Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Orthogonal photo-switching of supramolecular patterned surfaces†
Dongsheng
Wang
a,
Frank
Schellenberger
b,
Jonathan T.
Pham
c,
Hans-Jürgen
Butt
b and
Si
Wu
 *bd
*bd
aSchool of Optoelectronic Science and Engineering of UESTC, University of Electronic Science and Technology of China, No. 4, Section 2, North Jianshe Road, 610054, Chengdu, China
bMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: wusi@mpip-mainz.mpg.de
cDepartment of Chemical and Materials Engineering, University of Kentucky, 177 F. Paul Anderson Tower, Lexington, KY 40506, USA
dCAS Key Laboratory of Soft Matter Chemistry, Key Laboratory of Optoelectronic Science and Technology, Innovation Centre of Chemistry for Energy Materials, Department of Polymer Science and Engineering, University of Science and Technology of China Hefei, 230026, China. E-mail: siwu@ustc.edu.cn
First published on 10th March 2018
Abstract
We used Azo/α-CD and ipAzo/γ-CD host–guest complexes to demonstrate that four independent stable states can be orthogonally photo-switched by UV (365 nm), blue (470 nm), green (530 nm) and red light (625 nm). A supramolecular patterned surface was fabricated and orthogonally photo-switched by light with different wavelengths.
Orthogonal switching can be used to control multiple functions within a single material system by two or more independent external stimuli (e.g., pH, heat, light and chemical).1 In contrast to traditional stimuli-responsive systems, which are switched between two states, orthogonal systems can be triggered into three or more states having a wider range of possible functions.1a,2 However, the reported orthogonal switchable systems are controlled by stimuli with different types (i.e., light/heat,1a,1c pH/chemical1b and heat/chemical1d,1e), and the research on orthogonal switching systems by one-type of stimulus remains an unsolved challenge.
Light has been widely used to control processes and functions in chemistry and material science as an external stimulus because it can be applied with high spatial and time resolution.3 Photoresponsive materials have been successfully applied in the fields of biomedicine, nanoscience, hydrogels, and surfaces.3b,3d,4 Typically, chromophores responsive to light are integrated in the material systems, which can be switched between two independent states upon light exposure, and hence can display two different functions. However, these “switch”-type photoresponsive materials are limited to only two functional states (“on” and “off”), and are insufficient for functioning in an environment with a complex range of light stimuli. On the other hand, orthogonal photo-controllable systems possess a “mode dial”-type property that can switch between ≥3 states under control of ≥3 lights with different wavelengths. However, most photoresponsive chromophores have heavily overlapped absorption in the ultraviolet (UV)-visible region, which is one of the biggest challenges in the advancement of photoresponsive orthogonal systems.3e,5
Azobenzene (Azo) and cyclodextrin (CD) have been extensively investigated for designing photoresponsive supramolecular materials.6 Under dark or visible light irradiation, trans Azo can enter the hydrophobic cavity of α-CD to form a strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex. Under UV light irradiation (∼365 nm), trans Azo switches to cis form, and the host–guest complex dissociates due to hydrophilicity of the cis Azo and unfitted molecular scale with the α-CD cavity.6 In our previous work, we synthesized tetra-ortho-isopropoxy-substituted Azo (ipAzo), which shows trans-to-cis isomerization under green or red light irradiation (530 or 625 nm), and cis-to-trans isomerization under heating, UV or blue light irradiation (365 or 470 nm). cis ipAzo forms a 1
1 host–guest complex. Under UV light irradiation (∼365 nm), trans Azo switches to cis form, and the host–guest complex dissociates due to hydrophilicity of the cis Azo and unfitted molecular scale with the α-CD cavity.6 In our previous work, we synthesized tetra-ortho-isopropoxy-substituted Azo (ipAzo), which shows trans-to-cis isomerization under green or red light irradiation (530 or 625 nm), and cis-to-trans isomerization under heating, UV or blue light irradiation (365 or 470 nm). cis ipAzo forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex with γ-CD, while the host–guest interaction between trans ipAzo and γ-CD is weak.7 The result is the reverse of Azo/α-CD complex.
1 host–guest complex with γ-CD, while the host–guest interaction between trans ipAzo and γ-CD is weak.7 The result is the reverse of Azo/α-CD complex.
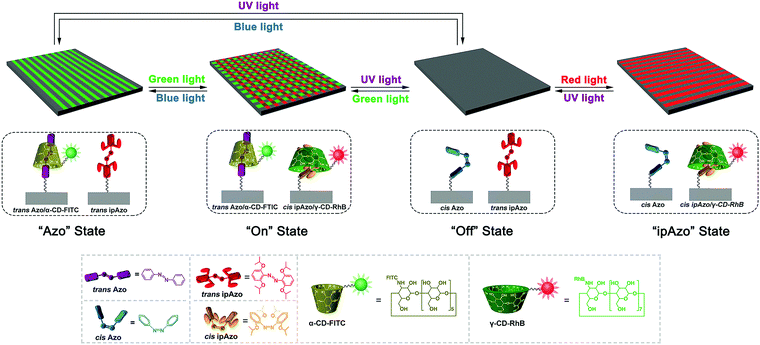
Here, we combined ipAzo/γ-CD with the Azo/α-CD host–guest complex to fabricate photo-switchable supramolecular micropatterned surfaces. 4 states can be orthogonally controlled by UV, blue, green and red light. Patterned surfaces are important for a number of fields related to materials and nano science.8 In particular, photoresponsive patterned surfaces can be fabricated with switchable wettability, morphology, and functions under precise control of external light irradiation.9 This makes the photoresponsive patterned surfaces applicable in molecular motors and cell culture.10 However, till now, studies on orthogonal photo-switching of micro/nano-patterned surfaces are lacking that develop the surfaces to be intelligent and applicable in complex light environments. Herein, we show that 4 independent states of Azo/CD micropatterns are achievable by simply irradiating the surface with light of different wavelengths (Scheme 1):
1. “Azo” state: trans Azo/trans ipAzo, trans Azo/α-CD on surface;
2. “On” state: trans Azo/cis ipAzo, trans Azo/α-CD and cis ipAzo/γ-CD on surface;
3. “Off” state: cis Azo/trans ipAzo, no complex on surface;
4. “ipAzo” state: cis Azo/cis ipAzo, cis ipAzo/γ-CD on surface.
The orthogonally photo-controlled isomerization of Azo/ipAzo was investigated by UV/vis spectroscopy and 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. 1). Azo-Si and ipAzo-Si were used as the model molecules and dissolved together (see ESI† (SI), Scheme S1). Our results demonstrated that Azo and ipAzo isomerization can be triggered in different directions by 4 light irradiations (Table 1). UV light at 365 nm excites the π–π* transition of trans Azo and triggers a trans-to-cis isomerization, inducing a sharp decrease of the π–π* transition band and an increase of the n–π* transition band (Fig. 1a, Fig. S3a, ESI†). In contrast, the trans-to-cis isomerization of ipAzo under UV light irradiation is minimal. Only ∼25% of trans ipAzo switched to the cis form after UV light irradiation (Fig. S3b, ESI†). Irradiation with blue light at 470 nm excites the n–π* transition and induces a cis-to-trans isomerization for both Azo and ipAzo (Fig. S4, ESI†). Green light (530 nm) induces a cis-to-trans isomerization for Azo, and trans-to-cis isomerization for ipAzo (Fig. S5, ESI†). Moreover, irradiation with red light at 625 nm triggers trans-to-cis isomerization of ipAzo, while Azo is not significantly affected (Fig. S6, ESI†). This might be attributed to the slightly higher absorbance of trans ipAzo than cis ipAzo in red light region (Fig. 1a).7,11 A summary of the photoisomerization of Azo and ipAzo under the different light irradiations is presented in Table 1.
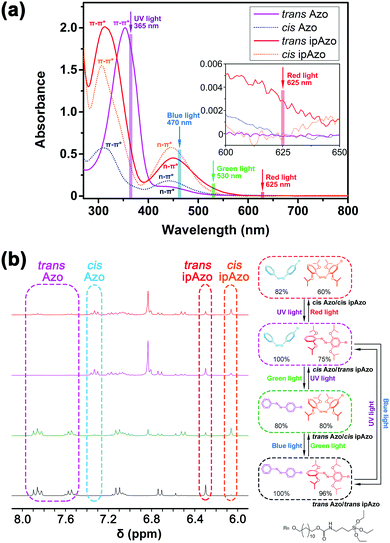 | ||
| Fig. 1 (a) UV/vis spectra of Azo and ipAzo ([Azo-Si] = [ipAzo-Si] = 0.25 mM in dimethyl sulfoxide (DMSO)): trans Azo and trans ipAzo were obtained after heating at 60 °C for 1 h in dark, absorbance of trans Azo at λ = 351 nm and trans ipAzo at λ = 310 nm were normalized; the UV/vis spectra of cis Azo and cis ipAzo were obtained from calculation (see detail in ESI†). (b) 1H NMR spectra of Azo/ipAzo under UV, blue, green and red light irradiations ([Azo-Si] = [ipAzo-Si] = 1.5 mM in DMSO-d6, 300 MHz at 298 K. UV, blue and green light = 30 min, red light = 20 min). | ||
The Azo/ipAzo combination can therefore be orthogonally controlled by light with various wavelengths, which was demonstrated by 1H NMR. Azo-Si and ipAzo-Si with the same concentrations in DMSO-d6 was used during the investigation (Fig. 1b). After blue light irradiation, the Azo/ipAzo system was in the photostationary trans Azo/trans ipAzo state with approximately 100% and 96% of trans Azo and trans ipAzo, respectively (integral of 1H NMR spectra). Green light irradiation transformed the system to the trans Azo/cis ipAzo while UV light irradiation transformed it to the cis Azo/trans ipAzo. Each of these photostationary states are easily obtained from any of the others by application of blue light (trans Azo/trans ipAzo), green light (trans Azo/cis ipAzo), and UV light (cis Azo/trans ipAzo). On the other hand, the fourth state (cis Azo/cis ipAzo) can only be achieved by starting with the cis Azo/trans ipAzo and irradiating with red light, which is reversible upon UV light irradiation (Fig. 1b). The results obtained by 1H NMR are consistent with our UV/vis data and Table 1.
To fabricate photoresponsive orthogonal micropatterned surfaces, Azo-Si and ipAzo-Si were micro-contact printed onto a glass surface (Scheme S3, ESI†). A polydimethylsiloxane (PDMS) stamp with 3 μm wide stripes and 5 μm wide valleys was used for the micropattern preparation (Fig. S9, ESI†). Azo and ipAzo micro-stripes were immobilized on glass surfaces by printing, which are denoted as Glass-Azo and Glass-ipAzo, respectively (see detail in ESI†). The Glass-ipAzo was used to further prepare a photoresponsive orthogonal micropatterned surface, which is denoted as Glass-ipAzo/Azo. To prepare this pattern, an Azo-Si coated PDMS stamp was rotated by 90° and then stamped onto the Glass-ipAzo surface, which leads to a crossed grid of Azo/ipAzo micro-stripe structures (Scheme S3, ESI†).
To visualize the microstructures, a green fluorescent dye, fluorescein isothiocyanate (FITC), was used to modify α-CD to obtain α-CD–FITC (Scheme S2, ESI†). The 2D fluorescent micro-stripes could be imaged by confocal microscopy on the Glass-Azo substrate after introducing an aqueous solution of α-CD–FITC, due to the formation of a host–guest complex between trans Azo and α-CD–FITC (Fig. S10a, ESI†). Irradiation by UV light leads to the disappearance of the fluorescent micro-stripes on the Glass-Azo surface. This is driven by the trans-to-cis photoisomerization of Azo, as described in Table 1, resulting in the dissociation of the host–guest complex, and further release of α-CD–FITC from the surface. The green fluorescent micro-stripes could be visible again on Glass-Azo surface by blue light irradiation and further treating with the α-CD–FITC aqueous solution (Fig. S10a and S11, see detail in ESI†). As suggested by Table 1, the Glass-ipAzo could be controlled by green and blue light irradiation in an opposite manner (Fig. S10b, ESI†). To demonstrate this, a red fluorescent dye, rhodamine B (RhB), was modified with γ-CD to obtain γ-CD–RhB (Scheme S2, ESI†). Upon immersing the Glass-ipAzo substrate in a γ-CD–RhB aqueous solution in the dark, no fluorescent micro-stripes are visible due to the weak host–guest interaction between trans ipAzo and γ-CD–RhB. By irradiating the Glass-ipAzo substrate with green light and further treating with the γ-CD–RhB aqueous solution, 2D fluorescent micro-stripes become visible. This is attributed to the green light induced trans-to-cis isomerization of ipAzo on the surface (Table 1). While cis ipAzo shows a strong host–guest interaction with γ-CD, the γ-CD–RhB could be visualized on Glass-ipAzo surface. Furthermore, by irradiating the surface with blue light, the fluorescent micro-stripes can be erased (Fig. S10b and S12, see detail in ESI†).
The Azo/α-CD and ipAzo/γ-CD host–guest complexes have been demonstrated to be orthogonal in our previous work.7 Considering that the Azo/ipAzo system can be controlled by different light wavelengths to 4 different photostationary states, we demonstrated a photoresponsive orthogonal micropatterned surface using the Glass-ipAzo/Azo crossed micro-stripes described above (Scheme S3, ESI†).
A mixed aqueous solution of α-CD–FITC and γ-CD–RhB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in molar ratio) was used for the orthogonal photo-switching process of Glass-ipAzo/Azo. The green fluorescent FITC and red fluorescent RhB can be monitored independently using different channels by confocal microscopy (ESI†). The Glass-ipAzo/Azo substrate surface was marked by a diamond cutter to make sure that the orthogonal photo-switching process occurred in situ. Under blue light irradiation, the Glass-ipAzo/Azo surface is in the “Azo” state (Scheme 1). Fluorescent micro-stripes are therefore visible in the FITC channel after treating with the α-CD–FITC/γ-CD–RhB mixture (Fig. 2 and Fig. S13, ESI†). Green light irradiation switched the Glass-ipAzo/Azo to the “On” state, both α-CD–FITC and γ-CD–RhB are thus immobilized on the substrate surface, and a crossed FITC/RhB micro-stripe structure is observed. Upon UV light irradiation, the Glass-ipAzo/Azo is transformed to the “Off” state, neither α-CD–FITC nor γ-CD–RhB are immobilized on the substrate surface. Therefore, no fluorescent micro-stripes are visible on the surface (Fig. S13, ESI†). Each of these states is easily obtained from any of the others by the application of blue light (“Azo” State, FITC micro-stripes), green light (“On” State, crossed FITC/RhB micro-stripes), or UV light (“Off” State, no micro-stripes) (Scheme 1 and Fig. 2), which are as expected by our UV/vis and NMR results. The fourth state (“ipAzo” State) is only obtained by irradiating the Glass-ipAzo/Azo substrate with UV light and red light in sequence; then the γ-CD–RhB is immobilized on the surface and fluorescent micro-stripes are only visible in the RhB channel (Fig. 2 and Fig. S13, ESI†). UV light irradiation induces the back switching from the “ipAzo” state to the “Off” state. This demonstrates that the supramolecular micropatterned system, formed by the Azo/α-CD and ipAzo/γ-CD, can be photo-controlled orthogonally by UV light, blue light, green light and red light irradiation, and switched between 4 different states (Scheme 1 and Fig. 2).
1, in molar ratio) was used for the orthogonal photo-switching process of Glass-ipAzo/Azo. The green fluorescent FITC and red fluorescent RhB can be monitored independently using different channels by confocal microscopy (ESI†). The Glass-ipAzo/Azo substrate surface was marked by a diamond cutter to make sure that the orthogonal photo-switching process occurred in situ. Under blue light irradiation, the Glass-ipAzo/Azo surface is in the “Azo” state (Scheme 1). Fluorescent micro-stripes are therefore visible in the FITC channel after treating with the α-CD–FITC/γ-CD–RhB mixture (Fig. 2 and Fig. S13, ESI†). Green light irradiation switched the Glass-ipAzo/Azo to the “On” state, both α-CD–FITC and γ-CD–RhB are thus immobilized on the substrate surface, and a crossed FITC/RhB micro-stripe structure is observed. Upon UV light irradiation, the Glass-ipAzo/Azo is transformed to the “Off” state, neither α-CD–FITC nor γ-CD–RhB are immobilized on the substrate surface. Therefore, no fluorescent micro-stripes are visible on the surface (Fig. S13, ESI†). Each of these states is easily obtained from any of the others by the application of blue light (“Azo” State, FITC micro-stripes), green light (“On” State, crossed FITC/RhB micro-stripes), or UV light (“Off” State, no micro-stripes) (Scheme 1 and Fig. 2), which are as expected by our UV/vis and NMR results. The fourth state (“ipAzo” State) is only obtained by irradiating the Glass-ipAzo/Azo substrate with UV light and red light in sequence; then the γ-CD–RhB is immobilized on the surface and fluorescent micro-stripes are only visible in the RhB channel (Fig. 2 and Fig. S13, ESI†). UV light irradiation induces the back switching from the “ipAzo” state to the “Off” state. This demonstrates that the supramolecular micropatterned system, formed by the Azo/α-CD and ipAzo/γ-CD, can be photo-controlled orthogonally by UV light, blue light, green light and red light irradiation, and switched between 4 different states (Scheme 1 and Fig. 2).
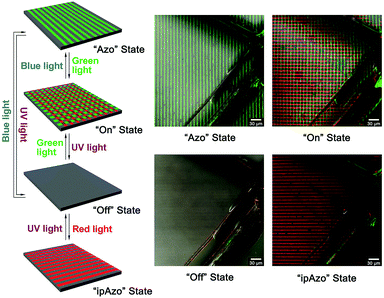 | ||
| Fig. 2 Confocal microscopic images of Glass-ipAzo/Azo after treating with UV, blue, green and red light irradiations. Scale bar: 30 μm. | ||
In summary, we demonstrated orthogonal switching of an Azo/ipAzo system under pure photo-control by UV (365 nm), blue (470 nm), green (530 nm) and red light (625 nm) irradiation. 4 independent photostationary states of Azo/ipAzo combinations are realized and switched between each other by light. A photoresponsive orthogonal supramolecular micropatterned surface was fabricated by combining the Azo/α-CD host–guest complex with ipAzo/γ-CD. The successful design of photoresponsive orthogonal supramolecular systems offers more applications of light-controlled materials in complex irradiation environments. Although the reported structures are in the microscopic scale, we envision that photoresponsive orthogonal supramolecular systems should also be applicable to macroscopic systems. For example, Shi et al. proposed a novel methodology of macroscopic supramolecular assembly to fabricate 3D ordered structures.12 This method can mildly load necessary bioactive species to designated locations within the structure during fabrication, which is significant for the increasing demand in constructing chemically or biologically specific tissue scaffolds. Our design may lead to more intelligent photoresponsive microscopic and macroscopic systems in the future.
D. W. was supported by the CSC program. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, WU 787/2-1). J. T. P was supported by an Alexander von Humboldt Fellowship.
Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. M. Lerch, M. J. Hansen, W. A. Velema, W. Szymanski and B. L. Feringa, Nat. Commun., 2016, 7, 12054 CrossRef CAS PubMed; (b) S. Schoder and C. A. Schalley, Chem. Commun., 2017, 53, 9546–9549 RSC; (c) J. Steinkoenig, M. M. Zieger, H. Mutlu and C. Barner-Kowollik, Macromolecules, 2017, 50, 5385–5391 CrossRef CAS; (d) Y. Liu, L. Shangguan, H. Wang, D. Xia and B. Shi, Polym. Chem., 2017, 8, 3783–3787 RSC; (e) X. Wu, Y. Yu, L. Gao, X. Hu and L. Wang, Org. Chem. Front., 2016, 3, 966–970 RSC; (f) F. Rachdi, L. Hajji, C. Goze, D. J. Jones, P. Maireles-Torres and J. Rozière, Solid State Commun., 1996, 100, 237–240 CrossRef CAS.
- (a) W. Xu, P. A. Ledin, Z. Iatridi, C. Tsitsilianis and V. V. Tsukruk, Angew. Chem., Int. Ed., 2016, 55, 4908–4913 CrossRef CAS PubMed; (b) C. Y. Ang, S. Y. Tan, S. Wu, Q. Qu, M. F. E. Wong, Z. Luo, P. Li, S. T. Selvan and Y. Zhao, J. Mater. Chem. C, 2016, 4, 2761–2774 RSC.
- (a) H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC; (b) M. M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed; (c) K. Ichimura, S. K. Oh and M. Nakagawa, Science, 2000, 288, 1624–1626 CrossRef CAS PubMed; (d) W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed; (e) F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37–54 RSC; (f) J. Lv, Y. Liu, J. Wei, E. Chen, L. Qin and Y. Yu, Nature, 2016, 537, 179–184 CrossRef CAS PubMed; (g) S. Sun, D. Thompson, U. Schmidt, D. Graham and G. J. Leggett, Chem. Commun., 2010, 46, 5292–5294 RSC; (h) C. Wu, Q. Cheng, S. Sun and B. Han, Carbon, 2012, 50, 1083–1089 CrossRef CAS.
- (a) D. P. Ferris, Y. L. Zhao, N. M. Khashab, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 1686–1688 CrossRef CAS PubMed; (b) N. Fomina, J. Sankaranarayanan and A. Almutairi, Adv. Drug Delivery Rev., 2012, 64, 1005–1020 CrossRef CAS PubMed; (c) I. Tomatsu, K. Peng and A. Kros, Adv. Drug Delivery Rev., 2011, 63, 1257–1266 CrossRef CAS PubMed; (d) J. Deng, X. Liu, W. Shi, C. Cheng, C. He and C. Zhao, ACS Macro Lett., 2014, 3, 1130–1133 CrossRef CAS; (e) M. Chen and F. Besenbacher, ACS Nano, 2011, 5, 1549–1555 CrossRef CAS PubMed.
- (a) M. Natali and S. Giordani, Chem. Soc. Rev., 2012, 41, 4010–4029 RSC; (b) A. S. Lubbe, W. Szymanski and B. L. Feringa, Chem. Soc. Rev., 2017, 46, 1052–1079 RSC.
- (a) A. Harada, Y. Takashima and M. Nakahata, Acc. Chem. Res., 2014, 47, 2128–2140 CrossRef CAS PubMed; (b) L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196–7239 CrossRef CAS PubMed; (c) S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520–1529 RSC; (d) G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed; (e) D. Wang, D. Xie, W. Shi, S. Sun and C. Zhao, Langmuir, 2013, 29, 8311–8319 CrossRef CAS PubMed; (f) Y. Zhou, D. Wang, S. Huang, G. Auernhammer, Y. He, H.-J. Butt and S. Wu, Chem. Commun., 2015, 51, 2725–2727 RSC.
- D. Wang, M. Wagner, A. K. Saydjari, J. Mueller, S. Winzen, H.-J. Butt and S. Wu, Chem. – Eur. J., 2017, 23, 2628–2634 CrossRef CAS PubMed.
- (a) D. Chanda, K. Shigeta, S. Gupta, T. Cain, A. Carlson, A. Mihi, A. J. Baca, G. R. Bogart, P. Braun and J. A. Rogers, Nat. Nanotechnol., 2011, 6, 402–407 CrossRef CAS PubMed; (b) S. Park, D. H. Lee and T. P. Russell, Adv. Mater., 2010, 22, 1882–1884 CrossRef CAS PubMed; (c) J. Rodríguez-Hernández, Prog. Polym. Sci., 2015, 42, 1–41 CrossRef; (d) M. Jaschke and H.-J. Butt, Langmuir, 1995, 11, 1061–1064 CrossRef CAS.
- (a) W. Jiang, G. Wang, Y. He, X. Wang, Y. An, Y. Song and L. Jiang, Chem. Commun., 2005, 3550–3552 RSC; (b) H. Zhou, C. Xue, P. Weis, Y. Suzuki, S. Huang, K. Koynov, G. K. Auernhammer, R. Berger, H.-J. Butt and S. Wu, Nat. Chem., 2017, 9, 145–151 CrossRef CAS PubMed; (c) A. A. Brown, O. Azzaroni and W. T. S. Huck, Langmuir, 2009, 25, 1744–1749 CrossRef CAS PubMed.
- (a) Y. Wang and Q. Li, Adv. Mater., 2012, 24, 1926–1945 CrossRef CAS PubMed; (b) Y. Gong, J. Yang, F. Cao, J. Zhang, H. Cheng, R. Zhuo and X. Zhang, J. Mater. Chem. B, 2013, 1, 2013–2017 RSC; (c) Z. Ming, X. Ruan, C. Bao, Q. Lin, Y. Yang and L. Zhu, Adv. Funct. Mater., 2017, 27, 1606258 CrossRef.
- (a) D. Wang and S. Wu, Langmuir, 2016, 32, 632–636 CrossRef CAS PubMed; (b) D. Wang, M. Wagner, H.-J. Butt and S. Wu, Soft Matter, 2015, 11, 7656–7662 RSC.
- M. Cheng, Y. Wang, L. Yu, H. Su, W. Han, Z. Lin, J. Li, H. Hao, C. Tong, X. Li and F. Shi, Adv. Funct. Mater., 2015, 25, 6851–6857 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc00770e |
| This journal is © The Royal Society of Chemistry 2018 |