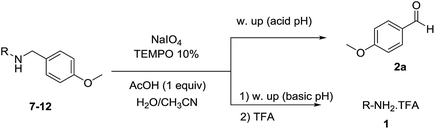
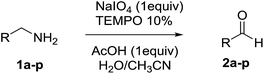Open Access Article
Open Access ArticleSodium periodate/TEMPO as a selective and efficient system for amine oxidation†
P. Galletti *,
G. Martelli,
G. Prandini,
C. Colucci and
D. Giacomini
*,
G. Martelli,
G. Prandini,
C. Colucci and
D. Giacomini *
*
Department of Chemistry “G. Ciamician” University of Bologna, Bologna 40126, Italy. E-mail: paola.galletti@unibo.it; daria.giacomini@unibo.it
First published on 7th March 2018
Abstract
A new metal-free protocol for promoting oxidation of amines in aqueous-organic medium was developed. NaIO4 and TEMPO as the catalyst emerged as the most efficient and selective system for oxidation of differently substituted benzyl amines to the corresponding benzaldehydes without overoxidation. Unsymmetrical secondary amines underwent selective oxidation only at the benzylic position thus realising an oxidative deprotection of a benzylic group with an easy amine recovery.
Introduction
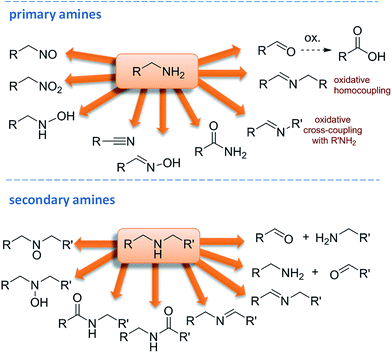
Oxidation chemistry plays a central role in organic synthesis and many industrial processes rely on oxidations as key steps. Oxidation reactions are indeed an important tool for the interconversion of functional groups and are largely exploited for alkane or alcohol transformations.1 Oxidation of amines has been less explored but it can afford a large panel of products (Fig. 1), some oxidations aim at the nitrogen atom, other at both nitrogen and carbon atoms. Thus, selective reagents and controlled conditions are required to get a specific functional group. Moreover, pressure from society and regulation is placing important restrictions on industrial oxidation technology, with emphasis on the need for sustainable and environmentally friendly processes.The oxidation of amines to get aldehydes is a particularly interesting transformation and despite its efficiency, the most common protocols suffer from the required use of stoichiometric amounts of toxic metal-containing reagents, such as KMnO4,2 argentic picolinate,3 ZnCr2O7,4 and nicotinium dichromate,5 or palladium-,6 copper-,7 and ruthenium-based8 catalysts. In addition, these methodologies are sometimes affected by over oxidation of aldehydes to carboxylic acids.
The synthesis of imines via oxidative coupling of primary amines or oxidation of secondary amines were recently explored using metals or metal-complexes as catalysts.9 Recently, photocatalysts such as titanium or niobium salts by UV irradiation, and mesoporous-C3N4,10 CdS,11 Au–Pd/ZrO2,12 conjugated microporous poly(benzooxadiazole) networks13 and hollow microporous organic networks14 by visible irradiation have been reported as active and highly selective catalysts for this oxidation. However, pure oxygen at high pressure is required. In recent contributions Au/TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 BiVO4 with a copper complex,16 and goldthiolates/TiO2 nanoclusters using atmospheric oxygen under visible light were used for benzylamine oxidation to secondary imines.17
15 BiVO4 with a copper complex,16 and goldthiolates/TiO2 nanoclusters using atmospheric oxygen under visible light were used for benzylamine oxidation to secondary imines.17
Many of these oxidation systems still require harsh reaction conditions and produce metal-containing wastes.18 Therefore, as an improvement, some metal-free methodologies were studied. Recently synthetic quinone-based catalysts for the efficient aerobic oxidation of amines to imines were reviewed.19 These methods have been inspired by copper amine oxidases, a family of metallo-enzymes which selectively converts primary amines into aldehydes, using molecular oxygen through the cooperation of a quinone-based cofactor. As an example of oxidation with enzymes from our research group,20 we reported a selective bio-oxidation of amines to aldehydes or imines using laccase by Trametes vs. and TEMPO as mediator.21 Stable nitroxyl radical TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) plays a salient role as catalyst in metal-, organo- or bio-catalysed oxidation processes and significant progress in terms of catalytic efficiency and substrate applicability has been achieved,22 including oxidation of amines.23 Concerning the bio-oxidation of amines, Contente et al. reported an application of a flow-based biocatalysis in the oxidation of amines to aldehydes by an immobilized transaminase with sodium pyruvate as co-oxidant,24 and Zheng et al. reported the α-oxydation of cyclic amines to amides by whole-cell biotransformation.25
Using metal-free oxidants, Gaspa et al. reported a mild and solvent-free oxidation of primary amines to aldehydes, ketones, and nitriles by N-chlorosuccinimide under ballmilling conditions.26 Very recently Brisar et al. described the use of pyrazine cation for the aerobic oxidation of amines to imines.27 Concerning the use of inorganic metal-free oxidants, choline peroxydisulfate was successfully applied to a selective oxidation of secondary amines to hydroxylamines.28 De Souza et al. reported a selective synthesis of imines and amides by oxidative coupling of amines using NaOCl.29 Hypochlorite indeed is a widely used and cheap oxidant but its solutions liberate toxic gases such as chlorine when acidified or heated. Moreover, when chlorine-based oxidants are used in conjunction with organic compounds the formation of potentially harmful organo-chlorine compounds is often an inevitable side reaction (chloramines, dioxines, etc), thus favoring the development and use of chlorine-free oxidant systems.30
From this point of view, NaIO4 can be considered a promising oxidative agent, since it is a relatively cheap reagent, exploitable in water or aqueous solvents.31 Moreover, it is active at neutral pH and under mild conditions which is compatible with a wide range of functionalities and for this reason it has been extensively used in oxidation reactions for organic synthetic applications. Sodium periodate has also been often used in combination with other more expensive oxidants, in this case the use of periodates in stoichiometric amounts as primary oxidants allows the use of these expensive oxidants in catalytic amounts.31
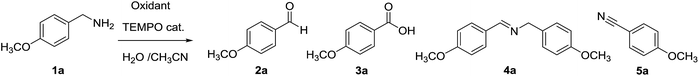
As an ongoing interest in sustainable oxidation methodologies, we now report on the use of the stable radical TEMPO as catalyst in combination with metal-free oxidation systems in aqueous medium to oxidize amines. We focused on the promising system NaIO4/TEMPO to selectively transform amines into the corresponding aldehydes and exploiting the reaction selectivity towards benzylic amines to develop a new protocol for removal of pMeO-benzylic group on secondary amines. Application of the oxidation system to cyclic amines in turn gave preferentially the unsaturated derivatives.
Results and discussion
Starting from our previous results with laccase Tv in the presence of TEMPO as catalyst, we initially investigated some inorganic oxidants for a selective oxidation of amines.21 To increase the sustainability of the process, we chose as reaction solvent an homogeneous aqueous-organic mixture able to dissolve both the starting amine and the oxidant, and mixtures of water and acetonitrile turned out to be suitable. We started our investigation using pOMe-benzylamine 1a as model substrate in H2O/CH3CN (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1) and some inorganic oxidants were screened such as NaClO, NaClO2/NaClO(cat), NaClO/NaBr (cat) following the Anelli-Montanari's protocol,32 Na2S2O8, and NaIO4. Data in Table 1 illustrate the effect of some parameters on the reaction efficiency and on the distribution of the oxidation products.
1) (Table 1) and some inorganic oxidants were screened such as NaClO, NaClO2/NaClO(cat), NaClO/NaBr (cat) following the Anelli-Montanari's protocol,32 Na2S2O8, and NaIO4. Data in Table 1 illustrate the effect of some parameters on the reaction efficiency and on the distribution of the oxidation products.
| Entry | Oxidant | TEMPO (mol%) | Additive | Time (h) | Conv. (%) | Productb (Y%) |
|---|---|---|---|---|---|---|
| a See GP1 in the Experimental section.b Isolated yields by solvent extraction after acid work-up (see Experimental section).c Solvent volume 6 mL. | ||||||
| 1 | NaClO2 (2 eq.)/NaClO (0.05 eq.) | 10 | 96 | 15 | 4a (12) | |
| 2 | NaClO2 (2 eq.)/NaClO (0.05 eq.) | 10 | AcOH, 1 eq. | 144 | 98 | 2a (10), 3a (40), 5a (50) |
| 3 | NaClO (1 eq) | 10 | AcOH, 1 eq. | 20 | >99 | 2a (23), 5a (71) |
| 4 | NaClO (1 eq.)/NaBr (0.15 eq.) | 2 | AcOH, 1 eq. | 72 | 44 | 2a (27) |
| 5 | NaClO (1 eq.)/NaBr (0.15 eq.) | 10 | AcOH, 1 eq. | 72 | 80 | 2a (73) |
| 6 | NaClO (1 eq.)/NaBr (0.15 eq.)c | 20 | AcOH, 1 eq. | 72 | 84 | 2a (77) |
| 7 | Na2S2O8 (1 eq.) | — | 48 | 50 | 2a (33) | |
| 8 | Na2S2O8 (1 eq.) | 10 | AcOH, 1 eq. | 72 | 76 | 2a (70) |
| 9 | NaIO4 (1 eq.) | — | 24 | 63 | 2a (42), 4a (16) | |
| 10 | NaIO4 (1 eq.) | 2 | 72 | 75 | 2a (45), 4a (49) | |
| 11 | NaIO4 (1 eq.) | 10 | 24 | 86 | 2a (73) | |
| 12 | NaIO4 (1 eq.) | — | AcOH, 1 eq. | 20 | 13 | 2a (12) |
| 13 | NaIO4 (1 eq.) | 10 | AcOH, 1 eq. | 20 | >99 | 2a (92) |
The aqueous reaction medium brought an initial drawback deriving from a basic pH resulting on dissolution of amine 1a in the aqueous-organic solvent mixture (observed pH = 12). This basicity deactivated in some cases the oxidation system, thus giving low conversions and yields (entries 1, 7, and 9, Table 1). To overcome this problem, the addition of one equivalent of acetic acid was successful in decreasing the initial pH to 7 and afforded a substantial improvement on both conversions and yields (entries 2, 3, 5, and 8, Table 1). The radical TEMPO showed to be an effective organocatalyst in promoting the reaction with almost all the oxidants with an optimized amount of 10 mol% to increase both yields and selectivity (Table 1, for NaClO–NaBr see entries 4–6, for Na2S2O8 see entries 7–8, for NaIO4 see entries 12 and 13). Concerning the effect of the oxidation system on products selectivity, NaClO2/NaClO or NaClO alone are efficient but poorly selective, affording almost complete conversions but a mixture of products: aldehyde/acid/nitrile with NaClO2/NaClO, and an aldehyde/nitrile 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture with NaClO (Table 1, entries 2 and 3). NaClO–NaBr and Na2S2O8 are very selective oxidants with an exclusive formation of the aldehyde 2a but do not reach complete conversions. NaIO4 alone or with TEMPO 2 mol% gave mixtures of aldehyde and imine (Table 1 entries 9 and 10), but on increasing the amount of TEMPO to 10% it yielded the aldehyde only. Finally, NaIO4 in the presence of 1 equiv. of acetic acid and 10% TEMPO gave complete conversion with an excellent isolated yield of 2a (Table 1, entry 13). Thus, from the initial screening, the system NaIO4/TEMPO/AcOH emerged as the most efficient and selective method to oxidize the model amine 1a to the aldehyde 2a. 1H NMR spectra representing products distribution in crude mixtures of selected entries of Table 1 are reported in Fig. 1S in ESI.†
3 mixture with NaClO (Table 1, entries 2 and 3). NaClO–NaBr and Na2S2O8 are very selective oxidants with an exclusive formation of the aldehyde 2a but do not reach complete conversions. NaIO4 alone or with TEMPO 2 mol% gave mixtures of aldehyde and imine (Table 1 entries 9 and 10), but on increasing the amount of TEMPO to 10% it yielded the aldehyde only. Finally, NaIO4 in the presence of 1 equiv. of acetic acid and 10% TEMPO gave complete conversion with an excellent isolated yield of 2a (Table 1, entry 13). Thus, from the initial screening, the system NaIO4/TEMPO/AcOH emerged as the most efficient and selective method to oxidize the model amine 1a to the aldehyde 2a. 1H NMR spectra representing products distribution in crude mixtures of selected entries of Table 1 are reported in Fig. 1S in ESI.†
We next examined the influence of solvent mixtures and the amount of TEMPO with NaIO4 on conversions and yields (Table 2). The mixture H2O/CH3CN 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gave the best result, but on shortening the reaction time, the efficiency decreased (Table 1, entries 1 and 2).
1 gave the best result, but on shortening the reaction time, the efficiency decreased (Table 1, entries 1 and 2).
| Entry | H2O/CH3CN, | Vol. (mL) | Time (h) | Conv. (%) | Yb (%) |
|---|---|---|---|---|---|
| a See GP1 in the experimental section.b Isolated yields of 2a as single compound by solvent extraction after acid work-up (see Experimental section).c Heterogeneous solution because of insolubility of periodate. | |||||
| 1 | 2/1 | 15 | 20 | >99 | 92 |
| 2 | 2/1 | 15 | 6 | 44 | 43 |
| 3 | 2/1 | 10 | 6 | 96 | 86 |
| 4 | 2/1 | 5 | 6 | 95 | 92c |
| 5 | 1/1 | 10 | 6 | 86 | 86 |
| 6 | 1/2 | 10 | 6 | 60 | 58 |
| 7 | CH3CN | 10 | 6 | 27 | 25c |
| 8 | H2O | 10 | 6 | 34 | 10 |
On increasing the concentration of 1a by diminishing the total solvent volume, the system recovered efficiency with good conversions and isolated yields in a shorter reaction time (Table 2, entries 3 and 4). An increase of the relative amount of CH3CN in H2O was not efficient (Table 2, entries 5 and 6) and worse results were obtained either in pure CH3CN because of the insolubility of NaIO4, or in H2O alone because of the insolubility of the starting amine 1a (Table 2 entries 7 and 8).
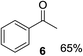
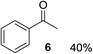
With the optimized conditions in hand, the oxidation protocol with NaIO4/TEMPO/AcOH was then applied to a series of commercial aldehydes to test the substrate scope (Table 3). Benzylamines 1a–f with different substituents on the aromatic ring were selectively and efficiently oxidized to the corresponding benzaldehydes 2a–f; both electron donating and electron-withdrawing substituents on the phenyl ring are well tolerated, giving access to the corresponding aldehydes in good to excellent isolated yields. 1-Phenyl ethylamine 1i gave acetophenone 6 as expected, whereas its structural isomer 2-phenylethylamine 1k afforded a mixture of products: 2-phenylacetaldehyde 2k isolated in low yields (10%), benzaldehyde 2b as the main product (40%) derived from an oxidative C–C cleavage of 2k,33 and traces of benzoic acid as over-oxidation of benzaldehyde. 2-Phenylpropylamine 1j yielded acetophenone 6 as the only product. Compound 6 probably derived from an initial oxidation of 1j to 2-phenyl-propanal which underwent an oxidative C–C cleavage to 6 as previously observed for phenylpropionic aldehydes.34 Tryptamine 1l provided only polymerized products, and we could not obtain a successful oxidation for any tested cyclic or linear aliphatic amine (Table 3, entries 13–16) thus revealing a strong selectivity towards the oxidation of benzylic moiety.
| Entry | Starting amine | Yb (%) | Entry | Starting amine | Yb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: see GP2 in the Experimental section.b Isolated yields by solvent extraction after acid work-up.c Solvent volume 10 mL.d An equivalent of trifluoroacetic acid (TFA) was added in the basic organic extract (see Experimental section). | |||||
| 1 | 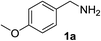 |
92 | 9 | 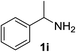 |
 |
| 2 | 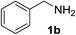 |
85 | 10c | 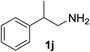 |
 |
| 3 | 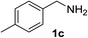 |
90 | 11 | 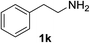 |
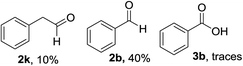 |
| 4 | 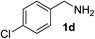 |
90 | 12 | 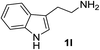 |
Polymerized products |
| 5 | 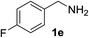 |
93 | 13d | 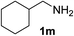 |
— |
| 6 | 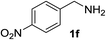 |
97 | 14d | 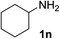 |
— |
| 7 | 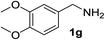 |
75 | 15d | 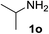 |
— |
| 8 | 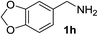 |
81 | 16d | 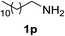 |
Traces |
We then evaluated a series of N-benzyl secondary amines 7–12, which were prepared via reduction of the corresponding imines or via a one-step reductive amination starting from the corresponding aldehydes and amines (see ESI†) (Table 4).
| Entry | Starting amine | NaIO4 (eq.) | R–NH2 (Y%) | 2ab (Y%) | Entry | Starting amine | NaIO4 (eq.) | R–NH2 (Y%) | 2ab (Y%) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: see GP3 in the Experimental section.b Isolated as single compound by solvent extraction after acid work-up (see Experimental section).c Amines were isolated as ammonium salts in the basic organic extract by adding an equivalent of trifluoroacetic acid (TFA).d 20% of TFA salt of 1a was obtained. | |||||||||
| 1 | 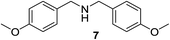 |
1 | Traces | 39 | 6 | 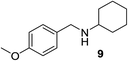 |
1.5 | 92c | 98 |
| 2 | 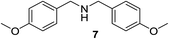 |
2.2 | 8 | 80 | 7 | 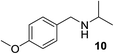 |
1 | 51c | 46 |
| 3 | 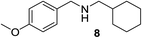 |
1 | 60c,d | 75 | 8 | 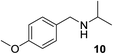 |
2 | 59c | 69 |
| 4 | 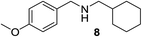 |
2 | 43c | 85 | 9 | 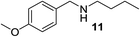 |
1.5 | 15c | 22 |
| 5 | 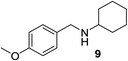 |
1 | 88c | 95 | 10 | 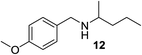 |
1.5 | 80c | 92 |
Oxidation of symmetrical bis-p-methoxy-benzylamine 7 exclusively gave the aldehyde 2a in good yields with 2.2 equiv. of periodate (Table 4, entry 2). In the presence of 1.1 equiv. of TEMPO (Table 4, entry 1) only traces of amine 1a, intermediate of the first oxidation step, were obtained. This could be due to a slower reactivity of the secondary amine 7 than the primary amine intermediate 1a, similarly to the oxidation with TEMPO of secondary alcohols compared to the primary ones.35 Unsymmetrical amines 8–12 were selectively oxidized only on the benzyl moiety, consequently yielding pOMe-benzaldehyde 2a and the aliphatic amine that were easily separated via liquid–liquid acid–base extraction (see Experimental section) as pure products in satisfactory to excellent yields. As a general observation, the yield of aldehyde 2a was in all cases increased by doubling the equivalents of sodium periodate (Table 4, entries 1–8). The developed protocol could be then used to obtain selective deprotection of benzylic groups on secondary amines.
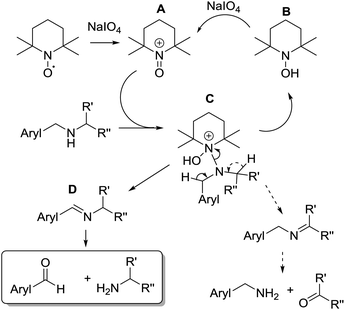
A tentative analysis of TEMPO-mediated oxidation pathway could account for the observed selectivity. TEMPO is a stable nitroxide radical which undergoes a one-electron oxidation to the active oxidizing agent, the oxammonium cation A (Scheme 1). The oxidation process provides the reduced form as the neutral hydroxylamine B. Nitroxide radicals can be used in a catalytic amount in the presence of a terminal oxidant and it has been already demonstrated the ability of sodium periodate to behave as terminal oxidant in alcohol oxidations with TEMPO as catalyst.36 Considering the mechanism proposed for alcohol oxidation by oxammonium cation,37 a tentative mechanism for the selective oxidation of secondary amines in aqueous medium could be formulated.
The reaction is initiated by the attack of the amine on A leading to the complex C, beta-hydrogen elimination then produces two possible imines as intermediates and the hydroxylamine B which is re-oxidized to oxammonium A by periodate. The H elimination selectively addressed the benzylic hydrogen rather than the aliphatic one probably on account of a lower C–H bond dissociation energy of the benzylic position.37,38 In the aqueous medium the aryl-conjugated imine D undergoes hydrolysis to give the target aryl-aldehyde and amine.
Finally, three aromatic bicyclic amines 1q–s were evaluated. Amine 1q underwent oxidative aromatization and quantitatively gave indole, as well as 1r provided quinoline 14 in 74% isolated yield with 2 equiv. of sodium periodate and 1 equiv. of acetic acid.
1,2,3,4-Tetrahydroisoquinoline 1s with 1 equiv. of periodate gave the imine 15 as the main product (78% yield) and small amounts of the amide 16 (Table 5 entry 4), but on enhancing the equivalents of periodate increased amounts of the amide 16 and the formation of the isoquinoline 17 were detected (Table 5 entries 5 and 6). 1H NMR spectra representing products distribution in crude mixture of entry 6 is reported in Fig. 3S in ESI.† Amide 16 could derive from an initial H2O addition to imine 15 and further oxidation of the intermediate aminol to amide, as recently reported in α-oxygenation of amines to amides catalyzed by gold nanoparticles in H2O.39
| En. | Amine | NaIO4 (eq.) | AcOH (eq.) | Product, Yb (%) |
|---|---|---|---|---|
a Reaction conditions: see GP4 in the Experimental section.b Isolated yields by solvent extraction after acid and basic work-up (see Experimental section); the yield ratios of products 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 were determined via 1H NMR analysis. 17 were determined via 1H NMR analysis. |
||||
| 1 | 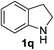 |
1 | — | 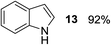 |
| 2 | 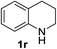 |
1 | — | 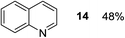 |
| 3 | 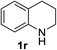 |
2 | 1 | 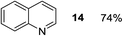 |
| 4 | 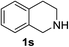 |
1 | 1 | 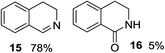 |
| 5 | 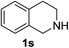 |
2 | 1 | 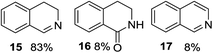 |
| 6 | 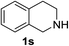 |
3 | 1 |  |
Conclusions
Selectivity and sustainable reaction conditions are key issues for the development of new eco-friendly methodologies for amine oxidation. Indeed, selectivity is often a critical point because of the large number of possible products and many systems for amine oxidation still need metal-containing reagents and produce toxic or hazardous wastes. According to this, we investigated some inorganic metal-free oxidants for oxidation of amines. NaIO4/TEMPO turned out to be the most efficient and selective system for benzylamines oxidation to benzaldehydes in aqueous–organic medium. If combined with 1 equiv. of acetic acid, this system provided complete conversions of substituted benzylamines to the corresponding benzaldehydes that were efficiently isolated in good to excellent yields. Oxidation of secondary amines gave selective oxidation on the benzylic portion thus realizing an oxidative deprotection of the N-benzyl group with an easy amine recovery. A mechanism considering the selectivity for secondary amines oxidation was formulated. Finally, application of the optimized oxidative method to some cyclic amines such as dihydroindole, tetrahydroquinoline and tetrahydroisoquinoline confirmed its efficiency mainly providing the corresponding aromatic derivatives.Experimental
General methods
Commercial reagents (reagent grade, >99%) were used as received without additional purification. Anhydrous solvents (CH2Cl2, MeOH, CH3CN) were obtained commercially. 1H and 13C NMR spectra were recorded with an INOVA 400 instrument with a 5 mm probe. All chemical shifts are quoted relative to deuterated solvent signals (δ in ppm and J in Hz). The purities of the target compounds were assessed as being >95% by HPLC-MS analysis. FTIR spectra: Brucker Alpha instrument, measured as films between NaCl plates; wave numbers are reported in cm−1. HPLC-MS: Agilent Technologies HP1100 instrument, equipped with a ZOBRAX-Eclipse XDB-C8 Agilent Technologies column; mobile phase: H2O/CH3CN, 0.4 mL min−1, gradient from 30 to 80% of CH3CN in 8 min, 80% of CH3CN until 25 min, coupled with an Agilent Technologies MSD1100 single-quadrupole mass spectrometer, full scan mode from m/z = 50 to 2600, in positive ion mode. GC-MS: Hewlett-Packard 5971 spectrometer with GC injection and EI ionization at 70 eV coupled with an Agilent Technologies MSD1100 single-quadrupole mass spectrometer, reported as: m/z (rel. intensity).Starting amines 1a–1t are commercially available; synthetized imines 4a, 4n, 4o, 4t and secondary amines 7–11 are known. The obtained oxidation products: aldehydes 2a–2k, acids 3a–3b, nitrile 5a, compounds 6, 13–17 are known. Structures and purities of all the obtained known compounds were assessed by 1H NMR and HPLC-MS analysis or by 1H NMR and GC-MS analysis for compounds 13–17 and were fully consistent with data reported in databases. Imines 4a, 4m, 4n, 4o, 4t and secondary amines 7–12 synthesis are reported in ESI.† 1H NMR and 13C NMR of new compounds 4m and 12 is reported in ESI† together with 1H NMR representative crude reaction mixtures (Fig. 1S and 2S†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of H2O/CH3CN (15 mL) (see Table 3). After 20 h the mixture was quenched with HCl 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The collected organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the corresponding aldehydes 2a–k or compounds 6 and 3b where specified. The aqueous phase was then basified with NaOH 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the residual starting amines 1a–1l, if present. The residual aliphatic amines 1m–1p were isolated as ammonium salts in the basic organic extract by adding one equivalent of trifluoroacetic acid (TFA) just before the final solvent evaporation.
1 ratio of H2O/CH3CN (15 mL) (see Table 3). After 20 h the mixture was quenched with HCl 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The collected organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the corresponding aldehydes 2a–k or compounds 6 and 3b where specified. The aqueous phase was then basified with NaOH 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the residual starting amines 1a–1l, if present. The residual aliphatic amines 1m–1p were isolated as ammonium salts in the basic organic extract by adding one equivalent of trifluoroacetic acid (TFA) just before the final solvent evaporation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of H2O/CH3CN (10 mL) (see Table 4). After 20 h the mixture was quenched with HCl 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The collected organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate p-anisaldehyde 2a. The aqueous phase was then basified with NaOH 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate amines RNH2 (1a, 1j, 1m, 1n, 1o, and 1t, see Table 4). When specified, amines were isolated as ammonium salts in the basic organic extract by adding one equivalent of trifluoroacetic acid (TFA) just before the final solvent evaporation.
1 ratio of H2O/CH3CN (10 mL) (see Table 4). After 20 h the mixture was quenched with HCl 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The collected organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate p-anisaldehyde 2a. The aqueous phase was then basified with NaOH 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate amines RNH2 (1a, 1j, 1m, 1n, 1o, and 1t, see Table 4). When specified, amines were isolated as ammonium salts in the basic organic extract by adding one equivalent of trifluoroacetic acid (TFA) just before the final solvent evaporation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of H2O/CH3CN (10 mL). After 20 h the mixture was quenched with HCl 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The collected organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the oxidation products 13, 15 (in its protonated form) or 16 (see Table 5). The aqueous phase was then basified with NaOH 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the starting amines 1q–1s if present, and the oxidation products 14, 15 and 17 (see Table 5).
1 ratio of H2O/CH3CN (10 mL). After 20 h the mixture was quenched with HCl 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The collected organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the oxidation products 13, 15 (in its protonated form) or 16 (see Table 5). The aqueous phase was then basified with NaOH 1 M and extracted with EtOAc or Et2O (3 × 10 mL). The organic layers were dried on anhydrous Na2SO4, filtered and concentrated to isolate the starting amines 1q–1s if present, and the oxidation products 14, 15 and 17 (see Table 5).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by University of Bologna research funding RFO 2016.Notes and references
-
(a) J. H. Teles, I. Hermans, G. Franz and R. A. Sheldon, Oxidation, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2015, DOI:10.1002/14356007.a18_261.pub
; (b) F. Cavani and J. H. Teles, ChemSusChem, 2009, 2, 508–534 CrossRef PubMed
.
- S. S. Rawalay and H. Shechter, J. Org. Chem., 1967, 32, 3129–3131 CrossRef CAS
.
- R. G. R. Bacon and W. J. W. Hanna, J. Chem. Soc., 1965, 4962–4968 RSC
.
- S. Sobhani and M. F. Maleki, Synlett, 2010, 383–386 CrossRef CAS
.
- S. Sobhani, S. Aryanejad and M. F. Maleki, Helv. Chim. Acta, 2012, 95, 613–617 CrossRef CAS
.
- S. Chandrasekhar, G. P. K. Reddy, C. Nagesh and C. R. Reddy, Tetrahedron Lett., 2007, 48, 1269–1271 CrossRef CAS
.
-
(a) J. Srogl and S. Voltrova, Org. Lett., 2009, 11, 843–845 CrossRef CAS PubMed
; (b) S. Naya, T. Niwa, R. Negishi, H. Kobayashi and H. Tada, Angew. Chem., Int. Ed., 2014, 53, 13894–13897 CrossRef CAS PubMed
.
- N. Iqbal and E. J. Cho, Adv. Synth. Catal., 2015, 357, 2187–2192 CrossRef CAS
.
-
(a) R. Tang, S. E. Diamond, N. Neary and F. Mares, J. Chem. Soc., Chem. Commun., 1978, 13, 562 RSC
; (b) A. J. Bailey and B. R. James, Chem. Commun., 1996, 20, 2343–2344 RSC
; (c) K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2003, 42, 1480–1483 CrossRef CAS PubMed
; (d) L. Aschwanden, T. Mallat, F. Krumeich and A. Baiker, J. Mol. Catal. A: Chem., 2009, 309, 57–62 CrossRef CAS
; (e) M. H. So, Y. Liu, C. M. Ho and C. M. Che, Chem.–Asian J., 2009, 4, 1551–1561 CrossRef CAS PubMed
; (f) M. Largeron, Eur. J. Org. Chem., 2013, 5225–5235 CrossRef CAS
; (g) H. Yu, Y. Zhai, G. Dai, S. Ru, S. Han and Y. Wei, Chem.–Eur. J., 2017, 23, 13883–13887 CrossRef CAS PubMed
.
- F. Su, S. C. Mathew, L. Mohlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657–660 CrossRef CAS PubMed
.
- W. Zhao, C. Liu, L. Cao, X. Yin, H. Xu and B. Zhang, RSC Adv., 2013, 3, 22944–22948 RSC
.
- S. Sarina, H. Zhu, E. Jaatinen, Q. Xiao, H. Liu, J. Jia, C. Chen and J. Zhao, J. Am. Chem. Soc., 2013, 13, 5793–5801 CrossRef PubMed
.
- Z. J. Wang, S. Ghasimi, K. Landfesterand and K. A. I. Zhang, Adv. Mater., 2015, 27, 6265–6270 CrossRef CAS PubMed
.
- J. H. Ko, N. Kang, N. Park, H.-W. Shin, S. Kang, S. M. Lee, H. J. Kim, T. K. Ahn and S. U. Son, ACS Macro Lett., 2015, 4, 669–672 CrossRef CAS
.
- S. Naya, K. Kimura and H. Tada, ACS Catal., 2013, 3, 10–13 CrossRef CAS
.
- S. Naya, T. Niwa, R. Negishi, H. Kobayashi and H. Tada, Angew. Chem., 2014, 126, 14114–14117 CrossRef
.
- H. Chen, C. Liu, M. Wang, C. Zhang, N. Luo, Y. Wang, H. Abroshan, G. Li and F. Wang, ACS Catal., 2017, 7, 3632–3638 CrossRef CAS
.
- D. Lenoir, Angew. Chem., Int. Ed., 2006, 45, 3206–3210 CrossRef CAS PubMed
.
- M. Largeron, Org. Biomol. Chem., 2017, 15, 4722–4730 CAS
.
- P. Galletti, M. Pori, F. Funiciello, R. Soldati, A. Ballardini and D. Giacomini, ChemSusChem, 2014, 7, 2684–2689 CrossRef CAS PubMed
.
- P. Galletti, F. Funiciello, R. Soldati and D. Giacomini, Adv. Synth. Catal., 2015, 357, 1840–1848 CrossRef CAS
.
-
(a) R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2010, 14, 245–251 CrossRef CAS
and references cited therein; (b) R. A. Sheldon and I. W. C. E. Arends, Adv. Synth. Catal., 2004, 346, 1051–1071 CrossRef CAS
.
- A. L. Bartelson, K. M. Lambert, J. M. Bobbitt and W. F. Bailey, ChemCatChem, 2016, 8, 3421–3430 CrossRef CAS
.
- M. L. Contente, F. Dall'Oglio, L. Tamborini, F. Molinari and F. Paradisi, ChemCatChem, 2017, 9, 3843–3848 CrossRef CAS
.
- D. Zheng, X. Zhou, B. Cui, W. Han, N. Wan and Y. Chen, ChemCatChem, 2017, 9, 937–940 CrossRef CAS
.
- S. Gaspa, A. Porcheddu, A. Valentoni, S. Garroni, S. Enzo and L. De Luca, Eur. J. Org. Chem., 2017, 5519–5526 CrossRef CAS
.
- R. Brisar, D. Hollmann and E. Mejia, Eur. J. Org. Chem., 2017, 5391–5398 CrossRef CAS
.
- A. Bana, H. Valizadeh, A. Heydari and A. Moghlml, Synlett, 2017, 28, 2315–2319 CrossRef
.
- G. F. P. de Souza, T. W. von Zuben and A. G. Salles Jr., ACS Sustainable Chem. Eng., 2017, 5, 8439–8446 CrossRef CAS
.
- T. J. Collins, Acc. Chem. Res., 2002, 35, 782–790 CrossRef CAS PubMed
.
-
(a) A. Sudalai, A. Khenkin and R. Neumann, Org. Biomol. Chem., 2015, 13, 4374–4394 RSC
; (b) A. Lauterbach, G. Ober, Iodine and iodine compounds, in Kirk Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Hoboken, NJ, 5th edn, 2005, vol. 14, pp. 353–380, DOI:10.1002/0471238961.0915040912012120.a01.pub2
.
- P. L. Anelli, C. Biffi, F. Montanari and S. Quici, J. Org. Chem., 1987, 52, 2559–2562 CrossRef
.
-
(a) Q. Xing, H. Lv, C. Xia and F. Li, Chem. Commun., 2016, 52, 489–492 RSC
; (b) S.-J. Jin, P. K. Arora and L. M. Sayre, J. Org. Chem., 1990, 55, 3011–3018 CrossRef CAS
.
- P. Galletti, M. Pori and D. Giacomini, Synlett, 2010, 17, 2644–2648 Search PubMed
.
- M. F. Semmelhack, C. S. Chou and D. A. Cortes, J. Am. Chem. Soc., 1983, 105, 4492–4494 CrossRef CAS
.
- M. Lei, R.-J. Hu and Y.-G. Wang, Tetrahedron, 2006, 62, 8928–8932 CrossRef CAS
.
-
(a) W. F. Bailey, J. M. Bobbitt and K. B. Wiberg, J. Org. Chem., 2007, 72, 4504–4509 CrossRef CAS PubMed
; (b) F. d'Acunzo, P. Baiocco, M. Fabbrini, C. Galli and P. Gentili, Eur. J. Org. Chem., 2002, 4195–4201 CrossRef
.
- Y.-R. Luo and J.-P. Cheng, Bond Dissociation Energies in Polyatomic Molecules in Handbook of Chemistry and Physics, CRC press, 98th edn, 2017–2018 Search PubMed.
- X. Jin, K. Kataoka, T. Yatabe, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2016, 55, 7212–7217 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures for the synthesis of the secondary amines 7–11 via imines 4a, 4m, 4n, 4o, and 4t, and NMR spectra of compounds. See DOI: 10.1039/c8ra01365a |
| This journal is © The Royal Society of Chemistry 2018 |