Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A generalized approach for NMR studies of lipid–protein interactions based on sparse fluorination of acyl chains†
Alfredo
De Biasio‡§
 *a,
Alain
Ibáñez de Opakua§
a,
Mark J.
Bostock
*a,
Alain
Ibáñez de Opakua§
a,
Mark J.
Bostock
 b,
Daniel
Nietlispach
b,
Daniel
Nietlispach
 b,
Tammo
Diercks
b,
Tammo
Diercks
 *a and
Francisco J.
Blanco
*a and
Francisco J.
Blanco
 *ac
*ac
aCIC bioGUNE, Derio, Spain. E-mail: tdiercks@cicbiogune.es; fblanco@cicbiogune.es
bDepartment of Biochemistry, University of Cambridge, Cambridge, UK
cIKERBASQUE, Basque Foundation for Science, Bilbao, Spain
First published on 15th June 2018
Abstract
Sparse lipid fluorination enhances the lipids' 1H signal dispersion, enables clean molecular distinction by 19F NMR, and evinces micelle insertion of proteins via fluorine-induced signal shifts. We present a minimal fluorination scheme, and illustrate the concept on di-(4-fluoro)-heptanoylphosphatidylcholine micelles and solubilised seven-helix transmembrane pSRII protein.
Lipid–protein interactions within cell membranes remain poorly characterized despite high relevance in physiological and pathological processes. Bound lipid molecules are rarely visible in the known membrane protein structures as the conformational heterogeneity and dynamics in lipid layers impede their orderly crystallisation for X-ray1 or fine classification for cryo-EM2 studies. In contrast, NMR spectroscopy does not require molecular order and generic lipid–protein contacts may be sampled more coarsely to gauge protein insertion into the lipid layer, e.g. by use of paramagnetic agents.3 High resolution analyses of lipid–protein contacts require more range-limited, directed, and unambiguous NMR indicators. Ultimately, specific lipid–protein contacts are revealed by intermolecular 1H, 1H NOE signals,4 but their identification and resolution are impeded by the generally poor 1H signal dispersion in lipid acyl chains. The classic workaround to correlate 1H with 13C frequencies is impractical for lipids, where 13C isotope enrichment may not resolve all overlap problems and provides no distinct molecular marker from a likewise 13C labelled protein to single out inter- from intramolecular NOEs.
We propose an approach for NMR studies of lipid–protein interactions that relies on sparse fluorination of the lipid acyl chains and exploits fluorine both indirectly, as a shift reagent affecting nearby NMR spins, and directly, as 19F isotope with unique NMR properties. The introduced fluorine atoms then solve the NMR resolution problem for acyl chains by (i) increasing their 1H signal dispersion via local deshielding, (ii) enabling clean molecular distinction via19F filtering, and (iii) allowing further resolution enhancement via19F editing. Fluorine may also induce chemical shift perturbation (CSP) in nearby protein spins to indicate protein insertion into lipid layers similar to paramagnetic markers, but with minimal steric impact. In this introductory study we focus on the indirect NMR effects of fluorine and demonstrate its utility as both intra- and intermolecular shift reagent. We derive a minimal fluorination scheme for acyl chains and show that a single hydrogen-to-fluorine substitution induces a fully resolved 1H spectrum for 4F-DHPC7 without impairing micelle formation or stabilisation of the phototaxis receptor sensory rhodopsin II (pSRII). The H/F substitution in the lipid provokes CSP for amide groups near both ends of the seven-helix bundle, elucidating its micelle insertion.
19F NMR studies on partially fluorinated membrane proteins have already yielded information on their structure in membrane mimicking environments.5,6 Complementary studies on fluorinated lipids focused on analyzing lipid phase properties.7–10 Use of fluorinated lipids and 19F NMR to investigate protein–lipid interactions was suggested before,11 but not explored presumably for technical deficiencies with regard to (i) preparing the required quantities of membrane protein with isotope enrichment and reconstituted in micelles or bicelles; (ii) synthesizing sparsely fluorinated lipids; and (iii) NMR methodology and hardware. Advances in these fields12 now make it possible to develop the experimental approach delineated here.
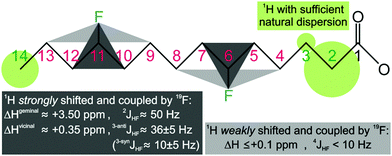
Replacing hydrogen by fluorine is a chemical modification that may affect the lipid's biophysical properties and interaction with membrane proteins. It also reduces the 1H density and, thus, coverage of (fluoro)lipid–protein contacts via1H, 1H NOE signals. Hence, the number of H/F substitutions must be minimized, but still ensure enough fluorine induced deshielding to separate all acyl chain 1H signals. Its reach may be deduced from the spectrum of 1-palmitoyl-2-(16-fluoropalmitoyl)phosphatidylcholine13 with one terminal fluorine on acyl chain 2 (Avanti Polar Lipids). Comparison of 1H, 1H-TOCSY traces of both chains (Fig. S1, ESI†) reveals that the single H/F substitution shifts the geminal 1H by ca. +3.5 ppm, vicinal 1H by +0.35 ppm, and the 1H four bonds away by +0.09 ppm. Also, heteronuclear JHF coupling is strong to geminal (2JHF ≈ 50 Hz) and vicinal 1H (3JHF ≈ 10–35 Hz), and still notable over four bonds (4JHF < 10 Hz). The induced 1H deshielding over ±2 carbon positions stipulates optimal fluorine spacing by 5 positions. Even unmodified acyl chains show resolved signals for the 1H bound to C2 (>2.0 ppm), C3 (>1.5 ppm), and the terminal Cω (<1.0 ppm), while those bound from C4 to Cω − 1 overlap at 1.4 ± 0.1 ppm. Acyl chains with zC > 5 carbon atoms, thus, require 1H dispersion enhancement by single H/F substitution at carbon atoms i ± m·5, starting ≤3 positions from the first (C3) or last (Cω) resolved CHn group, i.e. at positions i ≤ 6 or i ≥ ω − 3. This minimal fluorination scheme (Fig. 1) implies introduction of zF ≤ zC/5 fluorine atoms (rounded down), leaving zH = 2·zC − 1 − zF protons. Thus, less than 11% of all acyl chain protons need to be exchanged for fluorine.
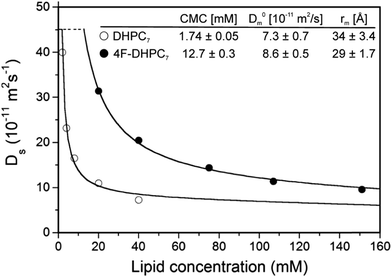
Our transmembrane test protein pSRII14 is best stabilized in diheptanoylphosphatidylcholine (DHPC7) micelles, where the minimal fluorination scheme suggests H/F substitution at C4 or C5. Opting for the former, we obtained di-(4-fluoro)heptanoylphosphatidylcholine (4F-DHPC7) by custom synthesis (Avanti Polar Lipids). NMR diffusion measurements proved stable micelle formation up to at least 323 K (Fig. S2, ESI†). A CMC of 12.7 mM was derived from the concentration dependence of diffusion coefficients15 (Fig. 2), about sevenfold higher than for unflourinated DHPC7 (1.7 mM) but similar to DHPC6 (14 mM).15 The also derived hydrodynamic micelle radii, rm, of some 29 Å for 4F-DHPC7 and 34 Å for DHPC7 may suggest smaller micelles for 4F-DHPC7, but still agree within errors.
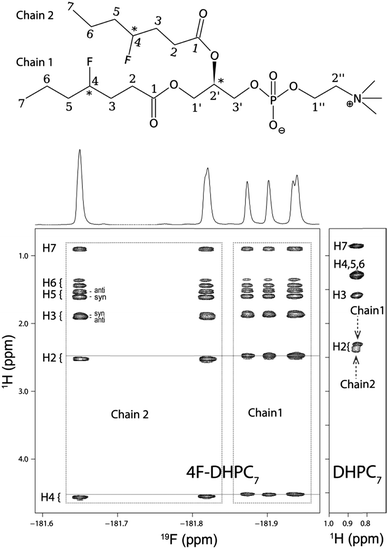
The acyl chain 1H NMR spectrum in the micelle (Fig. 3) shows the typical degeneracy of the central H4 to H6 protons for DHPC7, but full dispersion for 4F-DHPC7 induced by the fluorine atom. Even the diastereotopic H3, H5, and H6 methylene protons are resolved, and larger (36 ± 5 Hz) or smaller (10 ± 5 Hz) 3JHF coupling constants identify the H3 and H5 protons in anti- or syn-clinal position to the F4 atom, respectively. The latter enables further editing in a 19F dimension, where correlation with all acyl 1H signals is achieved in a 2D 1H, 19F TOCSY-COLOC16 spectrum (Fig. 3). Remarkably, the 19F spectrum of 4F-DHPC7 shows six distinct signals, with double integral for both downfield signals that correlate with also downfield shifted H2 to H4 signals. H2 correlation with glycerol C1′ or C2′ by long-range 13C HSQC then assigns the four upfield 19F signals to acyl chain 1 and both degenerate downfield 19F signals to chain 2. The distinct 19F signals most likely arise from the four stereoisomers of 4F-DHPC7 with its 3 chiral carbon atoms, where the sn2-glycerol C2′ has fixed R configuration while H/F substitution at both acyl chain C4 was not stereospecific. Only the acyl 13CO signals show dispersion similar to 19F and have higher shifts for chain 1, contrary to higher shifted 19F, 1H, and aliphatic 13C signals in chain 2. Table S1 (ESI†) lists the complete assignment for 4F-DHPC7 micelles.
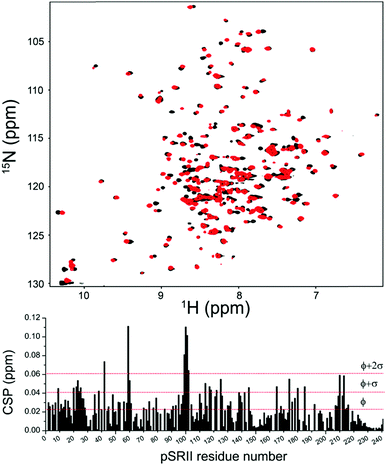
We next verified that 4F-DHPC7 micelles can stabilize pSRII as efficiently as DHPC7 for high resolution NMR studies.14 Overall similar TROSY spectra of pSRII in 4F-DHPC7 and DHPC7 micelles (Fig. 4) indicate matching protein structures, but some amide signals differ more clearly. After reassigning 194 signals (84% of expected 230 signals, cf. Table S2, ESI†) we quantified their CSP caused by the H/F substitution in 4F-DHPC7 (Fig. 4, below). 26 residues show significant CSP larger than the average of all 194 CSP values (ϕCSP = 0.023 ppm) plus their standard deviation (σCSP = 0.019 ppm), including 6 residues with a CSP > ϕCSP + 2·σCSP. These residues cluster within two bands near both ends of the transmembrane seven-helix bundle and indicate proximity to the fluorine atoms in 4F-DHPC7, thus guiding the sketched alignment of lipid molecules adjacent to embedded pSRII (Fig. 5). The picture for micelle insertion emerging from fluorine induced CSP mapping agrees in detail with that from paramagnetic relaxation enhancement:14 pSRII inserts asymmetrically into the micelle, while the rather large gap (ca. 10 Å) between opposed 4F-DHPC7 molecules allows for the suggested tail-on filling by further lipid molecules according to a prolate micelle model. This agreement validates fluorine induced CSP as an alternative indicator for lipid layer insertion. In contrast to bulky paramagnetic markers, fluorine may be inserted anywhere along the acyl chain, thus allowing to probe lipid layer insertion stepwise and with more precision owing to the shorter reach of fluorine induced CSP.
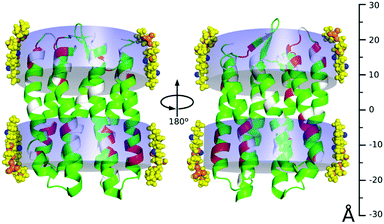 | ||
| Fig. 5 Model of pSRII (PDB 2KSY) insertion into 4F-DHPC7 micelles. Residues with a fluorine induced amide CSP above (red; below = green) the average plus one standard deviation cluster near both ends of the seven-helix bundle (unassigned residues in grey; the omitted disordered C-terminal residues 222–241 show no significant CSP). 4F-DHPC7 molecules were modeled adjacent to pSRII by aligning their fluorine atoms (blue; orange = oxygen, phosphorus, nitrogen) with the bands of induced CSP (transparent blue). The scale on the right is centered with the seven-helix bundle to underscore the indicated asymmetric micelle insertion of pSRII, where helices protrude farther on the cytosolic side (at the bottom). | ||
Besides deshielding through space, fluorine induced CSP might conceivably also derive from local rearrangements in the protein. Yet these should not be significant since even the major CSP amplitudes remain too small and mostly localise on amide groups forming part of well defined secondary structure in pSRII. There, any larger conformational changes would impair the stabilising hydrogen bond network and levy a high energetic penalty, which should also affect local dynamics and NMR relaxation rates. Yet the ratios of pSRII amide signal intensities in 4F-DHPC7versus DHPC7 micelles, which relate to their net T2 relaxation times, clearly do not correlate with the CSP values (Fig. S3, ESI†). Normalised pSRII signal intensities nevertheless appear to be ca. 10% lower on average in 4F-DHPC7 micelles, possibly due to some line broadening from more lipid exchange with the solution, as indicated by the higher CMC.
A next step along the proposed approach would be to explore the possibilities for 19F NMR enabled by the sparsely fluorinated lipids, where the main challenge is efficient inphase correlation of all acyl chain 1H with the sparse 19F spins for global selection by 19F filtering. This then allows implementation of diagonal-free 1H[19F], 1H[13C/15N] NOESY experiments with orthogonal isotope filtering for 19F (on the acyl chain) versus13C or 15N bound protons (on the protein) to only observe intermolecular lipid–protein contacts. While complete 1H, 19F correlation was obtained with the 1H, 19F TOCSY-COLOC16 experiment (Fig. 3), 1H, 19F Hetero-TOCSY mixing17 would achieve more efficient, robust, and directly inphase heteronuclear transfer with accompanying homonuclear 1H, 1H TOCSY transfer that is generally needed for correlation beyond the reach of heteronuclear nJHF coupling. Implementation, however, requires special NMR hardware (i.e. two high-frequency amplifiers for simultaneous 1H and 19F pulsing). The envisaged diagonal-free 1H[19F], 1H[13C/15N] NOESY experiment to selectively observe lipid–protein contacts then derives from a standard NOESY-(13C/15N)HSQC by prepending the NOESY with a heteronuclear 19F, 1H TOCSY transfer step. This concatenation corresponds to the 31P, 1H Hetero-TOCSY-NOESY experiment18 that was successfully used for efficient RNA assignment. The 19F, 1H analogue should be an order of magnitude more sensitive owing to the much larger heteronuclear couplings (nJHF ≤ 50 Hz vs.nJHP < 12 Hz) and polarisation (γF/γP ≈ 2.3) exploited.
The lipid chosen for this introductory study forms micelles that may not be the most appropriate membrane mimics. Nevertheless, two key results of this study have general validity: properly selected sparse fluorination of acyl chains greatly enhances their 1H signal dispersion, and also causes notable CSP in membrane proteins that gauge their insertion into the lipid phase. Other sparsely fluorinated lipids forming more membrane-like phases, such as nanodiscs,19 may next be tested to establish the generality of the proposed approach. The primary challenges expected for these larger, more asymmetric lipid systems arise from possibly faster 19F T2 relaxation. Yet while this would compromise 19F editing, 19F filtering may still be implemented with minimal sensitivity to T2 relaxation.
We thank MINECO for the Severo Ochoa Excellence Accreditation (SEV-2016-0644) and for the grant CTQ2017-83810-R to FJB. We dedicate this communication to the memory of Chris Spronk.
Conflicts of interest
There are no conflicts to declare.References
- S. H. White, A. S. Ladokhin, S. Jayasinghe and K. Hristova, J. Biol. Chem., 2001, 276, 32395–32398 CrossRef PubMed.
- S. Rawson, S. Davies, J. D. Lippiat and S. P. Muench, Mol. Membr. Biol., 2016, 33, 12–22 CrossRef PubMed.
- R. S. Prosser, F. Evanics, J. L. Kitevski and S. Patel, Biochim. Biophys. Acta, 2007, 1768, 3044–3051 CrossRef PubMed.
- C. Fernandez, C. Hilty, G. Wider and K. Wuthrich, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 13533–13537 CrossRef PubMed.
- J. L. Kitevski-LeBlanc and R. S. Prosser, Prog. Nucl. Magn. Reson. Spectrosc., 2012, 62, 1–33 CrossRef PubMed.
- T. Didenko, J. J. Liu, R. Horst, R. C. Stevens and K. Wuthrich, Curr. Opin. Struct. Biol., 2013, 23, 740–747 CrossRef PubMed.
- J. M. Sturtevant, C. Ho and A. Reimann, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 2239–2243 CrossRef.
- B. McDonough, P. M. Macdonald, B. D. Sykes and R. N. McElhaney, Biochemistry, 1983, 22, 5097–5103 CrossRef PubMed.
- P. M. Macdonald, B. D. Sykes and R. N. McElhaney, Biochemistry, 1985, 24, 4651–4659 CrossRef PubMed.
- Z. Y. Peng, N. Tjandra, V. Simplaceanu and C. Ho, Biophys. J., 1989, 56, 877–885 CrossRef PubMed.
- E. A. Pratt, J. A. Jones, P. F. Cottam, S. R. Dowd and C. Ho, Biochim. Biophys. Acta, 1983, 729, 167–175 CrossRef.
- J. Guimond-Tremblay, M. C. Gagnon, J. A. Pineault-Maltais, V. Turcotte, M. Auger and J. F. Paquin, Org. Biomol. Chem., 2012, 10, 1145–1148 RSC.
- D. J. Hirsh, N. Lazaro, L. R. Wright, J. M. Boggs, T. J. McIntosh, J. Schaefer and J. Blazyk, Biophys. J., 1998, 75, 1858–1868 CrossRef PubMed.
- A. Gautier, H. R. Mott, M. J. Bostock, J. P. Kirkpatrick and D. Nietlispach, Nat. Struct. Mol. Biol., 2010, 17, 768–774 CrossRef PubMed.
- J. J. Chou, J. L. Baber and A. Bax, J. Biomol. NMR, 2004, 29, 299–308 CrossRef PubMed.
- T. M. O'Connell and R. E. London, J. Magn. Reson., Ser. B, 1995, 109, 264–269 CrossRef.
- H. Hu, P. Kulanthaivel and K. Krishnamurthy, J. Org. Chem., 2007, 72, 6259–6262 CrossRef PubMed.
- G. W. Kellogg, A. A. Szewczak and P. B. Moore, J. Am. Chem. Soc., 1992, 114, 2727–2728 CrossRef.
- R. Puthenveetil, K. Nguyen and O. Vinogradova, Nanotechnol. Rev., 2017, 6, 111–126 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, figures and tables. See DOI: 10.1039/c8cc02483a |
| ‡ Present address: Department of Molecular and Cell Biology, Leicester Institute of Structural and Chemical Biology, University of Leicester, Leicester, UK. E-mail: adb43@leicester.ac.uk |
| § These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |