Coinage metal complexes of NHC-stabilized silyliumylidene ions†
Abstract
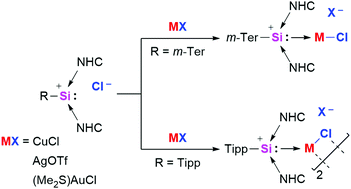
The first silyliumylidene ion coinage metal complexes are reported. Treatment of N-heterocyclic carbene (NHC) stabilized silyliumylidene ions (R = m-Ter, Tipp) with transition metal precursors (CuCl, AgOTf, (Me2S)AuCl) yields the first silyliumylidene coinage metal (M = Cu, Ag, Au) complexes, which have been fully characterized including X-ray diffraction analyses.


 Please wait while we load your content...
Please wait while we load your content...