Reversible sol–gel–sol medium for enzymatic optical biosensors†
Sofia M.
Safaryan
a,
Aleksandr V.
Yakovlev
 a,
Evgeny A.
Pidko
a,
Evgeny A.
Pidko
 ab,
Alexandr V.
Vinogradov
a and
Vladimir V.
Vinogradov
*a
ab,
Alexandr V.
Vinogradov
a and
Vladimir V.
Vinogradov
*a
aLaboratory of Solution Chemistry of Advanced Materials and Technologies, ITMO University, St. Petersburg 197101, Russian Federation. E-mail: vinogradov@scamt.ru
bInorganic Materials Chemistry group, Eindhoven University of Technology, Eindhoven, The Netherlands
First published on 10th November 2016
Abstract
In this paper we for the first time report a reversible sol–gel–sol approach to obtain optical enzymatic biosensors with improved enzyme stability and good sensitivity by using desktop inkjet printing. The developed technique is based on the bio-inorganic inks allowing for a sol–gel–sol transition of the inorganic matrix: from liquid ink to a solid alumina matrix with entrapped enzymes and a subsequent color response due to the enzymatic reaction upon the resuspension of the matrix. This approach improves the stability of the enzymes entrapped in the porous inorganic matrix, and at the same time maintains a high sensitivity of the biomolecules, whose facile release is ensured by the gel–sol transition. Rheological parameters of the developed bio-inorganic ink are highly adjustable making it suitable for the deposition on different surfaces by inkjet printing. The potential and utility of this approach is demonstrated by a successful production of optical biosensors for glucose and uric acid.
1 Introduction
Modern medicine relies heavily on the availability of robust and efficient methods for biochemical analysis of bodily fluids for diagnostic and therapeutic purposes. Among various available options diagnostic tools based on the spectrophotometry and enzymatic reactions are among the most popular and widely used because of their high specificity and sensitivity.1 The combination of these two approaches led to the development of enzymatic biosensors, which have been the subject of a number of recent studies.2,3 Modern biosensors may be differentiated according to the underlying working principles based on optical4 and electrochemical5,6 effects. However, the practical applications of such biochemical devices are limited due to the poor stability commonly exhibited by most enzyme mixtures when exposed to an alien environment. Conventionally, chemical stabilizers are introduced to the biological system to protect the enzyme and enhance its stability.7 However the general applicability of such an approach is hampered by the intrinsic activity of the chemical stabilizers and the complex mechanism of their interaction with the enzyme, which may affect the properties and sensitivity of the biosensors.8One of the most recent and very promising approaches for enzyme stabilization is their confinement in sol–gel inorganic matrices,9 for example, silica-based matrices. The choice of silica as a popular confining material for the biomolecules is mainly related to its bio-inertness,10 well-established sol–gel chemistry11 and the ability to transform into rigid solid and porous matrices necessary to ensure the appropriate characteristics of the final composite biosensor device.12,13
The sol–gel stabilization techniques are particularly attractive in view of the possibility of their utilization for inkjet printing, which opens up an avenue towards a facile and efficient approach for the production of cheap and robust biosensors.14,15 This emerging technology is heavily dependent on the availability of suitable bio-compatible sol–gel matrices with appropriate printing characteristics.
In our previous studies16,17 we developed an alumina sol–gel matrix based on boehmite nanorods as a superior carrier for enzyme immobilization. We demonstrated the possibility of the highly efficient stabilization of industrial and therapeutical enzymes by entrapment within alumina sol–gel derived matrices. By carrying out a detailed kinetic study complemented by the differential scanning colorimetry and circular dichroism analyses we showed that the encapsulation in the alumina matrices allows retaining the native structures of the proteins and prevents their unfolding upon exposure to elevated temperatures as high as 200 °C. At the same time, recent studies reported by other groups18 demonstrate the high-potential of alumina-based sol–gel inks for an economic and versatile inkjet printing technology allowing generating inorganic nanostructures on various substrates. Temperature stability and cost effectiveness are among the most crucial factors determining the ability of a technology to reach the consumer market and create an opportunity for the express tests under extreme climate conditions. For example, in many African regions it is almost impossible to ensure a strict control for the storage temperatures of biosensors. Yet, these regions are in an urgent need for quick and practical test methods to various diseases including, for example, diabetes.19
The application of silica-based bio-composites for the development of such biosensors is limited due to the fast gelation of the inorganic precursors and the strong impact of the inorganic phase on the protein structures.20 The entrapment in the silica matrix may lead to reduced enzymatic activity due to the complete encapsulation of the reactive moieties21 resulting in the reduced sensitivity of the biosensor. Sensitivity is an important parameter of noninvasive biosensors as it determines the possibility of fast, easy and reliable analysis of bodily fluids for particular diseases. The nature of the target bodily fluids effectively determines the required sensitivity of the biosensor. Although saliva is a very informative substance as it contains a wide set of important biomarkers22 the low concentration of these markers in saliva compared to that in blood raises dramatically the sensitivity levels required for the associated biosensors. Furthermore, when considered as a basis for inkjet technologies, silica-based sols present an important technical risk of the nozzle blockage due to the irreversible gelation23 and, related, they are not suitable for the long-term storage and re-suspension due to the formation of strong covalent structures within silica NPs.
We propose that by using non-silica crystalline matrices for enzyme encapsulation, an improved thermal stability and a high biocatalytic activity can be achieved along with the possibility of utilizing such biocomposites in inkjet printing of the actual biosensor devices. For example, because of the non-covalent bonding within Yoldas's sols24,25 they can be easily resuspended to prolong their long-term applicability and they are suitable to very simple desktop inkjet nanofabrication.26,27 The possibility of resuspending of these sols renders them unique inorganic matrices for the enzyme entrapment and detection of large target molecules. In the presence of a solvent (water or bodily fluids) these types of matrices can almost instantly release enzymes in their native conformation and bioactivity by resuspending of the gel matrix.
In view of these considerations, we aimed at the development of a robust technology for the production of optical biosensors based on the sol–gel–sol transition approach in the desktop inkjet printing. The practical applicability of this new method is demonstrated by producing working optical biosensors to uric acid and glucose.
2 Materials and methods
2.1 Reagents
The glucose oxidase (15000 U L−1)/peroxidase (1000 U L−1)/4-aminophenazone (2.6 mmol L−1) reagent (Trinder. GOD-POD), the glucose aqueous primary standard (100 mg dL−1), the uricase (60 U L−1)/peroxidase (660 U L−1)/4-aminophenazone (1 mmol L−1) reagent, the uric acid aqueous primary standard (6 mg dL−1), Tris buffer pH 4.8, containing 0.3 mmol L−1 of phenol and phosphate buffer pH 7.4, containing 2,4-dichlorophenol sulfonate (DCPS) 4 mmol L−1 were purchased from Spinreact (Girona, Spain). Aluminum isopropoxide (≤98%) was purchased from Sigma-Aldrich. Nitric acid (for analysis, ca. 65% solution in water) and NaOH (0.1 N) standard solution were obtained from Acros Organics.2.2 Synthesis of alumina sol
The boehmite sols were prepared using modified Yoldas24 procedures by peptizing an aluminum hydroxide precipitate with nitric acid. In a typical experiment, 8.2 g of Al(C3H7O)3 was added to 50 mL deionized water and a white precipitate was formed immediately. The precipitate was peptized with 0.65 mL concentrated nitric acid at 80 °C under vigorous stirring for 1 h to produce a stable boehmite sol. Then the sol was kept at 90 °C for 12 hours for drying. The resulting xerogel (3.3 g) was resuspended in 50 mL deionized water at 80 °C under vigorous stirring until complete dissolution. Sodium hydroxide (0.1 N) was used to adjust pH of the sol to 4.8.2.3 Preparation of enzyme@alumina mixtures
For the preparation of biocomposites, the solutions of enzymes with dye were produced from a dry mixture of the components: glucose oxidase GOD (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U L−1)/peroxidase POD (1000 U L−1)/4-aminophenazone (2.6 mmol L−1) for glucose biosensors and uricase UOD (60 U L−1)/peroxidase POD (660 U L−1)/4-aminophenazone (1 mmol L−1) for uric acid biosensors. In the case of a glucose biosensor 0.022 g dry mixture of enzymes was dissolved in 10 mL Tris buffer at a pH of 4.8, containing 0.3 mmol L−1 phenol. In the case of a uric acid biosensor 0.022 g dry mixture of enzymes was dissolved in 10 mL phosphate buffer at a pH 7.4, containing 4 mmol L−1 2,4-dichlorophenol sulfonate (DCPS). For both materials, the sol and enzyme solutions were then mixed in a ratio of 1
000 U L−1)/peroxidase POD (1000 U L−1)/4-aminophenazone (2.6 mmol L−1) for glucose biosensors and uricase UOD (60 U L−1)/peroxidase POD (660 U L−1)/4-aminophenazone (1 mmol L−1) for uric acid biosensors. In the case of a glucose biosensor 0.022 g dry mixture of enzymes was dissolved in 10 mL Tris buffer at a pH of 4.8, containing 0.3 mmol L−1 phenol. In the case of a uric acid biosensor 0.022 g dry mixture of enzymes was dissolved in 10 mL phosphate buffer at a pH 7.4, containing 4 mmol L−1 2,4-dichlorophenol sulfonate (DCPS). For both materials, the sol and enzyme solutions were then mixed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, so that the concentration of the enzymes in the enzyme–alumina biocomposite was equal to 3 wt% (for the dry substance).
1, so that the concentration of the enzymes in the enzyme–alumina biocomposite was equal to 3 wt% (for the dry substance).
2.4 Enzymatic activity and determination of thermal stability
To determine the enzymatic activity of the free and the entrapped enzymes, 0.1 mL of the free enzyme solution and 0.2 mL of the enzymes-alumina bio-composite, respectively, were transferred to a cuvette and then left in a vacuum desiccator at room temperature for 24 hours. The relative enzymatic activity was measured by using a spectrophotometer (Agilent Cary 8454 UV-Vis). In a typical experiment, 2.0 mL aqueous reducing substrate solution (glucose with concentration of 100 mg dL−1 and uric acid with concentration of 6 mg dL−1) were added to the cuvette with prepared enzymes and the biocatalytic activity was monitored by following the increase in absorbance at 505 nm due to the oxidized quinone. The associated chemical transformations are depicted in the ESI† (eqn (S1)–(S4)).2.5 Preparation of inks for printing
Inks were prepared with the same composition as described before but implied using sols with various nanoparticle concentrations. For this purpose, we first produced a sol using modified Yoldas procedures by peptizing an alumina precipitate with nitric acid as described above and keeping it in an oven for 12 hours at 90 °C. The resulting dry xerogel was resuspended in 50 mL deionized water. The weight of the resuspended xerogel was 1, 2, 3.25, and 4.5 g to achieve sols with concentrations of 2, 4, 6.5, and 9%, respectively. Then, a sodium hydroxide solution (0.1 N) was used to adjust the pH of the sol to the value of 4.8.2.6 Preparation of bio-inks
3.25 g xerogel prepared via the modified Yoldas procedure was resuspended in 50 mL of deionized water followed by the adjustment of pH to the value of 4.8 using a 0.1 NaOH solution and subsequent cooling of the sol to 4 °C. The cooled sols under constant stirring were mixed with the appropriate enzyme solutions to produce glucose and uric acid biosensors with the weight ratio of alumina NPs to enzymes kept at 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in both cases.
3 in both cases.
2.7 Printing of biosensors
To produce biosensors, we used a Canon Pixma IP 2840 inkjet desktop printer with standard PG-445 ink cartridges. The interior of the cartridges was removed and thoroughly washed with deionized water. The cartridge was then filled with the prepared bio-ink. Glass slides, filter paper, and glossy photo paper Lomond 230 g cm−2 were used as substrates.2.8 Enzymatic activity of printed biosensors
As a result of printing, we have prepared two types of biosensors for the detection of glucose and uric acid. To check the enzymatic activity after printing, we have prepared solutions with varying concentrations of the substrates. After printing, 10 mL of the substrate were deposited at the sensitive area of the biosensor and after 2 minutes the absorbance of this zone was analyzed using the Agilent Cary 60 UV-Vis spectrophotometer by a Video Barrelino measuring diffuse reflectance console.3 Results and discussion
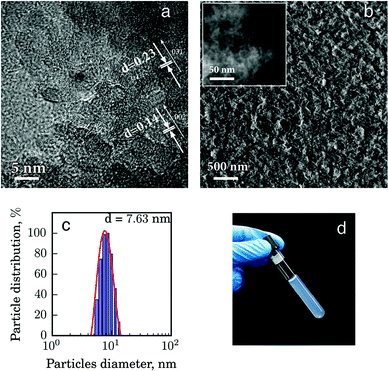
We have selected alumina-based inks for the design of the enzymatic printable biosensors. The entrapment within the sol–gel inorganic matrix is the basic principle for the current approach towards biosensors and the key element of the target technology allowing us to maintain a high stability of the enzymes. The basis for the inks is the alumina sols, which are composed of boehmite nanoparticles with a high degree of surface protonation acquired during the Yoldas synthesis method.25 As a result of synthesis modification (see experimental data) we reduced the concentration of acid and increased the pH of the final composite so as to make it suitable for the entrapment of enzymes and organic dyes. The high surface charge prevents covalent cross-linking of alumina nanoparticles upon the formation of xerogel and makes it easily resuspendable. At the same time, because of the high crystallinity and uniform size of the nanoparticles (see Fig. 1c and Fig. S1, ESI†), they can be used for the formation of a dense ceramic structure showing a uniform distribution of entrapped enzyme and dye molecules.17 High-resolution scanning and transmission electron microscopies (Fig. 1a and b) reveal that the alumina xerogel is composed of rod-like boehmite nanoparticles (Fig. 1a), which after the condensation produces a densely packed structure (Fig. 1b). X-ray diffraction (XRD) analysis confirms that boehmite is the only crystalline phase in the alumina matrix (see Fig. S1 in the ESI†) with an average Scherrer crystallite size of 2.95 nm. At the same time dynamic light scattering characterization showed that the alumina matrix particle size is 7.6 nm (Fig. 1c). This crystallite size matches well with the size of the enzymes selected for the construction of biosensors implying the possibility of an efficient entrapment of the biomolecules between the nanocrystallites in the target composite.The biomolecules in this work are the enzymes with a specific sensitivity to glucose and uric acid. In view of very minor differences in the textural properties of the selected enzymatic solutions, we will focus below on the detailed analysis of the characteristics of the glucose biosensor composite as a showcase example and compare them with those of the pure alumina matrix.
Co-condensation of alumina sol with the enzymes leads to a strongly reduced porosity of the xerogel and an increased mean pore size. The composite surface area (BET) decreased from 196 to 126 m2 g−1 for the glucose testing composite, compared to the pure alumina matrix (Fig. 2). The decrease of the surface area is most likely associated with the entrapment of the enzyme biomolecules between the nanoparticles in the xerogel matrix. The pronounced step in the desorption branch of the isotherm curve for p/p0 partial pressures in the range of 0.4 to 0.8 (Fig. 2) indicates the filling of the ink-bottle-type mesopores created by random particle packaging.17
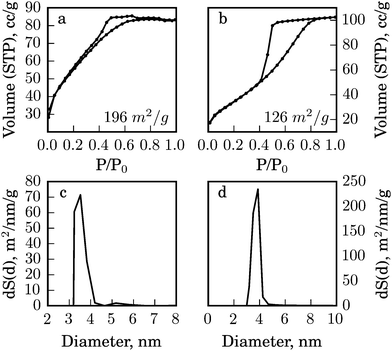 | ||
| Fig. 2 Gas (N2) adsorption analysis of (a) the alumina matrix; (b) bio-alumina for glucose detection; and BJH pore size distribution of: (c) the alumina matrix; (d) bio-alumina for glucose detection. | ||
At the same time the average pore size (calculated by BJH) of the biocomposite increased from 2.6 nm to 4.9 nm for the glucose sensing composite compared to pure alumina (Fig. 2c and d). Probably, only small pores that match the size of the protein become filled and the larger ones remain empty. This suggests an efficient and tight confinement of the protein globes that is necessary for their enhanced stability that will be demonstrated further.
We propose that during the introduction of biomolecules the alumina matrix provides entrapment conditions17 and the surface of the matrix ensures the preservation of the native structure and retains their stability even under heating, as we will show further. At the same time, because of the high degree of protonation of the nanoparticle surface, they can be easily resuspended to release the enzymes into the analytes and begin the sensing reaction (eqn (S1)–(S3), ESI†). Besides the thermal stability, we tested a long-term storage stability of the biocomposites. In our case, when the enzyme and dye mixture was entrapped in the alumina matrix, we observed the stability of the solid biocomposite for two months at room temperature storage.
To further demonstrate our basic concept of the enhanced stability of the biocomposites, we investigated their enzymatic activity in comparison with the free enzyme essays. We measured the relative activity of free and entrapped enzymes after heat treatment to assess the utility of the inorganic matrix as a stabilizing cage for enzymes. Here we used the mixtures of enzymes with a dye, which can be oxidized to a colored product as a result of the qualitative reaction with a biomarker substrate (glucose, uric acid). Two situations were considered, namely the one when these mixtures were entrapped in the alumina matrix and another one where an equal volume of the free mix was employed without the alumina. All samples were repeatedly exposed to 60 °C for 5 minutes for five heating–cooling cycles and the relative activity of the enzymes was measured by monitoring the color intensity during the oxidation of the dye after every heating cycle (see the experimental section for more details) (Fig. 3).
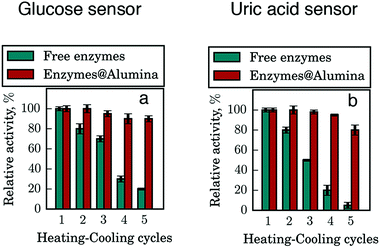 | ||
| Fig. 3 Thermal stability of entrapped enzymes. Tests of glucose sensors (a) and uric acid sensors (b). Alumina sol after condensation with enzymes provides exceptional stability of them. | ||
Both glucose and uric acid sensing activity of the free enzyme drastically deteriorates even after the short 5 min heating–cooling cycles (Fig. 3). After the first cycle, the free enzymes lost ca. 20% of the initial activity and it continued to decline with subsequent cycles. By the end of the experiment, the activity of the free enzymes decreased by 82% in the case of glucose and by 95% in the case of uric acid, while that of the entrapped enzymes dropped only by 9% and 20% respectively. For testing under more extreme conditions, the entrapped enzymes were exposed to prolonged (1 h) heating–cooling cycles. It is found that a 50% reduction of the enzymatic activity of the bio-composite could be achieved after 3 h heating, while after 5 cycles of heat treatment, 20% of the enzymatic activity remains. Thus, we can postulate that the entrapment in a sol–gel matrix shifts the point of enzyme denaturation toward higher temperatures due to a physical confinement between alumina nanoparticles. Besides the thermal stability, we have tested the activity of samples which were stored at room temperature for two months and we did not observe any loss of bioactivity, while in the free form the enzyme and dye solution is stable for seven days at room temperature.
With these promising results on the stability of the enzymatic complex in the alumina sol–gel–sol matrix in hand, we decided to move on to developing this concept for desktop inkjet printing sol–gel–sol biosensors. The key parameters of the inks are their rheological properties and the reproducibility of printing. We decided to start adjusting these properties by using a pure alumina matrix to determine the optimal parameters and to transfer our knowledge to the biocomposites at a later stage. We started with optimizing the printability of the inks by varying the sol-to-water ratio and selecting the optimum viscosity to achieve a stable ink-jet printing. For applying ink on substrates, we used desktop inkjet printer equipment with standard cartridges, whose contents after thorough washing were replaced with the sol–gel–sol ink.
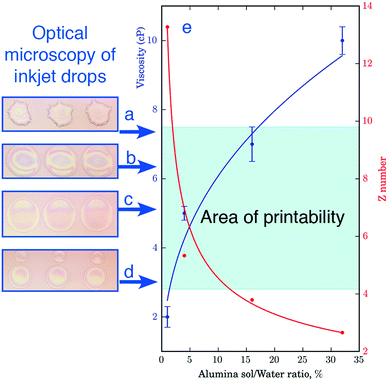
Fig. 4 summarizes the results of the printability optimization. We first prepared inks with different concentrations of alumina nanoparticles and, therefore, with varied viscosity of 2, 5, 7 and 10 cP (see the experimental part) and analyzed the print quality by analyzing individual drops by a combination of optical and atomic force microscopy. Fig. 4a–d depicts the deposited droplets with different viscosity that is the different sol concentration.
As can be seen from Fig. 4a–d, the variation in the viscosity of ink gives rise to a pronounced morphology difference in the resulting droplets. At the lowest sol concentration, the viscosity of the ink was not sufficient to form a stable droplet, it was torn during the flight and as a result formed small droplet-satellites (Fig. 4d). At high concentrations of the active substance printing was also possible, but upon drying the Marangoni flow effect was observed. The contents of the drops moved centripetally and thus hampered the uniform distribution of the substrate (Fig. 4a). To assess inkjet printability of ink, we employed the parameter Z-number 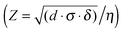 , where δ is the density, d is the diameter of the nozzle, σ is the surface tension, and η is the viscosity. According to the data presented in Fig. 4e, inkjet printing is possible in a wide range of dilution of the sol, while the optimal parameters correspond to the ink with a weight nanoparticle concentration of 4% and a viscosity of 5 cP. Such an ink allows the formation of uniform droplets with a high degree of repeatability yielding drops of proper shape without distortions when applied to a substrate.
, where δ is the density, d is the diameter of the nozzle, σ is the surface tension, and η is the viscosity. According to the data presented in Fig. 4e, inkjet printing is possible in a wide range of dilution of the sol, while the optimal parameters correspond to the ink with a weight nanoparticle concentration of 4% and a viscosity of 5 cP. Such an ink allows the formation of uniform droplets with a high degree of repeatability yielding drops of proper shape without distortions when applied to a substrate.
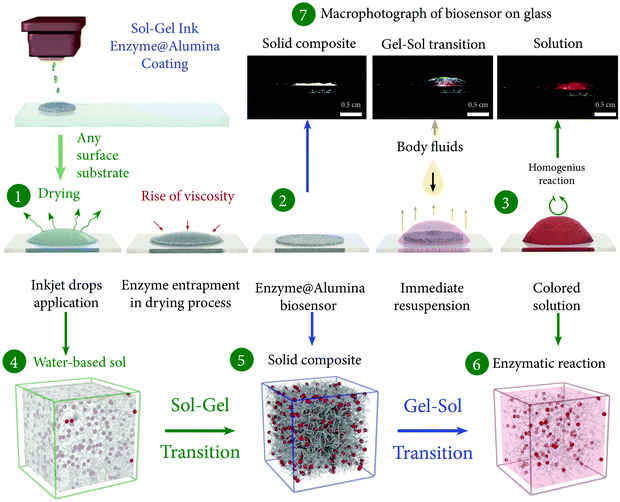
Having identified the optimal parameters for stable inkjet printing, we prepared biocomposite inks based on alumina with enzymes and dye solutions (see the experimental section) with a viscosity of 5 cP for inkjet printing sol–gel–sol biosensors. Fig. 5 presents schematically the general concept of our approach. During printing, the biocomposite is extruded from a cartridge nozzle and applied onto a substrate resulting in a uniform layer of sol with enzymes made of merged droplets (Fig. 51). Due to the fast drying and the concomitant increase of the concentration of the nanoparticles in the sol (Fig. 54), an alumina framework with entrapped biomolecules is formed (Fig. 52), which provides stability to globular enzyme molecules, similar to that observed for the dense solid composite. In this form the matrix of a biosensor is a suitable cage for enzymes (Fig. 55) that does not let them to unfold and denature even upon exposure to elevated temperatures.16 However, as we have already mentioned above, the key feature of this material is its ability to resuspend (Fig. 56) because of the presence of like-charged particles, which repel each other upon contact with the analyte. As soon as the xerogel is brought into contact with the biological fluid, the system swells instantaneously because of the high hydrophilicity of the protonated nanoparticles, which are only weakly bound with each other, so that they can accommodate large amounts of water in their interparticle space (Fig. 57). The resuspending process itself (up to 90 seconds) is accompanied by a release of the enzymes and the dye, making them more accessible to the analyte. With this mechanism, there is a classical interaction in solution of the analyte with the reagents (Fig. 53). The sensing is then clearly visible from the direct color change, even at the macroscale, as shown in Fig. 57.
A remarkable feature of this approach is the fact that the thickness of the xerogel layer is limited only by the amount of ink extruded from the nozzles, so that the film thickness can be easily increased making the amount of enzyme molecules that participates in the reaction larger. The increased amount of the fluid results in a more intensive coloring of the enzymatic biosensor reaction product, which is necessary for detecting even small amounts of substrate biomarkers, for example, glucose in saliva (0.03−0.6 mM28). Furthermore, such a material would be suitable for detection of relatively large target molecules, which in some cases cannot penetrate through the small pores of the matrix formed from the covalent crosslinking between for instance silica particles.
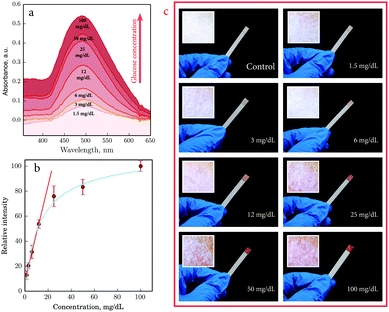
Based on the method described above, we prepared bioinks and enzymatic sol–gel–sol biosensors for the detection of glucose and uric acid. By means of the color reaction enzyme activity and sensitivity were tested after inkjet printing. Finally, we evaluated the relationship between the intensity of the optical response of inkjet-printed biosensors and the concentration of the analyte involved in the reaction (Fig. 6 and Fig. S2, ESI†). After analysis and visual evaluation, an experimental concentration scale was prepared.
As we can see in Fig. 6a the optical response can be easily detected by spectrophotometry in the diffuse reflectance mode. Glucose biosensors have a half-life exponential curve response depending on the concentration (Fig. 6b), with a decrease in sensitivity upon increasing glucose concentration. Direct dependence is observed at low concentrations, allowing for the most efficient signal at low concentrations and providing best quality detection by instrumental means. In spite of the nonhomogeneous distribution of the sol over the surface of the paper because of non-uniform density and porosity of the cellulosic fibers, coloration can be observed in macrophotographs of the biosensor surface, which is very evident on a macroscopic scale (Fig. 6c) by the naked eye. The uric acid biosensors also displayed a good linear relationship between the intensity of the color response and the concentration of uric acid (Fig. S2, ESI†).
Our experiments point to the applicability of the current generation of biosensors for the detection of concentrations as low as 0.02 mM at a time resolution that is the time required to achieve the maximum color intensity (reaction end) of no more than 90 seconds. These parameters are comparable with those of the commercial express blood tests.29 Normally, glucose concentrations in the blood of healthy adult human are in the range 4–5 mM29 and those in human saliva are 0.05–17 mM.28 These are well above the detection limits of the current generation of the printable biosensors making them potentially suitable for the express non-invasive analysis of the increased glucose level in such low-concentrated bodily fluids as saliva. We should however emphasize the necessity of more detailed and comprehensive tests involving the realistic biological liquids. The high complexity of bodily fluids including saliva may affect the sensitivity and selectivity of the printed device. Further studies should include the analysis and optimization of the encapsulated enzymes to other hexose isomers such as fructose and galactose potentially present in the biological samples. Furthermore, their performance as a function of the varied air humidity, viscosity and acidity of the sample liquids, etc.30 has to be carefully evaluated to enable the widespread application of the current technology. The analysis of these factors falls beyond the scope of this paper and will be the focus of our future works. Nevertheless, the presented data clearly show that a relatively small detection time and a high sensitivity to small concentrations of biomarkers in combination with the use of a modern inkjet printing method for the creation of biosensors render this technology attractive for the development of new matrices for biosensors and their application to reduce the loss of bioactivity to a minimum. The unique characteristics of the described approach are its simplicity and high efficiency, thus creating a new platform for preparation of optical sensing systems to other substrates, while opening a new direction for inkjet printing of sol–gel–sol biosensors.
4 Conclusion
In this work we presented a versatile sol–gel–sol approach for the production of optical enzymatic biosensors with inkjet printing. Due to the lack of the covalent bonding between matrix NPs, biocomposites can be easily resuspended while contacting with bodily fluids thus providing conditions for the interaction of biomolecules and analytes. At the same time, biocomposites exhibited outstanding stability compared with free form enzymes while showing an improved storage stability and prolonged action. Future development of the developed technology can help to produce biosensors based on the more fragile molecules and open up new opportunities for fast, easy and self-testing biosensors.Acknowledgements
This work was supported by the Russian Science Foundation Grant No. 16-19-10346.References
- M. Raja, A. Raja, M. Imran, A. Santha and K. Devasena, Biotechnology, 2011, 10, 51–59 CrossRef CAS.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Biotechnol. Adv., 2012, 30, 489–511 CrossRef CAS PubMed.
- J. D. Newman and S. J. Setford, Mol. Biotechnol., 2006, 32, 249–268 CrossRef CAS PubMed.
- M.-S. Steiner, A. Duerkop and O. S. Wolfbeis, Chem. Soc. Rev., 2011, 40, 4805–4839 RSC.
- J. Wang, Chem. Rev., 2008, 108, 814–825 CrossRef CAS PubMed.
- H. Yang, Curr. Opin. Chem. Biol., 2012, 16, 422–428 CrossRef CAS PubMed.
- J. K. Kaushik and R. Bhat, J. Biol. Chem., 2003, 278, 26458–26465 CrossRef CAS PubMed.
- L. S. Taylor, P. York, A. C. Williams, H. G. Edwards, V. Mehta, G. S. Jackson, I. G. Badcoe and A. R. Clarke, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1995, 1253, 39–46 CrossRef.
- C. Durrieu, Y. Ferro, M. Perullini, A. Gosset, M. Jobbágy and S. A. Bilmes, Environ. Sci. Pollut. Res., 2016, 23, 9–13 CrossRef CAS PubMed.
- M. Hasanzadeh, N. Shadjou, M. de la Guardia, M. Eskandani and P. Sheikhzadeh, TrAC, Trends Anal. Chem., 2012, 33, 117–129 CrossRef CAS.
- J. Livage, M. Henry and C. Sanchez, Prog. Solid State Chem., 1988, 18, 259–341 CrossRef CAS.
- B. J. Melde, B. J. Johnson and P. T. Charles, Sensors, 2008, 8, 5202–5228 CrossRef CAS PubMed.
- M. R. N. Monton, E. M. Forsberg and J. D. Brennan, Chem. Mater., 2011, 24, 796–811 CrossRef.
- J. Li, F. Rossignol and J. Macdonald, Lab Chip, 2015, 15, 2538–2558 RSC.
- O. De Los Cobos, B. Fousseret, M. Lejeune, F. Rossignol, M. Dutreilh-Colas, C. Carrion, C. Boissiere, F. Ribot, C. Sanchez and X. Cattoen, et al. , Chem. Mater., 2012, 24, 4337–4342 CrossRef CAS.
- A. Rutenberg, V. V. Vinogradov and D. Avnir, Chem. Commun., 2013, 49, 5636–5638 RSC.
- V. V. Vinogradov and D. Avnir, J. Mater. Chem. B, 2014, 2, 2868–2873 RSC.
- M. Kuang, L. Wang and Y. Song, Adv. Mater., 2014, 26, 6950–6958 CrossRef CAS PubMed.
- M. Azevedo and S. Alla, Int. J. Diabetes Dev. Countries, 2008, 28, 101 CrossRef PubMed.
- Y. Li, J. Rodrigues and H. Tomas, Chem. Soc. Rev., 2012, 41, 2193–2221 RSC.
- R. Gupta and N. Chaudhury, Biosens. Bioelectron., 2007, 22, 2387–2399 CrossRef CAS PubMed.
- R. S. Malon, S. Sadir, M. Balakrishnan and E. P. Córcoles, BioMed Res. Int., 2014, 962903 Search PubMed.
- H. Chen, S. Hou, H. Ma, X. Li and Y. Tan, Sci. Rep., 2016, 6, 20722 CrossRef PubMed.
- B. E. Yoldas, Am. Ceram. Soc. Bull., 1975, 54, 289–290 CAS.
- B. E. Yoldas, J. Mater. Sci., 1986, 21, 1087–1092 CrossRef CAS.
- A. V. Yakovlev, V. A. Milichko, V. V. Vinogradov and A. V. Vinogradov, ACS Nano, 2016, 10, 3078–3086 CrossRef CAS PubMed.
- A. V. Yakovlev, V. Milichko, V. Vinogradov and A. V. Vinogradov, et al. , Adv. Funct. Mater., 2015, 25, 7375–7380 CrossRef CAS.
- W. Zhang, Y. Du and M. L. Wang, Sensing and Bio-Sensing Research, 2015, 4, 23–29 CrossRef.
- S. Vaddiraju, D. J. Burgess, I. Tomazos, F. C. Jain and F. Papadimitrakopoulos, J. Diabetes Sci. Technol., 2010, 4, 1540–1562 CrossRef PubMed.
- N.-Y. Kim, K. K. Adhikari, R. Dhakal, Z. Chuluunbaatar, C. Wang and E.-S. Kim, Sci. Rep., 2015, 5, 7807 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns of a sensor based on the enzyme@alumina mixture, the optical response and additional characterization information. See DOI: 10.1039/c6tb02559e |
| This journal is © The Royal Society of Chemistry 2017 |