charge flux mechanism, where a certain amount of electron density is transferred between molecules according to the modulation of the hydrogen-bond length, similarly to the case of liquid water. This model is in a form that is suitable for use in molecular dynamics-based spectral simulations. From these spectral simulations, it is found that the observed spectral features of the far-IR spectrum of liquid formamide are reasonably reproduced, and that the inclusion of the effect of intermolecular charge flux is essential for it. Contrary to the case of liquid water, the molecular libration band (rather than the molecular translation band) is significantly enhanced by the intermolecular charge flux. It is discussed that this difference is related to the geometrical relation between the hydrogen bonds and the atomic displacements in the molecular translations and librations (rotations). It is considered that, in general, in hydrogen-bonding liquids, modulations of hydrogen-bond lengths occurring upon dynamics of molecules give rise to intermolecular charge fluxes, significantly affecting the dipole derivatives, and hence, the IR intensity distributions in the spectra.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...