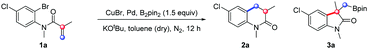Cu/Pd cooperatively catalyzed tandem intramolecular anti-Markovnikov hydroarylation of unsaturated amides: facile construction of 3,4-dihydroquinolinones via borylation/intramolecular C(sp3)–C(sp2) cross coupling†
Zhijie
Kuang
 ,
Bingnan
Li
and
Qiuling
Song
,
Bingnan
Li
and
Qiuling
Song
 *
*
Institute of Next Generation Matter Transformation, College of Chemical Engineering, College of Material Sciences Engineering, Huaqiao University, Xiamen, Fujian 361021, China. E-mail: qsong@hqu.edu.cn; Fax: +86-592-6162990
First published on 23rd November 2017
Abstract
An anti-Markovnikov hydroarylation of unsaturated amides via a Cu/Pd synergistically catalyzed cascade borylation/intramolecular sp2–sp3 cross-coupling has been disclosed. 3,4-Dihydroquinolinones, one type of important scaffold prevailing in pharmaceuticals and biologically active compounds, could be readily accessible with high regio-selectivity and high yields.
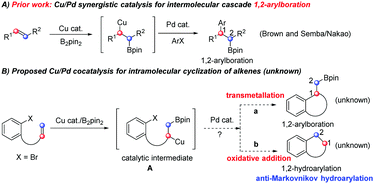
Transition-metal-catalyzed cross-coupling reactions of Csp3 nucleophiles have been proven to be a powerful strategy in chemical synthesis.1 Recently, Cu/Pd synergistic catalysis for 1,2-arylboration of alkenes has emerged as a successful and practical tool in Csp3 nucleophilic cross-couplings, which proceed via transmetallation by a catalytic nucleophile (Cu–R was converted into Pd–R, followed by arylation with ArX).2 Although significant progress has been achieved on intermolecular 1,2-arylboration (Scheme 1A), the Cu/Pd cooperatively catalyzed intramolecular cyclization of alkenes has not yet been reported.3 There are several advantages of intramolecular reactions over intermolecular counterparts: (1) versatile cyclic products will result, even some unusual cyclic products could be afforded by careful design; (2) unexpected and novel reaction modes might be disclosed due to the proximity; and (3) valuable drug-like heterocycles might be obtained.
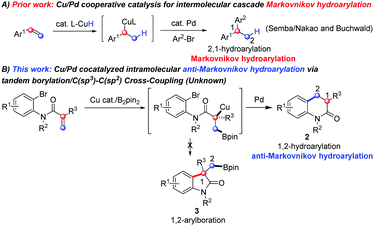
We envision that two plausible pathways might occurr from the catalytic intermediate A (Scheme 1B), which is formed by the attack of the in situ generated Cu–Bpin complex to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond upon Pd catalysis, during Cu/Pd cocatalyzed intramolecular cyclization of alkenes with B2pin2: (a) by transmetallation with active Cu species (a nucleophile), normal 1,2-arylboration will take place with the intramolecular ArX moiety and (b) instead of transmetallation, the oxidative addition of the Pd catalyst to the ArX moiety (an electrophile) occurs first, giving an active Pd species, which reacts with the in situ generated alkyl boronate moiety to lead to the 1,2-hydroarylative product. It is of note that the hydroarylative product is generated via an anti-Markovnikov hydroarylation mode (Scheme 1B). A literature survey indicates that intermolecular hydroarylations catalyzed by Cu/Pd cooperative catalysis were reported independently by Semba/Nakao4 and Buchwald5a in 2016 (Scheme 2A), and their intermolecular hydroarylations5b took place via a Markovnikov mode. Our hypothesis will provide a complementary pathway to their seminar methods in terms of the intramolecular pattern as well as the anti-Markovnikov mode if path b occurs first over path a (Scheme 1B). How to control the two competitive pathways in one reaction and make path b occur exclusively becomes an interesting but challenging subject. Herein, we report our new disclosure in addressing this gap by the formation of anti-Markovnikov hydroarylative products via Cu/Pd synergistic catalysis with B2pin2 in an intramolecular borylation/Csp3 cross-coupling protocol (Scheme 2B). This strategy is significant and in sharp contrast to prior reports on Cu/Pd co-catalyzed arylboration as well as intermolecular Markovnikov hydroarylation, therefore a novel reaction mode is resulted, which complements the existing methods in the hydroarylation of alkenes.
C bond upon Pd catalysis, during Cu/Pd cocatalyzed intramolecular cyclization of alkenes with B2pin2: (a) by transmetallation with active Cu species (a nucleophile), normal 1,2-arylboration will take place with the intramolecular ArX moiety and (b) instead of transmetallation, the oxidative addition of the Pd catalyst to the ArX moiety (an electrophile) occurs first, giving an active Pd species, which reacts with the in situ generated alkyl boronate moiety to lead to the 1,2-hydroarylative product. It is of note that the hydroarylative product is generated via an anti-Markovnikov hydroarylation mode (Scheme 1B). A literature survey indicates that intermolecular hydroarylations catalyzed by Cu/Pd cooperative catalysis were reported independently by Semba/Nakao4 and Buchwald5a in 2016 (Scheme 2A), and their intermolecular hydroarylations5b took place via a Markovnikov mode. Our hypothesis will provide a complementary pathway to their seminar methods in terms of the intramolecular pattern as well as the anti-Markovnikov mode if path b occurs first over path a (Scheme 1B). How to control the two competitive pathways in one reaction and make path b occur exclusively becomes an interesting but challenging subject. Herein, we report our new disclosure in addressing this gap by the formation of anti-Markovnikov hydroarylative products via Cu/Pd synergistic catalysis with B2pin2 in an intramolecular borylation/Csp3 cross-coupling protocol (Scheme 2B). This strategy is significant and in sharp contrast to prior reports on Cu/Pd co-catalyzed arylboration as well as intermolecular Markovnikov hydroarylation, therefore a novel reaction mode is resulted, which complements the existing methods in the hydroarylation of alkenes.
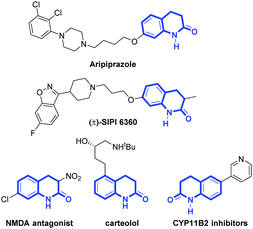
In order to verify our hypothesis, N-(2-bromo-4-chlorophenyl)-N-methylmethacrylamide (1a) was chosen as the template substrate, due to its ready accessibility (two-step synthesis) and the likelihood of cyclization (five-membered 2-oxindoline (path a) or six-membered 3,4-dihydroquinolinone (path b)), with the participation of Pd and Cu/B2pin2. It is noteworthy that 3,4-dihydroquinolinones are important molecular scaffolds in bioactive compounds and pharmaceuticals,6,7 such as in the antipsychotic drug Aripiprazole,8 atypical schizophrenia drug (±)-SIPI 6360,9 the expensive PMPA (NMDA antagonist),10 carteolol eye drops10 and CYP11B2 dual Inhibitors11 (Scheme 3). The development of efficient methods for the construction of this valuable skeleton therefore becomes highly desirable in organic synthesis.
It is not surprising that a significant amount of the five-membered 2-oxindoline 3a was generated in addition to the formation of six-membered 3,4-dihydroquinolinone 2a (Table 1, entry 1), because of the favorable propensity to the formation of a five-membered 3a from compound 1a. Thus the suppression of the formation of 3a becomes a big challenge in our hypothesis. Preliminary co-catalyst screening (entries 1–3) suggested that Pd(dba)2 and CuBr were able to control the selectivity for a single target product (2a) (entry 3). Subsequent changes in different variables, such as temperature (entries 4 and 6), time (entry 5) and the amount of base (entries 7–9) did not lead to significant improvements. Then the ratio of Cu and Pd catalysts was carefully adjusted since we realized that the Cu–Bpin complex addition over C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds should be prior to the competitive oxidative addition of the Pd catalyst into ArX, and the ratio of the Cu salt to Pd salt would be critical to the desired transformation. We simultaneously increased the amount of palladium and copper salts, but kept the amount of copper salt twice to that of palladium salt (entry 10). To our delight, the selectivity, conversions as well as yields were dramatically increased along with the changes. Based on the result in entry 10, we finely tuned the reaction conditions, resulting in the optimal one—entry 12. It is noteworthy that the reaction did not proceed at all in air (entry 13) or in the absence of B2pin2 (entry 14), which proves that B2pin2 is a prerequisite for the success of the transformation, and oxygen plays a deteriorative role in this reaction. More condition screenings are available in the ESI.†
C bonds should be prior to the competitive oxidative addition of the Pd catalyst into ArX, and the ratio of the Cu salt to Pd salt would be critical to the desired transformation. We simultaneously increased the amount of palladium and copper salts, but kept the amount of copper salt twice to that of palladium salt (entry 10). To our delight, the selectivity, conversions as well as yields were dramatically increased along with the changes. Based on the result in entry 10, we finely tuned the reaction conditions, resulting in the optimal one—entry 12. It is noteworthy that the reaction did not proceed at all in air (entry 13) or in the absence of B2pin2 (entry 14), which proves that B2pin2 is a prerequisite for the success of the transformation, and oxygen plays a deteriorative role in this reaction. More condition screenings are available in the ESI.†
| Entry | CuBr (x mol%) xantphos (x mol%) | Pd (x mol %) | KOtBu (x equiv.) | T (°C) | Yield (%) | |
|---|---|---|---|---|---|---|
| 2a | 3a | |||||
| a Reaction conditions: all reactions were performed on 0.2 mmol scale with respect to 1a; yields were determined by GC by utilizing dodecane as the internal standard. The reaction mixture contained H2O approximately 0.3% w/w. b For 24 h. c Isolated yield and Pd(dba)2 was used in 7.5 mol%. d No B2pin2. e In air. | ||||||
| 1 | 10 | Pd2(dba)3 (5) | 1.5 | 110 | 37 | 15 |
| 2 | 10 | Pd2(dba)3·CHCI3 (5) | 1.5 | 110 | 17 | 38 |
| 3 | 10 | Pd(dba)2 (5) | 1.5 | 110 | 41 | 1 |
| 4 | 10 | Pd(dba)2 (5) | 1.5 | 120 | 28 | — |
| 5b | 10 | Pd(dba)2 (5) | 1.5 | 110 | 43 | — |
| 6 | 10 | Pd(dba)2 (5) | 1.5 | 100 | 21 | — |
| 7 | 10 | Pd(dba)2 (5) | 1.2 | 110 | 37 | 5 |
| 8 | 10 | Pd(dba)2 (5) | 2.0 | 110 | 50 | — |
| 9 | 10 | Pd(dba)2 (5) | 3.0 | 110 | 35 | 7 |
| 10 | 20 | Pd(dba)2 (10) | 2.0 | 110 | 91 | 1 |
| 11 | 16 | Pd(dba)2 (8) | 2.0 | 110 | 89 | — |
| 12 | 15 | Pd(dba)2 (7.5) | 1.8 | 110 | 90 | — |
| 78c | — | |||||
| 13d | 15 | Pd(dba)2 (7.5) | 1.8 | 110 | — | — |
| 14e | 15 | Pd(dba)2 (7.5) | 1.8 | 110 | — | — |
|
||||||
With the optimal conditions in hand, we next examined the scope of this intramolecular anti-Markovnikov hydroarylation via Cu/Pd synergistically catalyzed tandem borylation/cross-coupling. (Scheme 4). We first tested the substrates with electron-withdrawing groups on the aromatic ring (2a–2f and 2h). Fluorine-containing compounds usually have different special properties and widely exist in pharmaceutical, materials science as well as agro-chemicals, so we especially investigated a series of fluorine-containing substrates (2b–2g), and medium to excellent yields (60–90%) were obtained with high selectivity. The cyano group was also tolerated under the standard conditions albeit in a low yield (2h, 32% yield). Subsequently, the substrates with electron-donating groups, such as CF3O, iPr, Me, and MeO, on the aromatic rings were also inspected (2g, 2i–2n), and they were well compatible under the standard conditions. It is of note that the positions of substituents on the phenyl ring did not significantly affect the efficiency of the reactions, as observed in products 2j–2l. We further examined the influence of the substituents on the N atom to investigate the universality of the reactions. With Et- (2o), Bn- (2p) or Me- (2r) substituents on the N atom, the reactions proceeded smoothly with excellent yields. However, the substituents on the α-position of carbonyls had a great influence on the smooth progress of the reaction (2q–2u). The H atom (2q) or Me group (2r) on the α-position promoted the conversion; however, the Bn group (2s) or phenyl group (2u) seriously affected the transformation and the corresponding yields were reduced to 40% or a trace amount, respectively. At the same time, we tried the compatibility of an internal olefin; the example of a phenyl group at the beta-position was not successful, and no desired product (2v) was formed. We also investigated benzyl amine (1w), as expected, 2w was not obtained due to the reluctant formation of the disfavored seven-membered ring.
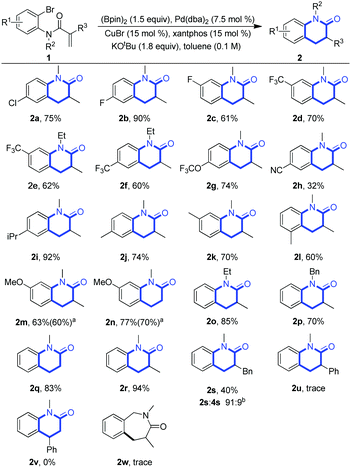 | ||
Scheme 4 Scope of the substrates. Reaction conditions: all reactions were performed under the standard conditions. The yields were isolated ones. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) On 1.5 mmol. b On 1.5 mmol. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The ratio was determined by NMR. The ratio was determined by NMR. | ||
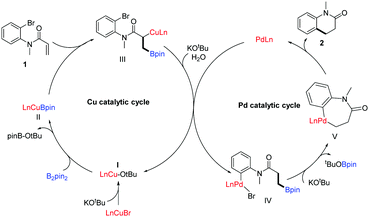
A tentative mechanism which contains two individual cycles for this transformation is depicted in Scheme 5, which is based on the previous work by Cazin's group.5b Initially, LnCuBr is activated by the base resulting in LnCuOtBu (I), which further reacts with B2pin2, giving the copper–boron species (II). Intermediate II attacks the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in substrate 1 to lead to the 1,2-copperboration product III or the 1,4-copperboration one.11 In the presence of KOtBu as well as water (from the solvent, reagents as well as glassware), intermediate I is regenerated to complete the Cu catalytic cycle with the release of the protonated boronate; meanwhile the oxidative addition of PdLn into the ArX moiety of the protonated boronate results in intermediate IV. Subsequent intramolecular cross-coupling delivers the seven-membered palladacycle intermediate V, and reductive elimination eventually affords the target product 2 as well as PdLn, completing the palladium catalytic cycle.
C bond in substrate 1 to lead to the 1,2-copperboration product III or the 1,4-copperboration one.11 In the presence of KOtBu as well as water (from the solvent, reagents as well as glassware), intermediate I is regenerated to complete the Cu catalytic cycle with the release of the protonated boronate; meanwhile the oxidative addition of PdLn into the ArX moiety of the protonated boronate results in intermediate IV. Subsequent intramolecular cross-coupling delivers the seven-membered palladacycle intermediate V, and reductive elimination eventually affords the target product 2 as well as PdLn, completing the palladium catalytic cycle.
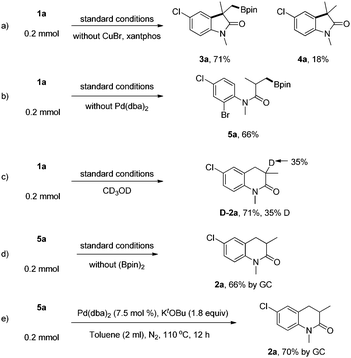
To gain more insights into the mechanism of the reaction, several control experiments were performed as shown below (Scheme 6): in order to thoroughly understand the roles of the copper catalyst and Pd catalyst in this transformation, the reactions under the standard conditions without copper salt and ligands as well as in the absence of the Pd catalyst were carried out respectively (Scheme 6a and b), and oxindoles 3a and 4a with the isolated yields of 71% and 18% were obtained in the former case, while acyclic boronate 5a was afforded in the latter case with the yield of 66%. These two reactions clearly demonstrated that both Cu and Pd are indispensable to the transformation and in the absence of the Cu catalyst, five-membered 2-oxindole compounds were exclusively formed via a typical Heck reaction pathway; however, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds were consumed without the Pd catalyst, affording the acyclic boronates obviously via the Cu–Bpin complex addition.
C bonds were consumed without the Pd catalyst, affording the acyclic boronates obviously via the Cu–Bpin complex addition.
By comparison with the above two experiments, we could draw the conclusion that the copper catalytic cycle took precedence over the palladium catalytic cycle. To prove our assumption, a deuterium-labeled experiment was conducted under the standard conditions with the addition of CD3OD, and to our delight, D-2a was obtained in 71% yield with 35% deuterium-labeling on the α-position of the carbonyl group (Scheme 6c). In order to validate that 5a was the key intermediate in this transformation, two more control experiments (Scheme 6d and e) were also performed, and as we expected the target product 2a was obtained well, irrespective of whether standard conditions (no B2pin2) were used or just palladium and base, which highlighted the validity of our conjecture.
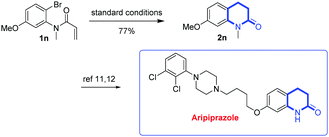
Considering the widespread existence of the product skeletons in biologically active compounds, drug molecules, and natural products, we provide an alternative transformation of one of the products (2n). Using our methodology, 7-methoxy-1-methyl-3,4-dihydroquinolin-2(1H)-one (2n) was obtained in 77%, which could be further converted into a highly complex aripiprazole after four steps of a known transformation12,13 (Scheme 7). It is worth noting that aripiprazole is a new atypical antipsychotic drug for the treatment of schizophrenia, which is one of the top 200 pharmaceutical products by US retail sales in 2012.
Quinolin-2(1H)-ones are the backbone of many biological enzymes14,15 and pesticides, such as HIV-1 integrase inhibitor elvitegravir, Penicillium and Aspergillums produce. The product (2a) was mildly oxidized16 to 6-chloro-1,3-dimethylquinolin-2(1H)-one with the isolated yield of 68% without expensive DDQ (Scheme 8).
In conclusion, we developed an unusual intramolecular hydroarylation of unsaturated amides via Cu/Pd cocatalyzed tandem borylation/cross-coupling with diboron reagents, affording 2-quinolones which are important skeletons in pharmaceuticals and biologically active compounds in high regioselectivity and good yields, and further transformations from 2-quinolones were also performed in our work.
Financial support from the Recruitment Program of Global Experts (1000 Talents Plan), the Natural Science Foundation of Fujian Province (2016J01064), the Program of Innovative Research Team of Huaqiao University (Z14X0047) and the Subsidized Project for Cultivating Postgraduates’ Innovative Ability in Scientific Research of Huaqiao University (for Z. Kuang) is gratefully acknowledged. We also thank the Instrumental Analysis Center of Huaqiao University for analysis support.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- (a) F.-Y. Yang, M.-Y. Wu and C.-H. Cheng, J. Am. Chem. Soc., 2000, 122, 7122–7123 CrossRef CAS; (b) J. Marco-Martínez, V. López-Carrillo, E. Buñuel, R. Simancas and D. J. Cárdenas, J. Am. Chem. Soc., 2007, 129, 1874 CrossRef PubMed; (c) K. M. Gligorich, S. A. Cummings and M. S. Sigman, J. Am. Chem. Soc., 2007, 129, 14193 CrossRef CAS PubMed; (d) M. Suginome, A. Yamamoto and M. Murakami, J. Am. Chem. Soc., 2003, 125, 6358–6359 CrossRef CAS PubMed; (e) Y. Zhou, W. You, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2014, 53, 3475 CrossRef CAS PubMed; (f) M. T. Pirnot, Y. M. Wang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 48 CrossRef CAS PubMed.
- (a) K. B. Smith, K. M. Logan, W. You and M. K. Brown, Chem. – Eur. J., 2014, 20, 12032–12036 CrossRef CAS PubMed; (b) K. Semba and Y. Nakao, J. Am. Chem. Soc., 2014, 136, 7567 CrossRef CAS PubMed; (c) K. M. Logan, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2015, 54, 5228–5231 CrossRef CAS PubMed; (d) T. Jia, P. Cao, B. Wang, Y. Lou, X. Yin, M. Wang and J. Liao, J. Am. Chem. Soc., 2015, 137, 13760 CrossRef CAS PubMed; (e) K. M. Logan and M. K. Brown, Angew. Chem., Int. Ed., 2017, 56, 851 CrossRef CAS PubMed; (f) S. R. Sardini and M. K. Brown, J. Am. Chem. Soc., 2017, 139, 9823–9826 CrossRef CAS PubMed.
- (a) D. D. Vachhani, H. H. Butani, N. Sharma, U. C. Bhoya, A. K. Shah and E. V. V. Eycken, Chem. Commun., 2015, 51, 14862 RSC; (b) F. Wei, L. Wei, L. Zhou, C.-H. Tung, Y. Ma and Z. Xu, Asian J. Org. Chem., 2016, 5, 971 CrossRef CAS.
- K. Semba, K. Ariyama, H. Zheng, R. Kameyama, S. Sakaki and Y. Nakao, Angew. Chem., Int. Ed., 2016, 55, 6275 CrossRef CAS PubMed.
- (a) S. D. Friis, M. T. Pirnot and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 8372 CrossRef CAS PubMed; (b) M. Lesieur, Y. D. Bidal, F. Lazreg, F. Nahra and C. S. J. Cazin, ChemCatChem, 2015, 7, 2108 CrossRef CAS.
- (a) B. Schmidt, F. Hölter, R. Berger and S. Jessel, Adv. Synth. Catal., 2010, 352, 2463–2473 CrossRef CAS; (b) L. Zhang, L. Sonaglia, J. Stacey and M. Lautens, Org. Lett., 2013, 15, 2128–2131 CrossRef CAS PubMed.
- S. Teramoto, M. Tanaka, H. Shimizu, T. Fujioka, F. Tabusa, T. Imaizumi, K. Yoshida, H. Fujiki, T. Mori, T. Sumida and M. Tominaga, J. Med. Chem., 2003, 46, 3033–3044 CrossRef CAS PubMed.
- Y. Yan, P. Zhou, D. P. Rotella, R. Feenstra, C. G. Kruse, J. H. Reinders, M. van der Neut, M. Lai, J. Zhang, D. M. Kowal, T. Carrick, K. L. Marquis, M. H. Pausch and A. Robichaud, J. Bioorg. & Med. Chem. Lett., 2010, 20, 2983 CrossRef CAS PubMed.
- X.-W. Chen, Y. Liu, X.-Q. Jin, Y.-Y. Sun, S.-L. Gu, L. Fu and J.-Q. Li, Org. Process Res. Dev., 2016, 20, 1662–1667 CrossRef CAS.
- W. Zhou, L. Zhang and N. Jiao, Tetrahedron, 2009, 65, 1982 CrossRef CAS.
- (a) A. R. Burns, J. Solana González and H. W. Lam, Angew. Chem., Int. Ed., 2012, 51, 10827 CrossRef CAS PubMed; (b) A. Welle, J. Petrignet, B. Tinant, J. Wouters and O. Riant, Chem. – Eur. J., 2010, 16, 10980 CrossRef CAS PubMed.
- Q. Hu, L. Yin and R. W. Hartmann, J. Med. Chem., 2012, 55, 7080–7089 CrossRef CAS PubMed.
- (a) M. Koreeda and J. I. Luengo, J. Org. Chem., 1984, 49, 2081 CrossRef; (b) H. Zhang, Z. Gu, Z. Li, C. Pan, W. Li, H. Hu and C. Zhu, J. Org. Chem., 2016, 81, 2122 CrossRef CAS PubMed; (c) X.-W. Chen, Y. Liu, X.-Q. Jin, Y.-Y. Sun, S.-L. Gu, L. Fu and J.-Q. Li, Org. Process Res. Dev., 2016, 20, 1662 CrossRef CAS.
- (a) S. J. Bonacorsi, S. C. Waller and J. Kent Rinehart, J. Labelled Compd. Radiopharm., 2006, 49, 1 CrossRef CAS; (b) Y. Oshiro, S. Sato, N. Kurahashi, T. Tanaka, T. Kikuchi, K. Tottori, Y. Uwahodo and T. Nishi, J. Med. Chem., 1998, 41, 658 CrossRef CAS PubMed.
- (a) J. Wu, S. Xiang, J. Zeng, M. Leow and X.-W. Liu, Org. Lett., 2015, 17, 222–225 CrossRef CAS PubMed; (b) R. Manikandan and M. Jeganmohan, Org. Lett., 2014, 16, 3568 CrossRef CAS PubMed.
- D. H. R. Barton and D. W. Jones, J. Chem. Soc., 1965, 3563 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc07180a |
| This journal is © The Royal Society of Chemistry 2018 |