Advanced smart-photosensitizers for more effective cancer treatment
Wooram
Park
 a,
Soojeong
Cho
a,
Soojeong
Cho
 a,
Jieun
Han
a,
Jieun
Han
 b,
Heejun
Shin
b,
Heejun
Shin
 b,
Kun
Na
b,
Kun
Na
 *b,
Byeongdu
Lee
*b,
Byeongdu
Lee
 *c and
Dong-Hyun
Kim
*c and
Dong-Hyun
Kim
 *ad
*ad
aDepartment of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois 60611, USA. E-mail: dhkim@northwestern.edu
bCenter for Photomedicine, Department of Biotechnology, The Catholic University of Korea, Bucheon-si, Gyeonggi-do 14662, Republic of Korea. E-mail: kna6697@catholic.ac.kr
cX-ray Science Division, Argonne National Laboratory, Lemont, Illinois 60439, USA. E-mail: blee@aps.anl.gov
dRobert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, Illinois 60611, USA
First published on 1st November 2017
Abstract
Photodynamic therapy (PDT) based upon the use of light and photosensitizers (PSs) has been used as a novel treatment approach for a variety of tumors. It, however, has several major limitations in the clinic: poor water solubility, long-term phototoxicity, low tumor targeting efficacy, and limited light penetration. With advances in nanotechnology, materials science, and clinical interventional imaging procedures, various smart-PSs have been developed for improving their cancer-therapeutic efficacy while reducing the adverse effects. Here, we briefly review state-of-the-art smart-PSs and discuss the future directions of PDT technology.
1. Introduction
Photodynamic therapy (PDT) utilizes light sensitive molecules to treat diseases. The light therapy has a history of more than 3000 years.1,2 The ancients used light to treat various diseases, such as psoriasis, rickets, edema, and skin cancer.3 Since the 1960s, PDT has been suggested as an alternative cancer therapy, and began to be used clinically for the cancer treatment in the 1970s.4,5 Cancers are treated using PDT by irradiating a cancer site with a specific wavelength of light after the administration of cancer-specific photosensitizers (PSs), which allows reducing the systemic side effects that conventional non-specific anticancer drugs might cause otherwise. Currently, PDT has been shown to be effective against early cancers and recommended for inoperable cancer patients having many complications.6,7 Clinical PDT has been frequently used for the treatment of breast cancer,8 gynecologic tumor,9 intraocular tumor,10 brain tumor,11 head and neck cancer,12 colorectal cancer,13 cutaneous malignancies,14 intraperitoneal cancer,15 cholangiocarcinoma16 and pancreatic cancer.17 Two non-toxic components, PSs and light of a specific wavelength, are utilized for killing specific cancer cells and tissues in an oxygen-dependent manner.18 PSs activated by the light of a specific wavelength transfer their energy to oxygen molecules, resulting in the production of cytotoxic reactive oxygen species (ROS) such as singlet oxygen (1O2) (Scheme 1a).19,20 In general, PSs produce ROS through type 1 (electron transfer) and/or type 2 (energy transfer) reactions.21 Type I reactions produce radical and radical anion species (e.g., O2˙− and HO˙), while type II reactions generate singlet oxygen (1O2).22,23 The generated ROS kills cancer cells through the oxidation of important biomolecules and organelles in the cancer cells.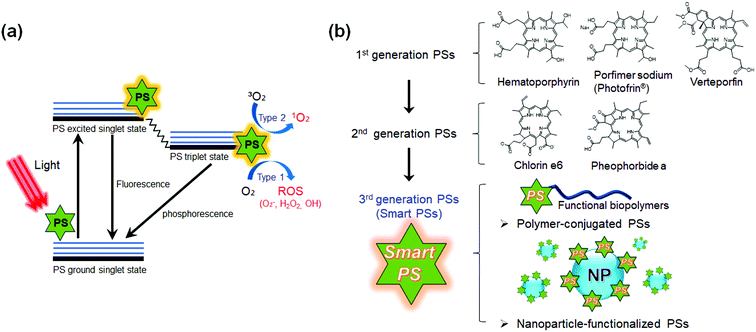 | ||
| Scheme 1 Schematic diagram of the principle of photodynamic therapy (PDT) and the classification of photosensitizers (PSs) developed to date. (a) The mechanism of action of PDT is illustrated by the Jablonski diagram.19 When light (photon) is shone on PS, the PS can be excited to the first excited singlet state that can relax to the more long-lived triplet state. The triplet PS can interact with oxygen molecules to produce reactive oxygen species (ROS) through the type 1 and type 2 mechanisms. (b) Classification of the generations of PSs developed so far. Currently developed PSs can be divided into first generation and second-generation based upon their physico-chemical properties. The third-generation of smart PSs functionalized with polymers or nanoparticles is currently under development. | ||
To date, various types of PSs have been developed for advancing PDT (Scheme 1b and Table 1).18,24 The first-generation PSs include porphyrin-based PSs such as Photofrin®.25,26 Various forms of hematoporphyrin derivatives (HpD) have been commercialized for over 30 years and have been used for thousands upon thousands of patients in clinical trials.24,27 However, the first-generation PSs were efficiently activated by only wavelengths shorter than 640 nm, so limited light tissue penetration is an issue.24 Furthermore, the patients treated with first-generation PSs had to stay in a dark room for a long time because the administrated PS remains in tissue for 4–6 weeks.24 The second-generation PSs were mostly developed in the late 1980s and consisted of porphyrinoid compounds with porphyrin or porphyrin-based macrocyclic structures.28 Typical second-generation PSs are chlorins, bacteriochlorins, phthalocyanines, pheophorbides, bacteriopheophorbides, texaphyrins, and aluminum phthalocyanine tetrasulfonate (AlPcS4). The second-generation PSs can be excited at wavelengths longer than 640 nm, which allows deeper light penetration and enhances tumor specificity.29,30 In addition, their short tissue accumulation times under 2 weeks allowed the early release of patients from the dark room.31,32 However, most of the second-generation PSs are still hydrophobic in nature with limited solubility in aqueous solution.33 Thus, they are apt to aggregate under physiological conditions, significantly reducing the quantum yields of ROS production. Even in the case of water-soluble PSs, the accumulation selectivity at malignant sites is not high enough for clinical use.33
| Platform | Trade name | Substance | Manufacturer | Potential applications |
|---|---|---|---|---|
| Porphyrin | Photofrin® | HpD | Axcan Pharma Inc. | Cervical, endobronchial, oesophageal, bladder, and gastric cancers, and brain tumors |
| Porphyrin | Levulan® | 5-ALA | DUSA | Basal-cell carcinoma, head and neck, and bladder tumors |
| Pharmaceuticals, Inc. | ||||
| Porphyrin | Metvix® | 5-ALA-methylester | PhotoCure ASA | Basal-cell carcinoma |
| Hexvix® | 5-ALA hexylester | PhotoCure ASA | Diagnosis of bladder tumors | |
| Porphyrin | Visudyne® | Verteporfin | Novartis | Basal-cell carcinoma |
| Pharmaceuticals | ||||
| Chlorine | LS11, Photolon®, Litx™, Apoptosin™, Laserphyrin | Talaporfin | Light sciences | Solid tumors from diverse origins |
| Chlorine | Photochlor | HPPH | RPCI | Basal-cell carcinoma |
| Phthalocyanines | Pc-4 | Phthalocyanine | CWRU | Cutaneous/subcutaneous lesions from diverse solid tumor origins |
In order for more diverse PSs to be used in clinical practice, several important points need to be satisfied;34 (1) the PS should dissolve well in water; (2) the PS should be specifically accumulated in cancer cells; (3) the PS should be excited at wavelengths longer than 650 nm, allowing deeper light penetration into the tissue; (4) the PS should be metabolized rapidly and discharged out of the body; and (5) the PS should be capable of accurately imaging disease sites through clinical imaging devices. In addition, a real-time system of dosimetry for the PSs should be developed to allow for optimal drug dose, light dose and drug light interval.
To meet these clinical demands, with advances in nanotechnology, materials science, and interventional imaging technology, various next-generation PSs are now being evaluated with the hope of their future use in patients who are suffering from cancer.35–39 Novel polymers or nanoparticles (NPs) have been combined with PSs for improving the solubility and photoactivity in the physiological environment. Smart PSs were designed to recognize the target tissue and regulate their photoactivity in the cancer micro-environment.40,41 They are a representative form of the third generation PSs. The smart PSs utilize stimuli responsive materials and multifunctional NPs for highly specific targeting and enhanced therapeutic response.42,43 Recently, various clinical image-guided interventions have been introduced into the PDT. With the smart PSs and multimodal imaging tools, the cancer site can be accurately imaged and simultaneously treated.44–46 Indeed, these efforts in third-generation PSs with image guidance have significantly improved the therapeutic efficacy while decreasing the side effects, compared to the conventional first and second generation PSs.47 This mini-review will summarize the trends of recently developed smart PSs, and their future clinical applications for more effective cancer treatment will be discussed.
2. Stimuli-responsive PSs
As stated in the previous studies, nonspecific activation of PSs in normal tissues not only reduces the therapeutic effect of PSs but also causes severe side effects such as skin burning,48,49 which limits their clinical applications. The development of smart PSs exhibiting an activity in response to specific stimuli at disease sites is a promising strategy for highly tumor-specific PDT. Stimuli-sensitive PSs with high water-solubility responding to tumor associated environments such as tumor specific enzymes or acidic pH have been developed for the targeted PDT.37,41,50–52 The tumor environment tends to become acidic due to abnormal fast metabolic processes of cancer cells such as the Warburg effect,53,54 and also increases the expression of specific enzymes such as matrix metalloproteinases (MMPs) and hyaluronidase for tumor cell metastasis.55,56 Recently, Na and Hyeon developed a nanocomposite PS that can diagnose and treat drug-resistant heterogeneous tumors.35 The hybrid nanocomposites combining self-assembling polymeric PSs and extremely small iron oxide NPs (ESIONs, ∼3 nm) allowed us to detect early tumors (approximately 3 mm in diameter) with fluorescence and magnetic resonance (MR) imaging, and successfully inhibited the growth of drug-resistant heterogeneous tumors. The Na group has also developed a tumor specific polymeric PS that reacts with hyaluronidase, which is highly expressed only in the tumor microenvironment.50,57 The PS molecules attached to one HA polymer lose their photoactivity due to the fluorescence resonance energy transfer (FRET) effect, but they regain the photoactivity through enzymatic degradation in cancer cells.57 As a result, the enzyme-responsive PSs have greater activity in cancer cells than in normal cells, thereby reducing the side effects and improving the therapeutic effects.57As another new approach, thermo-responsive smart PSs38,58–60 have been developed using temperature sensitive polymers. Recently, the Na, Lee, and Kim collaboration group reported a thermo-switchable polymeric PS (T-PPS) by conjugation of a conventional PS (i.e., pheophorbide-a) and a thermosensitive polymer (i.e., hydroxypropyl cellulose). This temperature-sensitive PS exhibited highly selective photoactivity only above the lower critical solution temperature (LCST) of the polymer (Fig. 1),38 at which the polymer's phase transition caused an intermolecular structural change in the PS that eventually enabled the PS to be photo-sensitive. The temperature sensitive PS can reduce non-specific photochemical activation in normal cells by means of a heat-triggering system. Specifically, this PS can be used for a combination therapy of PDT and hyperthermia. It is expected that the T-PPS has the considerable potential not only as a new class of photomedicines in the clinic but also as a biosensor and drug delivery platform based on temperature responsiveness.
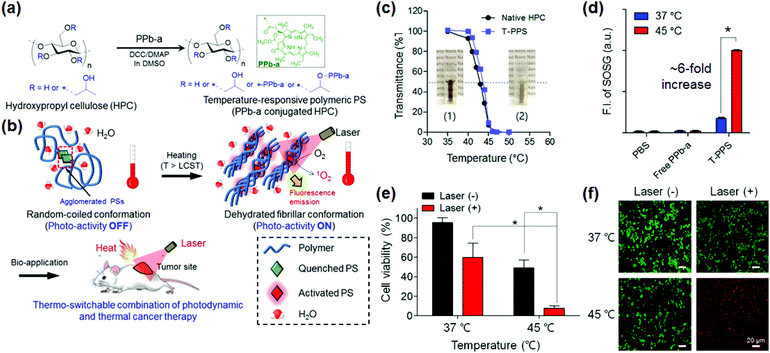 | ||
| Fig. 1 Temperature-responsive polymeric PS (T-PPS). (a and b) (a) Chemical structure of the T-PPS and (b) their bioapplications. (c and d) Temperature-dependent change in (c) transmittance of T-PPS and native HPC (inset: photograph of T-PPS aqueous solution (10 mg mL−1 in water) at (1) T < lower critical solution temperature (LCST) and (2) T > LCST), and (d) singlet oxygen generation of T-PPS in aqueous solution (n = 3, *p < 0.001). (e and f) In vitro photocytotoxicity of T-PPS at various temperatures on PANC-2 cells. (e) Cell counting based upon kit-8 (CCK-8) assay and (f) live/dead assay for cell viability of PANC-2 cells incubated with T-PPS (concentration of PPb-a, 1 μg mL−1) under laser irradiation (3.0 J cm−2) at 37 °C and 45 °C (n = 4, *p < 0.002). Reproduced with permission from ref. 38. Copyright 2016 American Chemical Society. | ||
Although various stimuli-sensitive PSs, as described above, have been developed to date, their sensitivity to the various stimuli does not meet our expectation. For these stimuli-sensitive PSs to be used in clinical applications, more highly sensitive materials having improved disease specificity must be developed, and various target markers must be discovered through the analysis of biological characteristics of diseases.
3. Nano–bio functionalized PSs for image-guided PDT
As NPs have unique characteristics such as optical/magnetic properties,61–63 shape, size, and chemical reactiveness, they have gained much attention in the field of PDT for the functionalization of conventional PSs.43,64–66 The use of NPs in conjunction with PSs offers the following representative advantages: (1) effective PS delivery to the targeted cancer cells,67 (2) easy phase transfer of hydrophobic PSs into amphiphilic, supporting unhindered circulation in bloodstream,68 (3) the enhanced permeability and retention (EPR) effect for the effective diffusion of PSs into tumors,69 (4) various surface chemistries for the functionalization with molecules enhancing the cellular uptake and targeting,70,71 and (5) multiple functionality by combining the original properties of NPs such as multi-modal imaging, with PSs.72,73As a new concept in cancer therapy, image-guided therapy is a promising optimization of the therapeutic effect of PDT by combining existing imaging technology and nanotechnology.46,74 In clinical trials, PSs such as 5-aminolevulinic acid (5-ALA) have actually been used to perform the image-guided PDT with an optical image device.75,76 Currently, various kinds of NPs are being tried to utilize in the image-guided PDT by taking advantage of the unique characteristics of NPs. For example, NPs enable the visualization of disease sites through optical/magnetic resonance imaging (MRI)/computed tomography (CT) imaging, while PSs on their surface generate ROS to treat the disease. The imaging properties of the NPs not only provide tumor size and location information, but also help in determining the optimal dose of a PDT. The monitoring of the therapeutic response of PDT can also help in deciding the future treatment options. A variety of inorganic and organic nanostructured PSs, such as gold NPs,77,78 magnetic NPs,35,79,80 carbon-based materials,81 mesoporous silica NPs,82,83 polymeric micelles,59,84 and upconversion NPs (UCNPs),85,86 have been actively developed by many researchers including our group for image-guided therapies.87–92 The imageable PSs can be visualized in real time through imaging devices to perform PDT, making sophisticated clinical procedures possible and convenient.
Regarding the recent progress in nanostructured PSs with various NPs, UCNPs have been highlighted as they not only solve the problem of low tissue penetration of light, which is the biggest limitation of conventional PSs, but also enable bio-imaging.86,93,94 The Hyeon group first reported the systemic administration of UCNP-based PSs followed by in vivo luminescence and MR imaging with the therapeutic effect of PSs upon indirect excitation with low energy near infrared (NIR) light (Fig. 2).85 The NIR-to-visible upconversion of NaYF4:Yb,Er/NaGdF4 core–shell UCNPs effectively induced PS activation for PDT via energy transfer and the accumulation of UCNP-based PSs was clearly visualized in the tumors by their upconversion luminescence and T1 contrast properties. More recently, the Zhang group employed UCNPs as light transducers to convert deeply penetrating NIR light to UV-visible wavelengths matching that of the absorption spectrum of photosensitive therapeutics for photodynamic cancer therapy65 and photo-activated control of gene expression.95
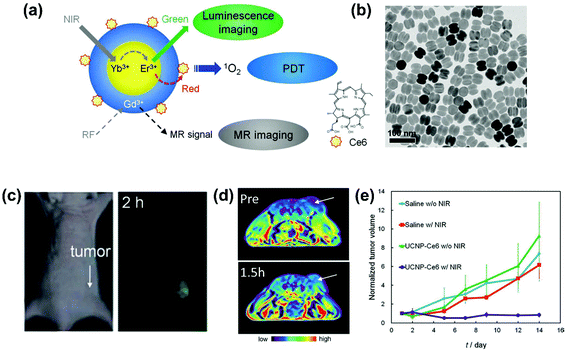 | ||
| Fig. 2 Upconverting nanoparticle (UCNP)-based smart photosensitizer (PS). (a) Schematic illustration of luminescence and magnetic resonance (MR) imaging with photodynamic therapy (PDT) using UCNP-based PSs. (b) Transmission electron microscopy (TEM) image of NaYF4:Yb,Er/NaGdF4 core–shell UCNPs. (c) Bright field and upconversion luminescence images of a nude mouse bearing tumor after intravenous injection of UCNP-based PSs. (d) T1-Weighted color maps of a tumor-bearing nude mouse before and 1.5 h after intravenous injection of UCNP-based PSs. Arrows indicate tumor sites. (e) Growth of tumors after treatments (n = 3 for each group). Reproduced with permission from ref. 85. Copyright 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Although the NP-functionalized PSs have shown many potential applications in PDT, they have several limitations in their commercialization and clinical utility. Difficulties in the quality control of NPs is one of the hurdles because subtle changes in the composition of NPs or multistep reaction and conjugation of PSs can affect the size, morphology, and surface properties of NPs, and loading of PSs. The lack of standardized evaluation procedures for nanomaterial-based agents is another limitation that impedes the development of clinically applicable NP-functionalized PSs. In addition, mechanistic studies of the bio-distribution and metabolism of NP-functionalized PSs in vivo are still lacking, which limits our ability in predicting in vivo long-term toxicity of NP-functionalized PSs and in determining dosage for clinical practice. If the in vivo toxicity and metabolic processes of NP and NP-functionalized PSs are obtained in the near future, the multifunctional NP-functionalized PSs could be used to treat a wide range of cancerous diseases in the clinic. Scientists and clinicians in this field should work in optimizing the NP-functionalized PSs while performing pharmacological analysis using appropriate animal models.
4. Multi-therapeutic PSs for combinational treatment of cancer
Although PDT with smart PSs is beneficial for the cancer treatment, it is difficult to completely treat tumors with mono-PDT. Combination PDT with chemotherapy,96,97 photothermal therapy (PTT),98–100 or cancer immunotherapy101,102 has shown an improved therapeutic effect compared to the conventional PDT alone. The improved synergistic therapeutic effects are attributed to mutual complementation of the disadvantages while sharing the advantages of each treatment.PDT has been tried to be combined with conventional chemotherapy using anticancer drugs.103 The primary goal of combination PDT with chemotherapy is to treat multi-drug resistant (MDR) cancer, which is difficult to treat with chemotherapeutic drugs alone.104 The Na group reported that the combination of PDT and chemotherapy exhibited effective therapeutic effects for the treatment of MDR cancer by exploiting the synergistic effects.105 Cancer cells are often resistant to anticancer drugs by effluxing anti-cancer drugs through the expression of P-glycoprotein (P-gp). The P-gp expressed in the drug-resistant cancer cells can be inactivated by PDT. In detail, the ROS generated from the polymeric PSs induces the peroxidation of the cellular membranes of the cancer cells, thereby inhibiting the activity of P-gp. This series of processes ultimately improves the anti-cancer effects of the combination PDT with chemotherapy in the MDR cells. However, there is room for improvement for the combination PDT with anti-cancer drugs in controlling the drug release rates and low drug encapsulation efficiency, which may limit their therapeutic effects.106
The combination therapy with PTT has also been frequently reported. NIR-PTT, a minimally invasive cancer treatment converting photon energy into heat, using an NIR wavelength (700–850 nm) absorption of NPs has shown promising anticancer effects with no significant heating of normal tissues.98,99 A recent study demonstrated a new type of combination PDT with PTT using organic NPs consisting of a polydopamine NP (PD-NP) core and a PS-conjugated hyaluronic acid (PS-HA) shell (Fig. 3).107 The PD-NP acts as both a quencher for PSs and a PTT agent. The PS-HA consists of a PS and cancer targeting hyaluronic acid (HA) that can not only cause PDT effects but also target cancer cells. In response to cancer-specific intracellular enzymes (e.g., hyaluronidase), the PS-HA-coated PD-NPs exhibited an excellent singlet oxygen generation capacity for PDT. Furthermore, an efficient photothermal conversion capacity for PTT was also shown in the PS-hyaluronic acid conjugate shielded polydopamine nanoparticles (PHPD-NPs). These properties provide superior therapeutic efficacy against cancer cells. In a mouse tumor model, the photo-mediated therapeutic effects of the PHPD-NPs were much higher in cancer microenvironments compared to that in the normal tissue. As a result, the PHPD-NPs showed a significant antitumor activity in the in vivo mouse tumor model. The superior therapeutic effect of the combination of PDT and PTT was consistent with the recently published results by the Lin group.100
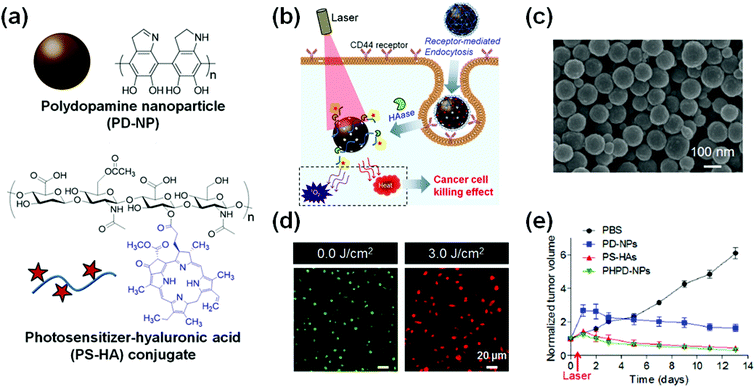 | ||
| Fig. 3 Smart photosensitizer (PS) for combination photodynamic therapy (PDT) and photothermal therapy (PTT). (a) Chemical structure of polydopamine nanoparticle (PD-NP) and PS-conjugated hyaluronic acid (PS-HA) conjugates. (b) Schematic illustration of synergistic cancer therapy effect of PDT and PTT using PS-hyaluronic acid conjugate shielded polydopamine nanoparticles (PHPD-NPs). (c) Scanning electron microscopy (SEM) image of PHPD-NPs (scale bar: 100 nm). (d) Live/dead assay of MDA-MB-231 cells treated with PHPD-NP with or without laser irradiation at 3.0 J cm−2 power. (e) Tumor growth inhibition after intratumoral injection of phosphate-buffered saline (PBS), PD-NP, PS-HAs, or PHPD-NPs with laser irradiation (n = 4). Reproduced with permission from ref. 107. Copyright 2016 American Chemical Society. | ||
Combination therapies with PDT and chemotherapy or NIR-PTT could improve the therapeutic benefits in cancer treatment, through overcoming the limitations of traditional chemotherapy or PDT, such as the drug resistance and the limited light tissue penetration. In the future, it is expected that multiple combination methods integrating more than two treatment methods will be developed to overcome the disadvantages of each treatment and superior therapeutic effects. Most recently, tri-modal therapy combining PDT, PTT, and chemotherapy has been developed along with multiple image guidance, and this technology has been reported to have therapeutic effects beyond that of conventional PDT.108–110
5. Nano-scintillator combined PSs for X-ray PDT
Porphyrin, a typical PS used for PDT, exhibits a strong absorbance at 400 nm (Soret band) and a weak absorbance at 600–800 nm (Q-band).111 Therefore, the ROS production in response to the Soret band is much higher than that by the Q-band.98 However, because the ultraviolet (UV) wavelength has the disadvantage of a very low tissue transmittance, a NIR wavelength with relatively high tissue transmittance has been mainly used for PDT. Although greater than UV, the NIR's skin permeability is still not satisfactory. It can only penetrate 5–6 mm, which makes it difficult to transmit light to deeply locate cancers under clinical conditions.98,112As an alternative to those low transmittance light sources for PDT, X-rays have recently drawn attention.98,113 Because conventional PSs cannot directly absorb X-rays, a scintillator that can convert X-rays to an appropriate wavelength becomes essential. Scintillating NPs can convert X-ray energy to light energy of UV wavelength, which activates the surrounding PSs through fluorescence resonance energy transfer (FRET).98 In this regard, the X-ray PDT can treat deeply seated tumor tissues.98 Furthermore, X-rays can be used as a source for radiation therapy that demonstrates synergetic therapeutic effects of X-ray PDT.112
The scintillators used in the X-ray PDT require some appropriate physical and biological properties for effective PDT. Effective scintillators should have high radiation sensitivity, high scintillation effect, efficient energy transfer to the PS, and excellent biocompatibility.114 To meet these requirements, several types of rare earth based scintillating NPs (BaFBr:Eu2+, BaFBr:Mn2+, LaF3:Ce3+, and LaF3:Tb3+) have been developed and used for X-ray PDT.98
In particular, terbium oxide (Tb2O3) NPs are well known as effective scintillators.114 They become biocompatible when coated with silica leading to core–shell nano-scintillators (Tb2O3@SiO2). A bio-inert silica layer is used to reduce the toxicity of NPs by preventing the release of rare-earth metal ions. Tb3+ ions exhibit fluorescence, which overlaps with the absorption wavelength of porphyrin and is therefore suitable for performing X-ray PDT. Bulin and co-workers recently reported the effectiveness of Tb2O3@SiO2 NPs with PSs in X-ray PDT for the treatment of cancer (Fig. 4).114 The Tb2O3@SiO2 NPs could activate the PS molecules through X-ray energy conversion and eventually kill cancer cells. This representative study demonstrated the strong potential of the X-ray PDT using scintillating NPs.114
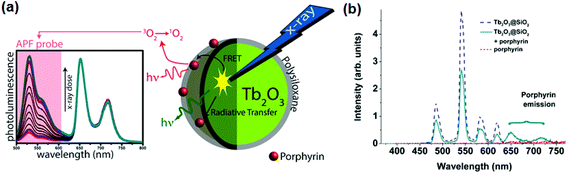 | ||
| Fig. 4 Smart PSs for X-ray photodynamic therapy (PDT). (a) Schematic illustration of the working mechanism of X-PDT. Under X-ray irradiation, nanoscintillator (Tb2O3) transfer energy toward the photosensitizer (PS, porphyrin), leading to singlet oxygen generation. (b) Fluorescence spectra measurement performed with 300 nm excitation. The emission spectrum of porphyrin was almost zero, while the spectrum of the porphyrin grafted Tb2O3@SiO3 nanoparticles (NPs) presents both the emission peaks at 650 and 725 nm. Reproduced with permission from ref. 114. Copyright 2013 American Chemical Society. | ||
There are challenges in using the scintillating NPs for X-ray PDT. Precise X-ray irradiation at the site of a local cancer is difficult. Thus, if the scintillating NPs and porphyrins are not co-localized to the cancer site, the diffuse X-ray radiation would cause adverse effects on normal tissues. Therefore, it is crucial to develop a nano-scintillator capable of accurately targeting cancer. It is also important to maintain the stability of the scintillating NPs in vivo for the course of treatments. The NPs injected into the body must be rapidly degraded and excreted. Combining X-ray PDT with multimodal image-guidance79,89,91 allows accurate X-ray irradiation to target cancer tissues, which would help reduce the side effects in normal tissue.
6. Immune-modulatory PSs for photodynamic immunotherapy
Cancer immunotherapy consists of harnessing our innate immune system to treat cancer.87,115 Due to recent promising results in the survival rate and recurrence rate,115,116 immunotherapy has been regarded as one of the most promising cancer therapies and various studies are on-going. It has been thought that PDT may enhance immune response and surveillance.117,118 Recent studies have reported that PDT can activate the anti-cancer immune cells and effectively inhibit tumor growth with preventing the metastasis of cancer cells and the recurrence of cancer.118,119 These motivating results have attracted many clinicians and researchers for photodynamic immunotherapy.101,118PDT kills cancer cells by apoptosis and necrosis, and stimulates the host immune system.118 It triggers acute inflammation and leukocyte infiltration to the tumors, which increases the presentation of tumor-derived antigens to T cells.101,118 Immune checkpoint blockade therapy, which targets regulatory pathways in T cells to enhance anti-tumor immune response, has recently emerged as a new strategy for cancer immunotherapy.120 Transmembrane protein PD-L1 expressed on tumor cells points to the PD-1/PD-L1 pathway as a valuable target for the checkpoint blockade immunotherapy121,122 Lin's group reported a new approach for photodynamic cancer immunotherapy using immunogenic NPs to augment the antitumor efficacy of PD-L1 antibody-mediated cancer immunotherapy (Fig. 5).123 Zn-Pyrophosphate NPs composed of the PS pyrolipid, a lipid conjugate of pyropheophorbide-a, (ZnP@pyro) effectively killed cancer cells upon light irradiation directly by inducing apoptosis and/or necrosis and indirectly by disrupting the tumor vasculature and increasing tumor immunogenicity. More importantly, the ZnP@pyro-mediated PDT was reported to induce an immunogenic environment in tumors and sensitize tumors to PD-L1 checkpoint blockade therapy. As a result, the ZnP@pyro PDT combined with anti-PD-L1 not only eradicated 4T1 breast primary tumors but also prevented lung metastasis and inhibited pre-existing metastatic tumors by generating systemic antitumor immunity. These results indicate that NP-mediated PDT can potentiate the systemic efficacy of checkpoint blockade immunotherapies by activating the innate and adaptive immune systems in the tumor microenvironment. Interestingly, this PDT-based immunotherapy could simultaneously treat distant tumors in bilateral syngeneic mouse tumor models by inducing an abscopal effect. The abscopal effect usually described with ionizing radiation refers to the regression of tumor outside of the irradiated volume.124 Although the mechanism is unknown, it is thought to be immune modulated.101,124
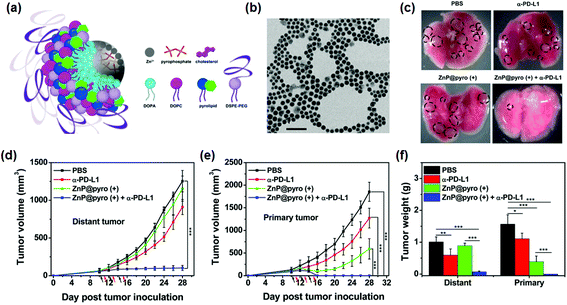 | ||
| Fig. 5 Immunogenic nanoparticles (NPs) for photodynamic cancer therapy. (a) Scheme illustration of the Zn-pyrophosphate (ZnP) core and the photosensitizer (PS) pyrolipid (a lipid conjugate of pyropheophorbide-a) shell of the immunogenic NP (ZnP@pyro). (b) Transmission electron microscopy (TEM) image showing the spherical and nearly monodispersed morphology of ZnP@pyro (scale bar = 200 nm). (c) Photographs of excised primary 4T1 tumors at the end point (23 days after tumor implantation). From top to bottom: phosphate-buffered saline (PBS), α-PD-L1, ZnP@pyro (+), and ZnP@pyro (+) plus α-PD-L1. The tumors indicated with dot-lined rectangle disappeared in the ZnP@pyro (+) + α-PD-L1 group. This result suggests that the ZnP@pyro PDT combined with PD-L1 blockade eradicates primary 4T1 tumors and prevents lung metastasis. (d and e) (d) Distant and (e) primary tumor growth curves of 4T1 models. The arrows represent the time of NP administration (black) and irradiation (red). (f) Weight of 4T1 tumors at the end point (28 days after tumor implantation) of the experiment. “(+)” in the figure legends refers to treatment with irradiation. *p < 0.05, **p < 0.01, ***p < 0.001. The combination of ZnP@pyro with irradiation and PD-L1 blockade induced complete eradication of the irradiated primary tumors (synergistic effect) and effective control of the nonirradiated distant tumors (abscopal effect), eliciting a 92% reduction in tumor size compared to the PBS control group. These results indicate that tumors can be sensitized to PD-L1 blockade immunotherapy by ZnP@pyro PDT-mediated tumor-specific immune responses. Reproduced with permission from ref. 123. Copyright 2016 American Chemical Society. | ||
Most recently, Liu's and Peng's groups have proposed using multi-functional NPs composed of UCNPs, PSs, and imiquimod (R837), a Toll-like-receptor-7 agonist simultaneously to overcome the disadvantages of conventional PDT such as limited penetration depth of light and low anticancer immune response.125 The multifunctional nanoparticles induced an effective PDT effect with high tissue permeability in a NIR wavelength laser due to the UCNPs, and served as an adjuvant, leading to a robust immune response induced by the tumor-associated antigen formed by the PDT treatment. More significantly, when combined with the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint blockade, it was shown to be more efficient in the antitumor immunities and inhibited the growth of distant tumors left behind after PDT treatment.
Although the immunotherapy combined with PDT is currently in its early stages, it is expected to have great potential in the future. In particular, the introduction of the aforementioned functional smart PSs into photodynamic immunotherapy could effectively treat various types of cancers as well as prevent cancerous diseases. However, detailed studies are needed to understand the biological role of PDT to improve the immune responses. In addition, the development of smart PSs with physicochemical properties suitable for immunotherapy will enable more effective combination cancer immunotherapy. The physicochemical properties can be the hydrophilicity/hydrophobicity, particle size, and surface charge of the smart PSs. These properties can affect the interaction with tumor-associated antigens or immune cells.126,127
7. Conclusions and perspectives
PDT has been clinically used to treat a variety of cancers such as skin, esophageal, lung, head and neck, liver, prostate, and bladder cancers. However, the classical first and second-generation PSs have several limitations in clinical application, such as poor water solubility, low cancer targeting efficacy, long-term phototoxicity, and limited light penetration of tissue. To overcome the shortcomings of conventional PSs, new types of smart PSs have been of great interest in PDT cancer therapeutics with the development of nanotechnology or materials science. Smart PSs with high water-solubility responding to various stimuli such as temperature, pH, and enzymes have been designed to more accurately target disease for improving the therapeutic efficacy in PDT. Nano-based PSs have also been studied to impart unique properties such as multi-modal medical imaging and therapeutic functions of NPs to the conventional PSs. The smart PSs, multifunctional NPs combined with stimuli responsive PSs, have great potential for various combinational therapies overcoming the drug resistance of cancer cells and enhancing therapeutic efficacy. Among the promising new combinational PDT therapies, X-ray PDT technology attracted great attention to mitigate the issue of limited light penetration in treating cancers located in deep tissues. Most recently, photodynamic immune therapy combined with the smart PSs and immune checkpoint blockade has also emerged as a new robust approach for treating cancers. This next-generation PS technology and its multidisciplinary convergence technology not only overcome the shortcomings of the conventional PSs but also evolve into a new concept of PDT technology.Thanks to the advances in the technology of third-generation PSs, first NP-based PS, Visudyne® (Verteporfin), was recently approved by the Food and Drug Administration (FDA) in 2000 for the treatment of age-related macular degeneration.128 In addition, two liposomal formulations of m-THPC, Foslip® and Fospeg®, were developed and are being evaluated.47 However, the third-generation PSs still need a lot of improvement for clinical use in a wide range of cancer diseases. The improvement can be made in progress of physicochemical properties of the PSs or clinical surgical procedure for accurate light source delivery. Currently, PSs suitable for NIR wavelengths using advanced nanotechnology are being developed. Based on state-of-the-art interventional image-guidance, new clinical techniques are also being tried to accurately deliver the light source to cancer in deep tissue using an optical fiber or an endoscope. Lastly, in-depth studies of the biological mechanisms of third generation PSs with various chemical structures will provide valuable information on the biological interactions between organisms and PSs to improve their therapeutic efficacy. These advanced techniques with better understanding in materials and clinical field will permit real-time monitoring and treatment of tumors allowing optimized and personalized PDT therapies for each patient.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the NIH/NIBIB and NIH/NCI for R21CA173491 (D.-H. K.), R21CA185274 (D.-H. K.), and R21EB017986 (D.-H. K.) is greatly acknowledged. This work was also supported by the National Research Foundation of Korea (NRF) (NRF-2017R1A2B3010038 and 2015R1A4A1042350). BL is supported by the DOE Office of Science through the Argonne National Laboratory under Contract No. DE-AC02-06CH11357.References
- M. Daniell and J. Hill, Aust. N. Z. J. Surg., 1991, 61, 340–348 CrossRef CAS PubMed.
- R. Ackroyd, C. Kelty, N. Brown and M. Reed, Photochem. Photobiol., 2001, 74, 656–669 CrossRef CAS PubMed.
- J. D. Spikes, Primary photoprocesses in biology and medicine, ed. R. V. Berghausen, G. Jori, E. J. Land, T. H. Truscott, Plenum Press, New York, 1985, pp. 209–227 Search PubMed.
- J. Kelly and M. Snell, J. Urol., 1976, 115, 150–151 CrossRef CAS PubMed.
- J. Kelly, M. Snell and M. Berenbaum, Br. J. Cancer, 1975, 31, 237 CrossRef CAS PubMed.
- M. Huggett, M. Jermyn, A. Gillams, R. Illing, S. Mosse, M. Novelli, E. Kent, S. Bown, T. Hasan and B. Pogue, Br. J. Cancer, 2014, 110, 1698–1704 CrossRef CAS PubMed.
- T. Yano, K. Hatogai, H. Morimoto, Y. Yoda and K. Kaneko, Ann. Transl. Med., 2014, 2, 29 Search PubMed.
- T. J. Dougherty, G. Lawrence, J. H. Kaufman, D. Boyle, K. R. Weishaupt and A. Goldfarb, J. Natl. Cancer Inst., 1979, 62, 231–237 CAS.
- B. G. Ward, I. J. Forbes, P. A. Cowled, M. M. McEvoy and L. W. Cox, Am. J. Obstet. Gynecol., 1982, 142, 356–357 CrossRef CAS PubMed.
- C. J. Gomer, D. R. Doiron, J. V. Jester, B. C. Szirth and A. L. Murphree, Cancer Res., 1983, 43, 721–727 CAS.
- M. A. Rosenthal, B. Kavar, J. S. Hill, D. J. Morgan, R. L. Nation, S. S. Stylli, R. L. Basser, S. Uren, H. Geldard and M. D. Green, J. Clin. Oncol., 2001, 19, 519–524 CrossRef CAS PubMed.
- V. G. Schweitzer, Otolaryngol.–Head Neck Surg., 1990, 102, 225–232 CrossRef CAS PubMed.
- H. Barr, N. Krasner, P. Boulos, P. Chatlani and S. Bown, Br. J. Surg., 1990, 77, 93–96 CrossRef CAS PubMed.
- R. R. Allison, T. S. Mang and B. D. Wilson, Semin. Cutaneous Med. Surg., 1998, 17, 153–163 CrossRef CAS.
- T. F. Delaney, W. F. Sindelar, Z. Tochner, P. D. Smith, W. S. Friauf, G. Thomas, L. Dachowski, J. W. Cole, S. M. Steinberg and E. Glatstein, Int. J. Radiat. Oncol., Biol., Phys., 1993, 25, 445–457 CrossRef CAS.
- M. A. E. Ortner, J. Liebetruth, S. Schreiber, M. Hanft, U. Wruck, V. Fusco, J. M. Müller, H. Hörtnagl and H. Lochs, Gastroenterology, 1998, 114, 536–542 CrossRef CAS.
- S. Bown, A. Rogowska, D. Whitelaw, W. Lees, L. Lovat, P. Ripley, L. Jones, P. Wyld, A. Gillams and A. Hatfield, Gut, 2002, 50, 549–557 CrossRef CAS PubMed.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- G. Tegos, T. Dai, B. B. Fuchs, J. J. Coleman, R. A. Prates, C. Astrakas, T. G. St Denis, M. S. Ribeiro, E. Mylonakis and M. R. Hamblin, Front. Microbiol., 2012, 3, 120 Search PubMed.
- N. L. Oleinick, R. L. Morris and I. Belichenko, Photochem. Photobiol. Sci., 2002, 1, 1–21 CAS.
- M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- W. Park, B.-c. Bae and K. Na, Biomaterials, 2016, 77, 227–234 CrossRef CAS PubMed.
- H. Ding, H. Yu, Y. Dong, R. Tian, G. Huang, D. A. Boothman, B. D. Sumer and J. Gao, J. Controlled Release, 2011, 156, 276–280 CrossRef CAS PubMed.
- R. R. Allison and C. H. Sibata, Photodiagn. Photodyn. Ther., 2010, 7, 61–75 CrossRef CAS PubMed.
- E. D. Sternberg, D. Dolphin and C. Brückner, Tetrahedron, 1998, 54, 4151–4202 CrossRef CAS.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS PubMed.
- T. J. Dougherty, Photochem. Photobiol., 1987, 45, 879–889 CrossRef CAS PubMed.
- A. B. Ormond and H. S. Freeman, Materials, 2013, 6, 817–840 CrossRef CAS PubMed.
- M. R. Detty, S. L. Gibson and S. J. Wagner, J. Med. Chem., 2004, 47, 3897–3915 CrossRef CAS PubMed.
- M. Wainwright, Anticancer Agents Med. Chem., 2008, 8, 280–291 CrossRef CAS PubMed.
- C. J. Gomer, Photochem. Photobiol., 1991, 54, 1093–1107 CrossRef CAS PubMed.
- M. O. Senge and J. C. Brandt, Photochem. Photobiol., 2011, 87, 1240–1296 CrossRef CAS PubMed.
- C.-K. Lim, J. Heo, S. Shin, K. Jeong, Y. H. Seo, W.-D. Jang, C. R. Park, S. Y. Park, S. Kim and I. C. Kwon, Cancer Lett., 2013, 334, 176–187 CrossRef CAS PubMed.
- H. Kataoka, H. Nishie, N. Hayashi, M. Tanaka, A. Nomoto, S. Yano and T. Joh, Ann. Transl. Med., 2017, 5, 183 CrossRef PubMed.
- D. Ling, W. Park, S.-j. Park, Y. Lu, K. S. Kim, M. J. Hackett, B. H. Kim, H. Yim, Y. S. Jeon and K. Na, J. Am. Chem. Soc., 2014, 136, 5647–5655 CrossRef CAS PubMed.
- S. Jeong, W. Park, C. S. Lee and K. Na, Macromol. Biosci., 2014, 14, 1688–1695 CrossRef CAS PubMed.
- C.-S. Lee, W. Park, Y. U. Jo and K. Na, Chem. Commun., 2014, 50, 4354–4357 RSC.
- W. Park, S.-J. Park, S. Cho, H. Shin, Y.-S. Jung, B. Lee, K. Na and D.-H. Kim, J. Am. Chem. Soc., 2016, 138, 10734–10737 CrossRef CAS PubMed.
- C.-S. Lee, W. Park, S.-j. Park and K. Na, Biomaterials, 2013, 34, 9227–9236 CrossRef CAS PubMed.
- H. Fan, G. Yan, Z. Zhao, X. Hu, W. Zhang, H. Liu, X. Fu, T. Fu, X. B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2016, 128, 5567–5572 CrossRef.
- S. Y. Park, H. J. Baik, Y. T. Oh, K. T. Oh, Y. S. Youn and E. S. Lee, Angew. Chem., Int. Ed., 2011, 50, 1644–1647 CrossRef CAS PubMed.
- B.-c. Bae and K. Na, Int. J. Photoenergy, 2012, 2012, 431975 CrossRef.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed.
- M. P. Melancon, M. Zhou and C. Li, Acc. Chem. Res., 2011, 44, 947–956 CrossRef CAS PubMed.
- J. Li, F. Liu, S. Gupta and C. Li, Theranostics, 2016, 6, 1393 CrossRef CAS PubMed.
- L. Cheng, D. Jiang, A. Kamkaew, H. F. Valdovinos, H. J. Im, L. Feng, C. G. England, S. Goel, T. E. Barnhart and Z. Liu, Adv. Funct. Mater., 2017, 27, 1702928 CrossRef.
- E. Paszko, C. Ehrhardt, M. O. Senge, D. P. Kelleher and J. V. Reynolds, Photodiagn. Photodyn. Ther., 2011, 8, 14–29 CrossRef CAS PubMed.
- M. Tarstedt, I. Rosdahl, B. Berne, K. Svanberg and A.-M. Wennberg, Acta Derm. –Venereol., 2005, 85, 424 CrossRef CAS PubMed.
- D. Rubel, L. Spelman, D. F. Murrell, J. A. See, D. Hewitt, P. Foley, C. Bosc, D. Kerob, N. Kerrouche and H. Wulf, Br. J. Dermatol., 2014, 171, 1164–1171 CrossRef CAS PubMed.
- W. Park, S.-j. Park and K. Na, Biomaterials, 2011, 32, 8261–8270 CrossRef CAS PubMed.
- B.-c. Bae and K. Na, Biomaterials, 2010, 31, 6325–6335 CrossRef CAS PubMed.
- S. j. Park, W. Park and K. Na, Adv. Funct. Mater., 2015, 25, 3472–3482 CrossRef CAS.
- V. Estrella, T. Chen, M. Lloyd, J. Wojtkowiak, H. H. Cornnell, A. Ibrahim-Hashim, K. Bailey, Y. Balagurunathan, J. M. Rothberg and B. F. Sloane, Cancer Res., 2013, 73, 1524–1535 CrossRef CAS PubMed.
- E. S. Lee, Z. Gao and Y. H. Bae, J. Controlled Release, 2008, 132, 164–170 CrossRef CAS PubMed.
- E. I. Deryugina and J. P. Quigley, Cancer Metastasis Rev., 2006, 25, 9–34 CrossRef CAS PubMed.
- R. A. Gatenby, E. T. Gawlinski, A. F. Gmitro, B. Kaylor and R. J. Gillies, Cancer Res., 2006, 66, 5216–5223 CrossRef CAS PubMed.
- F. Li, B.-c. Bae and K. Na, Bioconjugate Chem., 2010, 21, 1312–1320 CrossRef CAS PubMed.
- H. Koizumi, Y. Shiraishi, S. Tojo, M. Fujitsuka, T. Majima and T. Hirai, J. Am. Chem. Soc., 2006, 128, 8751–8753 CrossRef CAS PubMed.
- D. H. Kim, E. A. Vitol, J. Liu, S. Balasubramanian, D. J. Gosztola, E. E. Cohen, V. Novosad and E. A. Rozhkova, Langmuir, 2013, 29, 7425–7432 CrossRef CAS PubMed.
- D. H. Kim, T. Choy, S. Huang, R. M. Green, R. A. Omary and A. C. Larson, Acta Biomater., 2014, 10, 742–750 CrossRef CAS PubMed.
- D. H. Kim, Y. Guo, Z. Zhang, D. Procissi, J. Nicolai, R. A. Omary and A. C. Larson, Adv. Healthcare Mater., 2014, 3, 714–724 CAS.
- D. H. Kim, J. Chen, R. A. Omary and A. C. Larson, Theranostics, 2015, 5, 477 CrossRef CAS PubMed.
- D. H. Kim and A. C. Larson, Biomaterials, 2015, 56, 154–164 CrossRef CAS PubMed.
- D. K. Chatterjee, L. S. Fong and Y. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1627–1637 CrossRef CAS PubMed.
- N. M. Idris, M. K. Gnanasammandhan, J. Zhang, P. C. Ho, R. Mahendran and Y. Zhang, Nat. Med., 2012, 18, 1580–1585 CrossRef CAS PubMed.
- D. Bechet, P. Couleaud, C. Frochot, M.-L. Viriot, F. Guillemin and M. Barberi-Heyob, Trends Biotechnol., 2008, 26, 612–621 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. FaroKHzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- M. E. Wieder, D. C. Hone, M. J. Cook, M. M. Handsley, J. Gavrilovic and D. A. Russell, Photochem. Photobiol. Sci., 2006, 5, 727–734 CAS.
- E. Blanco, H. Shen and M. Ferrari, Nat. Biotechnol., 2015, 33, 941–951 CrossRef CAS PubMed.
- M. E. Davis, Z. Chen and D. M. Shin, Nat. Rev. Drug Discovery, 2008, 7, 771–782 CrossRef CAS PubMed.
- K. J. Cho, X. Wang, S. M. Nie, Z. Chen and D. M. Shin, Clin. Cancer Res., 2008, 14, 1310–1316 CrossRef CAS PubMed.
- K. Park, S. Lee, E. Kang, K. Kim, K. Choi and I. C. Kwon, Adv. Funct. Mater., 2009, 19, 1553–1566 CrossRef CAS.
- J. Kim, Y. Piao and T. Hyeon, Chem. Soc. Rev., 2009, 38, 372–390 RSC.
- C. J. Zhang, Q. Hu, G. Feng, R. Zhang, Y. Yuan, X. Lu and B. Liu, Chem. Sci., 2015, 6, 4580–4586 RSC.
- C. Chi, Y. Du, J. Ye, D. Kou, J. Qiu, J. Wang, J. Tian and X. Chen, Theranostics, 2014, 4, 1072 CrossRef PubMed.
- W. Stummer, A. Novotny, H. Stepp, C. Goetz, K. Bise and H. J. Reulen, J. Neurosurg., 2000, 93, 1003–1013 CrossRef CAS PubMed.
- J. Lin, S. Wang, P. Huang, Z. Wang, S. Chen, G. Niu, W. Li, J. He, D. Cui and G. Lu, ACS Nano, 2013, 7, 5320 CrossRef CAS PubMed.
- D. H. Kim and A. C. Larson, Biomaterials, 2015, 56, 154–164 CrossRef CAS PubMed.
- D. H. Kim, J. Chen, R. A. Omary and A. C. Larson, Theranostics, 2015, 5, 477–488 CrossRef CAS PubMed.
- D. H. Kim, Y. Guo, Z. Zhang, D. Procissi, J. Nicolai, R. A. Omary and A. C. Larson, Adv. Healthcare Mater., 2014, 3, 714–724 CrossRef CAS PubMed.
- P. Huang, J. Lin, X. Wang, Z. Wang, C. Zhang, M. He, K. Wang, F. Chen, Z. Li and G. Shen, Adv. Mater., 2012, 24, 5104–5110 CrossRef CAS PubMed.
- H. L. Tu, Y. S. Lin, H. Y. Lin, Y. Hung, L. W. Lo, Y. F. Chen and C. Y. Mou, Adv. Mater., 2009, 21, 172–177 CrossRef CAS.
- J. Sun, D.-H. Kim, Y. Guo, Z. Teng, Y. Li, L. Zheng, Z. Zhang, A. C. Larson and G. Lu, J. Mater. Chem. B, 2015, 3, 1049–1058 RSC.
- L. Cheng, A. Kamkaew, H. Sun, D. Jiang, H. F. Valdovinos, H. Gong, C. G. England, S. Goel, T. E. Barnhart and W. Cai, ACS Nano, 2016, 10, 7721–7730 CrossRef CAS PubMed.
- Y. Park, H. M. Kim, J. H. Kim, K. C. Moon, B. Yoo, K. T. Lee, N. Lee, Y. Choi, W. Park, D. Ling, K. Na, W. K. Moon, S. H. Choi, H. S. Park, S. Y. Yoon, Y. D. Suh, S. H. Lee and T. Hyeon, Adv. Mater., 2012, 24, 5755–5761 CrossRef CAS PubMed.
- J. Lee, A. C. Gordon, H. Kim, W. Park, S. Cho, B. Lee, A. C. Larson, E. A. Rozhkova and D. H. Kim, Biomaterials, 2016, 109, 69–77 CrossRef CAS PubMed.
- W. Park, A. C. Gordon, S. Cho, X. Huang, K. R. Harris, A. C. Larson and D. H. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 13819–13824 CAS.
- M. J. Jeon, A. C. Gordon, A. C. Larson, J. W. Chung, Y. I. Kim and D. H. Kim, Biomaterials, 2016, 88, 25–33 CrossRef CAS PubMed.
- D. H. Kim, W. Li, J. Chen, Z. Zhang, R. M. Green, S. Huang and A. C. Larson, Sci. Rep., 2016, 6, 29653 CrossRef CAS PubMed.
- W. Park, J. Chen, S. Cho, S. J. Park, A. C. Larson, K. Na and D. H. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 12711–12719 CAS.
- W. Park, S. Cho, X. Huang, A. C. Larson and D. H. Kim, Small, 2017, 13, 1602722 CrossRef PubMed.
- S. Cho, W. Park and D. H. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 101–111 CAS.
- Y. I. Park, K. T. Lee, Y. D. Suh and T. Hyeon, Chem. Soc. Rev., 2015, 44, 1302–1317 RSC.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- M. K. Gnanasammandhan, N. M. Idris, A. Bansal, K. Huang and Y. Zhang, Nat. Protoc., 2016, 11, 688–713 CrossRef CAS PubMed.
- X. Feng, D. Jiang, T. Kang, J. Yao, Y. Jing, T. Jiang, J. Feng, Q. Zhu, Q. Song and N. Dong, ACS Appl. Mater. Interfaces, 2016, 8, 17817–17832 CAS.
- G. Chen, R. Jaskula-Sztul, C. R. Esquibel, I. Lou, Q. Zheng, A. Dammalapati, A. Harrison, K. W. Eliceiri, W. Tang and H. Chen, Adv. Funct. Mater., 2017, 27, 1604671 CrossRef PubMed.
- A. Kamkaew, F. Chen, Y. Zhan, R. L. Majewski and W. Cai, ACS Nano, 2016, 10, 3918–3935 CrossRef CAS PubMed.
- J. Han, H. Xia, Y. Wu, S. N. Kong, A. Deivasigamani, R. Xu, K. M. Hui and Y. Kang, Nanoscale, 2016, 8, 7861–7865 RSC.
- K. Deng, Z. Hou, X. Deng, P. Yang, C. Li and J. Lin, Adv. Funct. Mater., 2015, 25, 7280–7290 CrossRef CAS.
- C. He, X. Duan, N. Guo, C. Chan, C. Poon, R. R. Weichselbaum and W. Lin, Nat. Commun., 2016, 7, 12499 CrossRef CAS PubMed.
- D. Wang, T. Wang, J. Liu, H. Yu, S. Jiao, B. Feng, F. Zhou, Y. Fu, Q. Yin and P. Zhang, Nano Lett., 2016, 16, 5503–5513 CrossRef CAS PubMed.
- H.-C. Huang, S. Mallidi, J. Liu, C.-T. Chiang, Z. Mai, R. Goldschmidt, N. Ebrahim-Zadeh, I. Rizvi and T. Hasan, Cancer Res., 2016, 76, 1066–1077 CrossRef CAS PubMed.
- B. Q. Spring, I. Rizvi, N. Xu and T. Hasan, Photochem. Photobiol. Sci., 2015, 14, 1476–1491 CAS.
- H. Park, W. Park and K. Na, Biomaterials, 2014, 35, 7963–7969 CrossRef CAS PubMed.
- N. Kamaly, B. Yameen, J. Wu and O. C. Farokhzad, Chem. Rev., 2016, 116, 2602–2663 CrossRef CAS PubMed.
- J. Han, W. Park, S.-j. Park and K. Na, ACS Appl. Mater. Interfaces, 2016, 8, 7739–7747 CAS.
- B. Liu, C. Li, G. Chen, B. Liu, X. Deng, Y. Wei, J. Xia, B. Xing, P. a. Ma and J. Lin, Adv. Sci., 2017, 4, 1600540 CrossRef PubMed.
- S. Fang, J. Lin, C. Li, P. Huang, W. Hou, C. Zhang, J. Liu, S. Huang, Y. Luo and W. Fan, Small, 2017, 13, 1602580 CrossRef PubMed.
- K. Hayashi, T. Maruhashi, M. Nakamura, W. Sakamoto and T. Yogo, Adv. Funct. Mater., 2016, 26, 8613–8622 CrossRef CAS.
- M. Vicente, Curr. Med. Chem. Anticancer Agents, 2001, 1, 175–194 CrossRef CAS PubMed.
- C. Zhang, K. Zhao, W. Bu, D. Ni, Y. Liu, J. Feng and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 1770–1774 CrossRef CAS PubMed.
- S. Lu, D. Tu, P. Hu, J. Xu, R. Li, M. Wang, Z. Chen, M. Huang and X. Chen, Angew. Chem., Int. Ed., 2015, 54, 7915–7919 CrossRef CAS PubMed.
- A.-L. Bulin, C. Truillet, R. Chouikrat, F. Lux, C. Frochot, D. Amans, G. Ledoux, O. Tillement, P. Perriat and M. Barberi-Heyob, J. Phys. Chem. C, 2013, 117, 21583–21589 CAS.
- J. Couzin-Frankel, Science, 2013, 342, 1432–1433 CrossRef CAS PubMed.
- M. M. Gubin, X. Zhang, H. Schuster, E. Caron, J. P. Ward, T. Noguchi, Y. Ivanova, J. Hundal, C. D. Arthur and W.-J. Krebber, Nature, 2014, 515, 577–581 CrossRef CAS PubMed.
- M. Korbelik, J. Clin. Laser Med. Surg., 1996, 14, 329–334 CAS.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS PubMed.
- P. Mroz, J. T. Hashmi, Y.-Y. Huang, N. Lange and M. R. Hamblin, Expert Rev. Clin. Immunol., 2011, 7, 75–91 CrossRef CAS PubMed.
- P. Sharma and J. P. Allison, Science, 2015, 348, 56–61 CrossRef CAS PubMed.
- X. Meng, Z. Huang, F. Teng, L. Xing and J. Yu, Cancer Treat. Rev., 2015, 41, 868–876 CrossRef CAS PubMed.
- Y. Iwai, M. Ishida, Y. Tanaka, T. Okazaki, T. Honjo and N. Minato, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12293–12297 CrossRef CAS PubMed.
- X. Duan, C. Chan, N. Guo, W. Han, R. R. Weichselbaum and W. Lin, J. Am. Chem. Soc., 2016, 138, 16686–16695 CrossRef CAS PubMed.
- M. A. Postow, M. K. Callahan, C. A. Barker, Y. Yamada, J. Yuan, S. Kitano, Z. Mu, T. Rasalan, M. Adamow and E. Ritter, N. Engl. J. Med., 2012, 366, 925–931 CrossRef CAS PubMed.
- J. Xu, L. Xu, C. Wang, R. Yang, Q. Zhuang, X. Han, Z. Dong, W. Zhu, R. Peng and Z. Liu, ACS Nano, 2017, 11, 4463–4474 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, H. Guo, A. Emili and W. C. Chan, J. Am. Chem. Soc., 2012, 134, 2139–2147 CrossRef CAS PubMed.
- K. Saha, M. Rahimi, M. Yazdani, S. T. Kim, D. F. Moyano, S. Hou, R. Das, R. Mout, F. Rezaee and M. Mahmoudi, ACS Nano, 2016, 10, 4421–4430 CrossRef CAS PubMed.
- N. M. Bressler, Arch. Ophthalmol., 1999, 117, 1329–1345 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |






