Open Access Article
Open Access ArticleRuthenium-catalyzed C–H oxygenation of quinones by weak O-coordination for potent trypanocidal agents†
Gleiston G.
Dias
ab,
Torben
Rogge
b,
Rositha
Kuniyil
b,
Claus
Jacob
 c,
Rubem F. S.
Menna-Barreto
d,
Eufrânio N.
da Silva Júnior
c,
Rubem F. S.
Menna-Barreto
d,
Eufrânio N.
da Silva Júnior
 *a and
Lutz
Ackermann
*a and
Lutz
Ackermann
 *b
*b
aInstitute of Exact Sciences, Department of Chemistry, Federal University of Minas Gerais, Belo Horizonte, MG, 31270-901, Brazil. E-mail: eufranio@ufmg.br
bInstitut für Organische und Biomolekulare Chemie, Georg-August-Universität, Göttingen, Tammannstraße 2, 37077 Göttingen, Germany. E-mail: lutz.ackermann@chemie.uni-goettingen.de
cDivision of Bioorganic Chemistry, School of Pharmacy, University of Saarland, D-66123 Saarbruecken, Germany
dOswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, RJ, 21045-900, Brazil
First published on 16th October 2018
Abstract
Ruthenium-catalysis enabled the C-5 selective C–H oxygenation of naphthoquinones, and also sets the stage for the site-selective introduction of a hydroxyl group into anthraquinones. A-ring modified naphthoquinoidal compounds represent an important class of bioactive quinones for which the present study encompasses the first C–H oxygenation strategy by weak O-coordination.
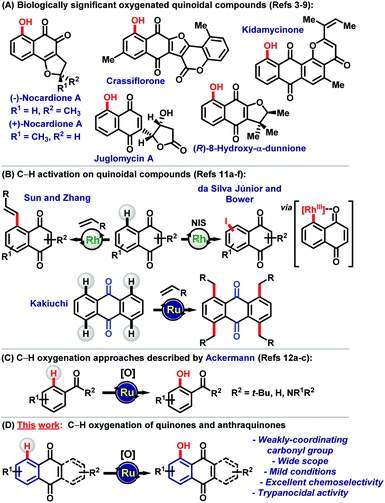
Among the versatile scaffolds studied in Medicinal Chemistry, quinones represent a privileged class of substance.1 Thus, quinoidal compounds are involved in biologically relevant processes, such as in the electron transport chain, redox mediators, and as alkylating agents in a broad spectrum of biological processes.2 For example, nocardiones A are notable bioactive naphthoquinoidal molecules. (−)-Nocardione A is a Cdc25B tyrosine phosphatase inhibitor, while (+)-nocardione A presents antifungal and cytotoxic activity with the ability to kill human myeloid leukemia cell lines via a mechanism of action associated with apoptosis.3 Remarkable 1,4-naphthoquinones (1,4-NQs) include crassiflorone,4 a natural product isolated from the stem bark of the African ebony (persimmon) tree Diospyros crassiflora with anti-mycobacterial and anti-gonorrhoeal properties,5 among others. Furthermore, juglomycin A,6 (R)-8-hydroxy-R-dunnione7 and kidamycinone8 are examples of important quinoidal compounds with diverse bioactivity9 (Scheme 1A).
The quinones depicted in Scheme 1A bear a hydroxyl group as a recurring structural motif in the benzenoid A-ring, which is usually installed in the first steps of their previously reported respective syntheses.3,4,8 The preparation of such molecules via late-stage functionalization by C–H activation logic has thus far not been addressed.10 This C–H activation strategy can be an instrument to maximize the synthesis process with the preparation of the target molecules in a reduced number of steps with overall significantly improved yields. Late-stage functionalization of quinones is not easily achieved due to the following aspects, including: (a) generally deactivated quinoidal systems present low nucleophilicity at the benzenoid A-ring; (b) the weakly coordinating ability of the B-ring carbonyls towards transition metal catalysts and (c) the difficulty in developing general reaction protocols capable of providing favorable results with the use of different classes of quinonoid systems.10
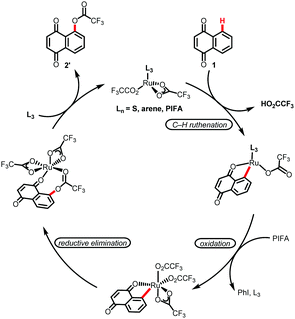
In this context, Sun and Zhang, Kakiuchi, Bower and da Silva Júnior groups11 have recently developed useful methods for C–H functionalization of naphthoquinones and anthraquinones (Scheme 1B). Despite the recent development in the area, direct C–H oxygenations of quinonoid compounds have been proven to be elusive. Given our recent success in terms of ruthenium-catalyzed C–H oxygenations with weakly coordinating ketones, amides and aldehydes12 (Scheme 1C), herein, we describe an efficient process for the direct, site-selective installation of a hydroxyl group in a broad range of naphthoquinones and anthraquinones. For the first time, a C–H bond oxygenation strategy was thus established for the synthesis of quinonoid derivatives with unique bioactivity (Scheme 1D).
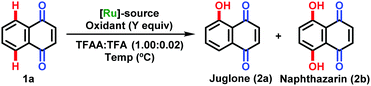
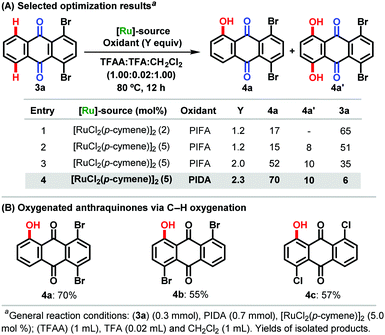
Preliminary studies involved the reaction of 1,4-naphthoquinone (1a) with PhI(CF3CO2)2 (PIFA) as an oxidant and [RuCl2(p-cymene)]2 as the catalyst (Table 1, entry 1). Here, juglone (2a) and naphthazarin (2b) were obtained in 53 and 42% yield, respectively. Further refinement aiming at minimizing the amount of the ruthenium-based catalyst afforded products 2a and 2b in 87 and 5% yield when 2 mol% of [RuCl2(p-cymene)]2 and 1.2 equivalents of PIFA were used (entry 2). Despite the efficiency of the catalyst, we continued our efforts towards alternative oxidation sources. Hence, PIDA and NH4S2O8 were also evaluated as oxygenation agents, albeit with limited success (entries 3 and 4). We also tested alternative sources of catalysts with the use of rhodium, cobalt and palladium complexes, but promising results were not observed (entries 5–8). A control experiment confirmed the essential role of the ruthenium catalyst (entry 9). Fine tuning of the reaction temperature enabled the formation of juglone (2a) in 92% yield (entry 10). Viable substrates for the new C–H oxygenation strategy are outlined in Scheme 2.
| Entry | [Ru]-Source (mol%) | Oxidant | Y | Temp (°C) | 2a | 2b |
|---|---|---|---|---|---|---|
| a General reaction conditions: (1a) (0.4 mmol), PIFA (0.5 mmol), [RuCl2(p-cymene)]2 (5.0 or 2.0 mol%); (TFAA) (1 mL) and TFA (0.02 mL), 18 h. b 12 h. PIFA = PhI(CF3CO2)2. PIDA = PhI(CH3CO2)2. NR = for all cases starting material 1a was recovered, with the exception of the reaction with Pd(OAc)2, here degradation of the products was observed. Yields of isolated products. | ||||||
| 1 | [RuCl2(p-cymene)]2 (5) | PIFA | 2.0 | 110 | 53 | 42 |
| 2 | [RuCl2(p-cymene)]2 (2) | PIFA | 1.2 | 80 | 87 | 5 |
| 3 | [RuCl2(p-cymene)]2 (2) | PIDA | 1.2 | 80 | 71 | Traces |
| 4 | [RuCl2(p-cymene)]2 (2) | NH4S2Os | 1.2 | 80 | NR | NR |
| 5 | RuCl3·H20 (5) | PIFA | 1.2 | 80 | NR | NR |
| 6 | [RhCp*Cl2]2 (5) | PIFA | 1.2 | 80 | NR | NR |
| 7 | CoCp*(CO)I2(5) | PIFA | 1.2 | 80 | NR | NR |
| 8 | Pd(OAc)2 (5) | PIFA | 1.2 | 80 | NR | NR |
| 9b | — | PIFA | 1.2 | 80 | NR | NR |
| 10 | [RuCl 2 (p-cymene)] 2 (2) | PIFA | 1.2 | 80 | 92 | 3 |
Structural assignments were based on detailed NMR analysis and X-ray diffraction analysis of products 2c–2f, 2h, 2i and 2j.
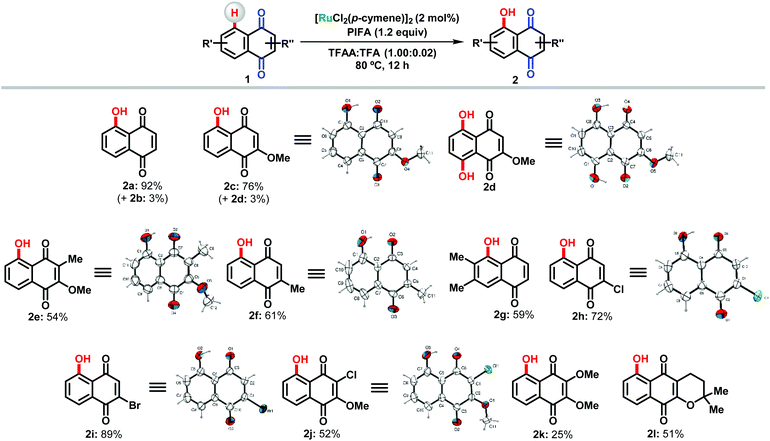
In general, quinones were prepared in moderate to high yields, tolerating the presence of electron withdrawing and donating groups. In most cases, the dihydroxylated product was not formed or was observed in only 3% yield (2b and 2d). Our method proved effective even for the preparation of the hydroxylated derivative of α-lapachone (2l), a natural product related to the lapachol family with remarkable antitumor activity.13
Subsequently, the synthetic utility of the ruthenium(II)-catalyzed C–H oxygenation was reflected by the chemo-selective diversification of anthraquinones 3. Here, a slight modification of the reaction conditions featured the use of PIDA, allowing for the C–H oxygenations with excellent levels of positional and chemo-selectivities in terms of mono-functionalization, fully tolerating, inter alia, chloro and bromo functional groups (Scheme 3).
Based on our previous reports, a plausible catalytic cycle is initiated by carboxylate-assisted C–H ruthenation,12,14 along with subsequent oxidation-induced reductive elimination (Scheme 4).14,15
Neglected tropical diseases (NTDs) comprehend a group of seventeen illnesses that affect more than one billion people. Among NTDs, Chagas disease caused by the hemoflagellate protozoa Trypanosoma cruzi presents significant mortality and morbidity, especially in low-income populations in Latin America endemic countries.16,17 Recently, the intense flux of immigration due to globalization has changed the distribution of Chagas disease patients, resulting in the presence of infected people in non-endemic countries such as European countries, the United States, Japan or Australia, where the transmission is sustained by blood transfusion or congenital routes.18,19 Since the 1960s up to now, the clinical chemotherapy for Chagas disease has been based on two nitroderivatives, benznidazole and nifurtimox, that present important side effects and debatable anti-T. cruzi activity in chronic patients.20 The absence of alternatives for the most severe phase of this illness leads to the continuous search for novel trypanocidal drugs.21 In this context, in the last few years, we have evaluated diverse naphthoquinones against the parasite and proved the potential trypanocidal effect of these compounds.11f To understand the biological importance of A-ring modified quinoidal compounds and to continue our efforts towards the development of potent trypanocidal agents, we herein disclose the evaluation of 1,4-NQs against T. cruzi. Thus, compounds 2c, 2e, 2k and 2l were not active against the parasite with IC50/24 h > 1000.0 μM. To our delight, six compounds showed activity against T. cruzi with IC50 in the range of 176.1 to 29.5 μM (Table S1), highlighting 2h (IC50 = 32.9 μM) and 2i (IC50 = 29.5 μM). In comparison with the standard drug benznidazole (IC50 = 103.6 μM), 2h and 2i are 3.1 and 3.5-fold more active, respectively (Fig. 1).
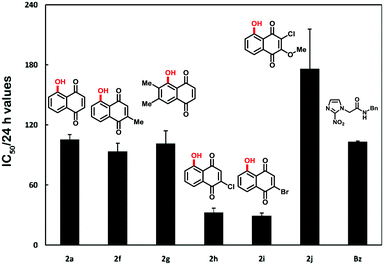 | ||
| Fig. 1 Most active naphthoquinones on trypomastigote forms of T. cruzi. Benznidazole, a standard drug for the treatment of chagasic patients, was used as a control. IC50/24 h values in μM. | ||
In conclusion, we have reported on an efficient C–H oxygenation strategy for naphthoquinones and anthraquinones. Thus, carboxylate-assisted22 ruthenium(II) catalysis enabled the step-economical synthesis of potent bioactive compounds with ample scope. The obtained C–H hydroxylated naphthoquinones displayed unique trypanocidal activities.
E. N. S. J. acknowledges funding from CNPq 404466/2016-8 and PQ 305741/2017-9, CAPES and DAAD for exchange PROBRAL program (99999.008126/2015-01) and Capes-Humboldt research fellowship programme for experienced researchers (Proc. No. 88881.145517/2017-01). L. A. acknowledges funding from the DFG (Gottfried-Wilhelm-Leibniz prize).
Conflicts of interest
There are no conflicts to declare.References
- (a) R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow and D. A. Pippin, Comb. Chem. High Throughput Screening, 2004, 7, 473 CrossRef CAS; (b) L. Costantino and D. Barlocco, Curr. Med. Chem., 2010, 5, 381 CAS; (c) C. D. Duarte, E. J. Barreiro and C. A. M. Fraga, Mini-Rev. Med. Chem., 2007, 7, 1108 CrossRef CAS PubMed; (d) K. Bondensgaard, M. Ankersen, H. Thøgersen, B. S. Hansen, B. S. Wulff and R. P. Bywater, J. Med. Chem., 2004, 47, 888 CrossRef CAS PubMed.
- (a) R. H. Thomson, Naturally Occurring Quinones, Academic Press, London, 2nd edn, 1971 Search PubMed; (b) R. H. Thomson, Naturally Occurring Quinones III Recent Advances, Chapman and Hall, London, 3rd edn, 1987 Search PubMed; (c) G. Powis, Pharmacol. Ther., 1987, 35, 57 CrossRef CAS PubMed; (d) P. J. O'Brien, Chem.-Biol. Interact., 1991, 80, 1 CrossRef; (e) E. A. Hillard, F. C. Abreu, D. C. Ferreira, G. Jaouen, M. O. F. Goulart and C. Amatore, Chem. Commun., 2008, 2612 RSC.
- (a) T. Otani, Y. Sugimoto, Y. Aoyagi, Y. Igarashi, T. Furumai, N. Saito, Y. Yamada, T. Asao and T. Oki, J. Antibiot., 2000, 53, 337 CrossRef CAS PubMed; (b) D. L. J. Clive and S. P. Fletcher, Chem. Commun., 2003, 2464 RSC.
- J. Padwal, W. Lewis and C. J. Moody, Org. Biomol. Chem., 2011, 9, 3484 RSC.
- E. Uliassi, G. Fiorani, R. L. Krauth-Siegel, C. Bergamini, R. Fato, G. Bianchini, J. C. Menéndez, M. T. Molina, E. L. Montero, F. Falchi, A. Cavalli, S. Gul, M. Kuzikov, B. Ellinger, G. Witt, C. B. Moraes, L. H. Freitas-Júnior, C. Borsari, M. P. Costi and M. L. Bolognesi, Eur. J. Med. Chem., 2017, 141, 138 CrossRef CAS PubMed.
- H. Maeda and G. A. Kraus, J. Org. Chem., 1996, 61, 2986 CrossRef CAS PubMed.
- X.-H. Cai, X.-D. Luo, J. Zhou and X.-J. Hao, J. Nat. Prod., 2005, 68, 797 CrossRef CAS PubMed.
- T. Mabit, A. Siard, M. Pantin, D. Zon, L. Foulgoc, D. Sissouma, A. Guingant, M. Mathé-Allainmat, J. Lebreton, F. Carreaux, G. Dujardin and S. Collet, J. Org. Chem., 2017, 82, 5710 CrossRef CAS PubMed.
- (a) K. Ushiyama, N. Tanaka, H. Ono and H. Ogata, Jpn. J. Antibiot., 1971, 24, 197 CrossRef CAS PubMed; (b) A. S. Soares, F. L. Barbosa, A. L. Rüdiger, D. L. Hughes, M. J. Salvador, A. R. Zampronio and M. E. A. Stefanello, J. Nat. Prod., 2017, 80, 1837 CrossRef CAS PubMed.
- (a) J. C. K. Chu and T. Rovis, Angew. Chem., Int. Ed., 2018, 57, 62–101 CrossRef CAS PubMed; (b) E. N. da Silva Júnior, G. A. M. Jardim, R. S. Gomes, Y.-F. Liang and L. Ackermann, Chem. Commun., 2018, 54, 7398 RSC; (c) P. Gandeepan and L. Ackermann, Chem, 2018, 4, 199–222 CrossRef CAS; (d) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Chem. Rev., 2017, 117, 9333–9403 CrossRef CAS PubMed; (e) D. L. Davies, S. A. Macgregor and C. L. McMullin, Chem. Rev., 2017, 117, 8649–8709 CrossRef CAS PubMed; (f) C. Borie, L. Ackermann and M. Nechab, Chem. Soc. Rev., 2016, 45, 1368–1386 RSC; (g) M. H. Shaw and J. F. Bower, Chem. Commun., 2016, 52, 10817–10829 RSC; (h) J. Li and L. Ackermann, Nat. Chem., 2015, 7, 686–687 CrossRef PubMed; (i) K. Shin, H. Kim and S. Chang, Acc. Chem. Res., 2015, 48, 1040–1052 CrossRef CAS PubMed; (j) S. I. Kozhushkov and L. Ackermann, Chem. Sci., 2013, 4, 886–896 RSC; (k) B. Li and P. H. Dixneuf, Chem. Soc. Rev., 2013, 42, 5744–5767 RSC; (l) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed; (m) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS PubMed; (n) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed; (o) H. M. Davies and J. R. Manning, Nature, 2008, 451, 417–424 CrossRef CAS PubMed , and references cited therein.
- (a) K. Kitazawa, T. Kochi, M. Sato and F. Kakiuchi, Org. Lett., 2009, 11, 195 CrossRef PubMed; (b) D. Matsumura, K. Kitazawa, S. Terai, T. Kochi, Y. Le, M. Nitani, Y. Aso and F. Kakiuchi, Org. Lett., 2012, 14, 3882 CrossRef CAS PubMed; (c) C. Zhang, M. Wang, Z. Fan, L.-P. Sun and A. Zhang, J. Org. Chem., 2014, 79, 7626 CrossRef CAS PubMed; (d) G. A. M. Jardim, E. N. da Silva Júnior and J. F. Bower, Chem. Sci., 2016, 7, 3780 RSC; (e) G. A. M. Jardim, T. L. Silva, M. O. F. Goulart, C. A. de Simone, J. M. C. Barbosa, K. Salomão, S. L. de Castro, J. F. Bower and E. N. da Silva, Júnior, Eur. J. Med. Chem., 2017, 136, 406 CrossRef CAS PubMed; (f) G. A. M. Jardim, W. X. C. Oliveira, R. P. de Freitas, R. F. S. Menna-Barreto, T. L. Silva, M. O. F. Goulart and E. N. da Silva Júnior, Org. Biomol. Chem., 2018, 16, 1686 RSC.
- (a) V. S. Thirunavukkarasu and L. Ackermann, Org. Lett., 2012, 14, 6206 CrossRef CAS PubMed; (b) F. Yang and L. Ackermann, Org. Lett., 2013, 15, 718 CrossRef CAS PubMed; (c) F. Yang, K. Rauch, K. Kettelhoit and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 11285–11288 CrossRef CAS PubMed; (d) Y.-H. Sun, T.-Y. Sun, Y.-D. Wu, X. Zhang and Y. Rao, Chem. Sci., 2016, 7, 2229–2238 RSC; (e) X. Yang, G. Shan and Y. Rao, Org. Lett., 2013, 15, 2334–2337 CrossRef CAS PubMed; (f) V. S. Thirunavukkarasu, S. I. Kozhushkova and L. Ackermann, Chem. Commun., 2014, 50, 29–39 RSC.
- D. C. M. Ferreira, I. Tapsoba, S. Arbault, Y. Bouret, M. S. A. Moreira, A. V. Pinto, M. O. F. Goulart and C. Amatore, ChemBioChem, 2009, 10, 528 CrossRef CAS PubMed.
- For examples of ruthenium-catalyzed C–H oxygenations under arene ligand-free conditions, see: V. S. Thirunavukkarasu, J. Hubrich and L. Ackermann, Org. Lett., 2012, 14, 4210–4213 CrossRef CAS PubMed.
- For arene ligand-free ruthenium catalysts for C–H activations, see: (a) L. Ackermann, A. Althammer and R. Born, Synlett, 2007, 2833–2836 CrossRef CAS; (b) L. Ackermann, R. Born and P. Álvarez-Bercedo, Angew. Chem., Int. Ed., 2007, 46, 6364–6367 CrossRef CAS PubMed; (c) L. Ackermann, A. Althammer and R. Born, Tetrahedron, 2008, 64, 6115–6124 CrossRef CAS; (d) L. Ackermann and R. Vicente, Org. Lett., 2009, 11, 4922–4925 CrossRef CAS PubMed; (e) P. Marce, A. J. Paterson, M. F. Mahon and C. G. Frost, Catal. Sci. Technol., 2016, 6, 7068–7076 RSC; (f) J. McIntyre, I. Mayoral-Soler, P. Salvador, A. Poater and D. J. Nelson, Catal. Sci. Technol., 2018, 8, 3174–3182 RSC; (g) M. Simonetti, D. M. Cannas, X. Just-Baringo, I. J. Vitorica-Yrezabal and I. Larrosa, Nat. Chem., 2018, 10, 724–731 CrossRef CAS PubMed; (h) K. Korvorapun, N. Kaplaneris, T. Rogge, S. Warratz, A. C. Stückl and L. Ackermann, ACS Catal., 2018, 8, 886–892 CrossRef CAS; (i) F. Fumagalli, S. Warratz, S.-K. Zhang, T. Rogge, C. Zhu, A. C. Stückl and L. Ackermann, Chem. – Eur. J., 2018, 24, 3984–3988 CrossRef CAS PubMed.
- (a) G. A. Schmunis and Z. E. Yadon, Acta Trop., 2010, 115, 14 CrossRef PubMed; (b) Y. Jackson, V. M. Herrera and J. Gascon, Bull. W. H. O., 2014, 92, 771 CrossRef PubMed.
- (a) L. Conteh, T. Engels and D. H. Molyneux, Lancet, 2010, 375, 239 CrossRef PubMed; (b) A. Rassi and J. A. Marin-Neto, Lancet, 2010, 375, 1388 CrossRef PubMed.
- (a) C. Bern and S. P. Montgomery, Clin. Infect. Dis., 2009, 49, e52 CrossRef PubMed; (b) C. Bern, S. Kjos, M. J. Yabsley and S. P. Montgomery, Clin. Microbiol. Rev., 2011, 24, 655 CrossRef PubMed.
- WHO. Expert Committee. Control of Chagas Disease, World Health Organization, Brasilia, Brazil, 2002. WHO technical report series 905 Search PubMed.
- (a) M. N. Soeiro and S. L. de Castro, Expert Opin. Ther. Targets, 2009, 13, 105 CrossRef CAS PubMed; (b) J. A. Urbina, Acta Trop., 2010, 115, 55 CrossRef PubMed.
- J. Rodriques Coura and S. L. de Castro, Mem. Inst. Oswaldo Cruz, 2002, 97, 3 CrossRef PubMed.
- L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data for all compounds. CCDC 1859287 (2i), 1859288 (2f), 1859289 (2c), 1859290 (2d), 1859453 (2j), 1859454 (2e) and 1859455 (2h). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc07572g |
| This journal is © The Royal Society of Chemistry 2018 |