 Open Access Article
Open Access ArticleInfluence of specific intermolecular interactions on the thermal and dielectric properties of bulk polymers: atomistic molecular dynamics simulations of Nylon 6
N. V.
Lukasheva
*a,
D. A.
Tolmachev
a,
V. M.
Nazarychev
a,
J. M.
Kenny
ab and
S. V.
Lyulin
ac
aInstitute of Macromolecular Compounds, Russian Academy of Science, Bol'shoi pr. 31 (V.O.), St. Petersburg, 199004, Russia. E-mail: luk@imc.macro.ru; Fax: +7 (812) 3286869
bMaterials Science and Technology Centre, University of Perugia, Loc. Pentima, 4, Terni, 05100, Italy
cDepartament of Physics, St. Peterburg State University, Ul'yanovskaya str. 1, Petrodvorets, St. Petersburg, 198504, Russia
First published on 21st November 2016
Abstract
Specific intermolecular interactions, in particular H-bonding, have a strong influence on the structural, thermal and relaxation characteristics of polymers. We report here the results of molecular dynamics simulations of Nylon 6 which provides an excellent example for the investigation of such an influence. To demonstrate the effect of proper accounting for H-bonding on bulk polymer properties, the AMBER99sb force field is used with two different parametrization approaches leading to two different sets of partial atomic charges. The simulations allowed the study of the thermal and dielectric properties in a wide range of temperatures and cooling rates. The feasibility of the use of the three methods for the estimation of the glass transition temperature not only from the temperature dependence of structural characteristics such as density, but also by using the electrostatic energy and dielectric constant is demonstrated. The values of glass transition temperatures obtained at different cooling rates are practically the same for the three methods. By proper accounting for partial charges in the simulations, a reasonable agreement between the results of our simulations and experimental data for the density, thermal expansion coefficient, static dielectric constant and activation energy of γ and β relaxations is obtained demonstrating the validity of the modeling approach reported.
I. Introduction
In order to develop new materials and compounds with designed new properties, it is essential that these properties can be predicted before preparation, processing, and characterization. The main goal of computational materials science is an accurate prediction of the properties of new materials before their development and production. Molecular dynamics simulations using atomistic force fields to describe molecular-level interactions are useful tools for this goal. This approach is of particular importance in the field of polymer/filler nanocomposites, where the material properties depend on its structure at the nano-scale level. However, before predicting the properties of nanocomposites based on a new polymer, it is necessary to reproduce correctly the properties of the polymer matrix.The importance of accounting accurately for the electrostatic interactions for evaluating the thermal properties of polymers with highly polar groups from computer simulations has been already reported.1 This is also true for polymers with specific intermolecular interactions, for example, for polyamides with H-bonding between chains providing good mechanical and thermal properties.2 Polyamides are very important engineering thermoplastics due to their high strength at elevated temperatures and toughness at low temperatures, combined with other important properties, such as stiffness, wear and abrasion resistance. The most common application of polyamides in the engineering industry is in textile fibres and in the replacement of traditional metals taking advantage of their great elasticity.3 Deforming under load they are able to return to their original shape up to 75 times faster than other materials. Nylon 6 is the most common type of polyamide accounting for 60% of the global production of this polymeric family and providing an excellent example for proper accounting for H-bonding in computer simulations of polymers.
Few molecular dynamic (MD) simulations of crystalline Nylon 6 have been already reported.8,9 However, simulations of amorphous Nylon 6 providing a wide analysis of its properties are absent. The modelling of nanocomposite materials with the Nylon 6 matrix has extensively been reported. In particular, the influence of the addition of silica nanoparticles,9 carbon nanotubes,10,11 graphene nanosheets12 and clay13 on the properties of the Nylon 6 matrix was studied. However, the major drawback of these studies is the short equilibration time in the creation of the starting system and in the analysis of the temperature dependence of the various characteristics studied. For this reason, only a qualitative correspondence has been obtained between the modelling results and experimental data. Moreover, none of the previous works have addressed the properties of pure amorphous Nylon 6. This paper reports the study of the structural, thermal and relaxation characteristics of polyamide Nylon 6 by using atomistic molecular dynamics simulations. To our knowledge, the present study is the first one in which the importance of the proper consideration of specific intermolecular interactions like hydrogen bonding is demonstrated in order to provide a realistic assessment of the thermal and dielectric properties of bulk Nylon 6. Moreover, the temperature dependence of the structural and dielectric characteristics of this polymer was investigated in a wide temperature range and cooling rates.
The paper is organized as follows. The simulation method, force field, models and procedures are described in Section II. Section III is devoted to the discussion of the simulation results and the structural characteristics of individual polymer molecules and compounds in the condensed state. Moreover, thermophysical properties such as the glass transition temperature estimated from temperature dependence of the density, electrostatic energy and reciprocal value of the dielectric constant, as well as the relaxation processes evaluated in terms of the time decay of the dipole moment autocorrelation functions are also calculated and reported. The main conclusions are presented in Section IV.
II. Method, force field, model, and simulation procedure
Method and force field
The simulations were performed by the atomistic MD approach using the GROMACS 5.0.7 package.14 To maintain a constant temperature and pressure in the system, the Berendsen thermostat and barostat with temporal constants τT = 0.2 ps and τp = 0.1 ps, were used, respectively. Long-distance Coulomb interactions were calculated via the particle mesh Ewald approach.15,16 The cut-off distance (rc) for the PME electrostatics was chosen to be 1.0 nm. We performed preliminary simulations for cut-offs from 0.8 nm up to 1.5 nm which demonstrated that the correction of the electrostatic energy by changing rc from 1.0 nm to 1.5 nm does not exceed the computation error (which is about 0.7%), but the time required for the calculations almost doubles. On the other hand, a comparison of the results obtained at rc = 1.0 nm and 0.8 nm showed a reduction of the calculation time but a more significant underestimation of the electrostatic energy. The bond length was limited by the LINCS algorithm.17The inter-atomic potentials of the force field AMBER99sb4 were used. AMBER is a family of force fields for MD simulations of biomolecules such as peptides, proteins, and nucleic acids. The choice of the ff99sb parameter set was dictated by the fact that it gives a better description of the hydrogen bonds (in comparison with other AMBER parameter sets) and allows for a better balance of secondary structure elements as evidenced by the improved distribution of backbone dihedrals. However, it should be noted that generally hydrogen bonds are not well described by various force fields and, on average, they are weaker than those suggested by experimental data.5 In the AMBER99sb force field, H-bond interactions are taken into account through electrostatic interactions. The use of the partial atomic charges calculated by the ab initio HF/6-31G* (RESP) and semi-empirical AM1-BCC6 is allowed. The AM1-BCC method quickly and efficiently generates high-quality atomic charges for use in condensed-phase simulations because it combines the speed of a semi-empirical calculation with the efficiency of the BCI (bond charge increment) approach. It was demonstrated that AM1-BCC yields charge sets of quality comparable to HF/6-31G* RESP-derived charges for the compounds with conjugated bonds and with cyclic fragments.7 However, the efficiency of the AM1-BCC approach for aliphatic polyamides has not been yet reported. In the present study, the effects of H-bonding on the structural and thermal characteristics of Nylon 6 are studied using two sets of partial atomic charges (HF/6-31G* (RESP) and AM1-BCC). Moreover, the correspondence of the simulated results with experimental data is examined.
Simulation model and procedure
The procedure for creating equilibrium samples of the polymer melt was similar to the procedure reported previously for polyimides.1,18 The cubic cell for simulated systems contains 27 polyamide chains with polymerization degree n = 30. To obtain statistically significant results in the study of the thermal properties of the simulated samples of Nylon 6, simulations were carried out during 1 μs that allowed using a proper distribution of starting configurations for the cooling procedure. Five starting configurations (at 300, 400, 500, 600, and 700 ns) were selected for each system. Gradual cooling with cooling rates of 1.5 × 1013 K min−1 (10 K/40 ps), 1.5 × 1012 K min−1 (10 K/400 ps) and 1.5 × 1011 K min−1 (10 K/4 ns) was performed for each of the configurations. The simulation with the lowest cooling rate (1.5 × 1010 K min−1 (10 K/40 ns)) was applied only to the configurations of the systems with atomic charges obtained by the HF/6-31G* (RESP) calculations.III. Results and discussion
Characteristics of individual molecules and simulated systems in melt
| N | H | C | O | |
|---|---|---|---|---|
| HF/6-31G* (RESP) | −0.55 | 0.29 | 0.67 | −0.6 |
| AM1-BCC | −0.32 | 0.15 | 0.21 | −0.28 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) shows that the density of the system with the HF/6-31G* (RESP) charges is much closer to the experimental value. It is necessary to point out that even for the system with HF/6-31G* (RESP) charges, the density in the molten state approaches its equilibrium value very fast (it takes about 400 ps). Thus, our equilibration during 4 ns, and even longer for 40 ns (upon cooling from 600 K down to 545 K with steps 10 and 5 degrees) was long enough to obtain the equilibrium density values.
20) shows that the density of the system with the HF/6-31G* (RESP) charges is much closer to the experimental value. It is necessary to point out that even for the system with HF/6-31G* (RESP) charges, the density in the molten state approaches its equilibrium value very fast (it takes about 400 ps). Thus, our equilibration during 4 ns, and even longer for 40 ns (upon cooling from 600 K down to 545 K with steps 10 and 5 degrees) was long enough to obtain the equilibrium density values.
| l p, nm | h, nm | R g, nm | ρ, kg m−3 at 545 K | |
|---|---|---|---|---|
| AM1-BCC | 0.53 | 5.6 | 2.26 | 876 |
| HF/6-31G* | 0.56 | 5.9 | 2.35 | 943 |
Thermal characteristics
The analysis of the curves for the density dependence for different systems and different cooling rates showed a strong difference in the position of the transition region (from melt to glass) for the systems with the values of the partial charges on the atoms calculated by the AM1-BCC and HF/6-31G* (RESP) methods (Fig. 2a and b, respectively). Moreover, there is a clear correlation between the changes in the electrostatic interaction energy in these systems and the changes in their density (see Fig. 2a–c).
This behavior is caused by the differences in the values of the partial atomic charges of the amide groups (Table 1). In the case of the charges calculated by HF/6-31G* (RESP), their values are higher, providing a larger contribution of the electrostatic interactions to the total energy of the system at the transition from the molten to the glassy state.
Since the main contribution to the intermolecular interactions in Nylon 6 is provided by H-bonds and in the AMBER99sb force field these interactions are taken into account through electrostatic interactions, the above conclusion is confirmed by comparing the pair radial distribution function (RDF) (g(r)) for the atoms involved in the hydrogen bond formation (Fig. 3a) and potentials of the mean force (A(r)) for them (Fig. 3b), designed for the systems in the glassy state (at 300 K). The pair distribution function was calculated through the following equation:
 | (1) |
| A(r) = −kBT·ln(g(r)) + B, | (2) |
The much higher maximum of the pair distribution function for atoms involved in the formation of hydrogen bonds and the shift of the maximum position to shorter distances can be seen for the system with the HF/6-31G* (RESP) partial atomic charges in Fig. 3a. This is evidence of the much stronger attractive forces in this system (Fig. 3b). The differences in the H-bond energy for the simulated systems are also manifested in the amount of hydrogen bonds per amide group in each simulated system (Fig. 3c). The H-bond is considered to be formed if the distance between the oxygen atom of the –CO group and the hydrogen atom of the –NH group does not exceed 0.3 nm and the H–N–O angle does not exceed 30°.21 Although the temperature dependence of the number of H-bonds per amide group (for the HF/6-31G* (RESP) charges) is very similar to the dependence of density, the intersection points of the two straight lines obtained by the linear fit of the high-temperature domain and the low temperature domain of these curves lie in the region of temperatures that are lower than Tg defined from the ρ(T) and Eel(T) curves. This is understandable, since the H-bonding energy is only for a part of the electrostatic energy.
Since the cooling rate in the MD simulations is several orders of magnitude higher than that in a real experiment, the glass transition temperature measured in the modeling must be higher than the experimental value.22 As reported in Table 3, the Tg values determined at different cooling rates for the system with the HF/6-31G* (RESP) partial atomic charges are in the range of temperatures which are significantly higher than the experimental data. In fact, dry Nylon-6 with the molecular mass of the polymer chains similar to the simulated systems has a glass transition temperature of 310 K.23,24 At the same time, for the systems with the partial charges calculated by AM1-BCC, the values of the glass transition temperature (at different cooling rates) are in the same interval as the experimental values, and even below (Table 3).
| Nylon 6 | 10 K/40 ps | 10 K/400 ps | 10 K/4 ns | 10 K/40 ns |
|---|---|---|---|---|
| AM1-BCC | 338 ± 3 K | 316 ± 4 K | <290 K | — |
| HF/6-31G* RESP | 427 ± 15 K | 413 ± 11 K | 401 ± 8 K | 387 ± 1 K |
The slower a liquid is cooled, the longer the time available for configurational sampling at each temperature, and hence the colder it can become before falling out of the liquid-state equilibrium. Consequently, Tg increases with the cooling rate.22
When comparing the simulation results with experimental data, it should be considered, of course, that in our simulations we are dealing with fully amorphous samples. In fact, additional peaks which would indicate the formation of any order are absent in the RDF curve. Nylon 6 is a semi-crystalline polymer, and in experimental studies samples with different crystallinity were investigated. The formation of crystalline regions in a sample obviously results in a reduction of the amorphous fraction and a restriction of the chain mobility in the amorphous regions by crystallites, that in turn, leads to an increasingly higher Tg with crystallinity development. Experimental data reported in the scientific literature regard samples with crystallinity values below 35%. In particular, the Tg for amorphous samples is 310 K and reaches a limiting value of about 316 K at 5–10% crystallinity.25
It is well known that the glass transition temperature depends on the cooling rate (γ) logarithmically:26,27
 | (3) |
 | ||
| Fig. 4 (a) Tg of the simulated system with HF/6-31G* (RESP) atomic charges as a function of cooling rate (black stars) and its approximation by eqn (3) (red line) with the following set of parameters: A = 1.9 × 10−20 min K−1, B = 768 K and T0 = 310 K. (b) Experimental temperature dependence of the coefficient of thermal expansion (CTE) of Nylon 6 (black line) and the dependence for the systems with partial charges HF/6-31G* (RESP) (green curve) and with partial charges AM1-BCC (red curve). | ||
It is known that the glass transition temperature obtained from MD simulations is not suitable for the verification of computational models against experimental data since extremely high CPU resources are required.18 However, our results suggest that the use of the AMBER99sb force field with HF/6-31G* (RESP) charges gives significantly more reasonable results than those obtained with the AM1-BCC atomic charges.
Measuring the coefficient of thermal expansion (CTE) of the polymer provides an additional way for verifying computational models.28 The CTE, β, was calculated from the temperature dependence of the polymer sample density via the following finite difference relation:
 | (4) |
Fig. 4b shows the temperature dependence of the CTE obtained for the two simulated systems in comparison with the experimental data obtained for Nylon 6 in a wide temperature interval.29,30 Our calculations over-estimate the experimental CTE in both cases but it can be seen that the CTE values for the system with the partial charges HF/6-31G* (RESP) are closer to the experimental data.
Thus, for the system with the HF/6-31G* (RESP) partial atomic charges both characteristics (Tg and CTE) are more realistic. For this reason, the systems with the HF/6-31G* (RESP) partial atomic charges were used to calculate the dielectric characteristics and to study the relaxation processes of Nylon 6.
In this section, we reported the Tg values estimated from temperature dependence of structural characteristics such as density and electrostatic energy. It is also possible to estimate Tg by monitoring the temperature dependence of relaxation times.31 However, this method is less accurate due to the limited range of relaxation times that can be addressed in computer simulations. For this reason, we did not estimate Tg by using the relaxation times deduced, for example, from the diffusion of chain fragments27 and in the next section, we also estimated Tg by using structural characteristics such as the dielectric constant.
Dielectric characteristics and dielectric relaxation
 | (5) |
The temperature dependence of the simulated dielectric constant is shown in Fig. 5 in comparison with the experimental data from ref. 36. The rotation of the polar groups is not possible below Tg and the permittivity is low, with a big increase around Tg. At higher temperatures the increased thermal vibrations cause the permittivity to drop again (in our simulations this can be seen at the slowest cooling rate).
 | ||
| Fig. 5 (a) Changes in the dielectric constant (ε) with temperature. The simulation results are shown in comparison with the experimental data in the inset.36 (b) Plot 1/(ε − 1) vs. temperature. | ||
The value of the dielectric constant depends on the cooling rate. In our simulations with the HF/6-31G* partial atomic charges and at the equilibration during 40 ns at each temperature the static dielectric constant is underestimated in comparison with the experimental values. But, for example, the additional equilibration at 340 K during 200 ns results in an increase in ε, from 2.48 up to 4.1, which is much closer to the experimental value (see the inset in Fig. 5a).
The dependence of the static dielectric constant on the cooling rate is even more pronounced than that for density (Fig. 2). It has been reported that the polymer glass transition temperature can be estimated from the temperature dependence of the inverse value of the dielectric permittivity.37 Moreover, it was experimentally assessed that for many polymer systems exhibiting the dependence of the reciprocal dielectric permittivity on temperature there are at least two regions which correspond to the system being in glassy and rubbery states, respectively. As seen in Fig. 5b, there is a change in the character of the temperature dependence of (1/(ε − 1)) at a certain critical temperature (as for polymer density). The values of this temperature at different cooling rates are very close to those obtained from the ρ(T) dependence.
The main relaxation processes in Nylon 6 (Fig. 6) are related to (i) the movement of extended CH2 sequences of the backbone with involvement of amide groups (the α relaxation), (ii) localized motions involving several monomer units, but influenced by hydrogen bonded amide groups (the β relaxation) (iii) the γ relaxation associated with the dynamics of single monomer units and corresponding to the vibrational motions of the methylene groups in the chains.38,39
The cooling rate expresses how much time is spent at each new temperature, and if the cooling process is faster than the relaxation time of a certain process the polymer segment will not be able to relax. According to the experimental data reported for Nylon 6,36,38,39 the α process relaxation time is tens of microseconds, the β process relaxation time in melt is about 1 ns and reaches ∼10 μs at the glass transition, but the relaxation time of the γ process is changed from about 100 ps in melt up to 100 ns in glassy state. Thus, from our simulation data (even for those obtained at the slowest cooling rate) we can reliably identify only the γ relaxation process.
The relaxation processes in the simulated Nylon 6 systems were evaluated for the case of the lowest cooling rate (10 grad/40 ns (1.5 × 1010 K min−1)) in terms of the time decay of dipole moment autocorrelation functions (DACF). The DACF are given as:
 | (6) |
The time decays of the DACF at different temperatures are reported in Fig. 7.
 | ||
| Fig. 7 The time decays of the DACF at different temperatures from 600 K to 300 K through each 20 K (in color representation from red to black). | ||
We applied two approaches to analyse the DACF. The DACF can be represented as the sum of multiple decaying exponential terms or the Kohlrausch–Williams–Watts (KWW) functions,40,41 stretched functions:
 | (7) |
| ACF(t) = A1f1(t) + A2f2(t), | (8) |
 | (9) |
It can be seen that the frequencies obtained when representing DACF by two relaxation processes in the high temperature region (from 600 K to 450 K) show a distinct linear dependence on temperature which means the manifestation of a certain relaxation process. However, the frequencies obtained when representing DACF by one relaxation process at lower temperatures show a distinct linear dependence on temperature.
Moreover, in order to identify the particular relaxation processes we applied the CONTIN approach,42 which gives the relaxation time density function (RTDF) from the autocorrelation function, as it is the Laplace transform of the RTDF. Obtaining these functions is not a straightforward process because there are essentially infinite solutions to the problem. After analyzing the relaxation time density functions (Fig. 9a and b) obtained by using the CONTIN procedure, the most physically feasible solutions were selected (Fig. 9c).
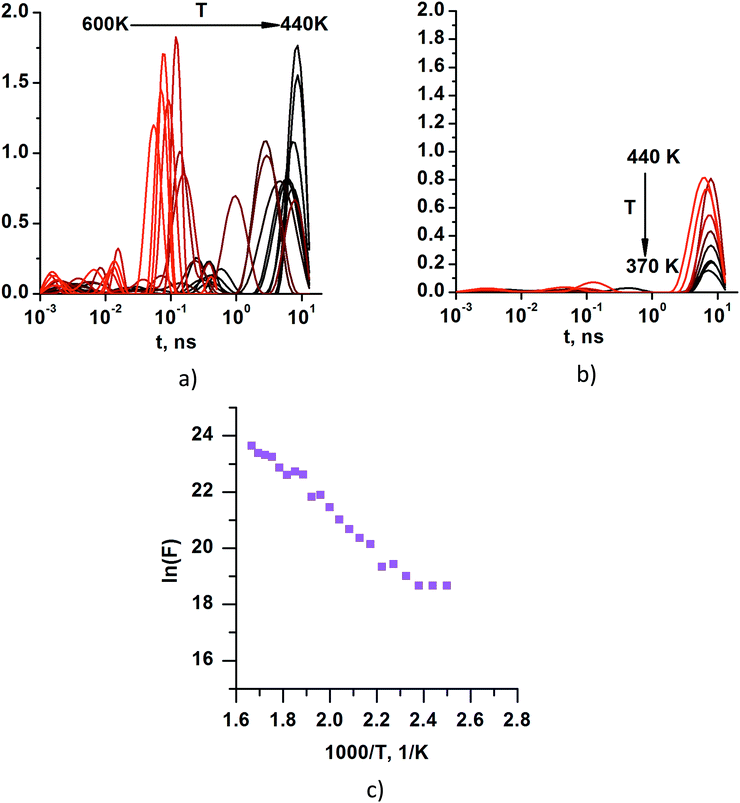 | ||
| Fig. 9 Relaxation time density functions at temperatures (a) 600 K 440 K and (b) 440 K 370 K. (c) Frequencies corresponding to the most intensive peaks of the relaxation time density functions. | ||
It is important to note that the DACF curves that do not decay below 0.8, namely, the curves at temperatures of 360 K and lower (Fig. 7) cannot provide reliable relaxation times with any kind of fit. Thus, we have performed the final analysis for the results obtained at temperatures higher than 360 K. Summarizing, it can be concluded that the temperature dependence of the frequencies of the most pronounced relaxation process in the KWW analysis (obtained by using the fitting of DACF with the sum of two stretched exponents) and in the CONTIN analysis is very close (Fig. 10a). The computed exponential results and experimental data of the relaxation processes38 (which were identified as γ relaxation) represented as a function of the inverse of the temperature demonstrates a linear approximation with very similar slopes (Fig. 10b, lines 2 and 3). The estimated value of the activation energy (where KB is the Boltzmann constant) of the relaxation process in our simulations is ∼0.517 eV and it is very close to the activation energy of the experimentally identified γ relaxation process of ∼0.477 eV.38
The linear fitting of the CONTIN and of the two-exponential data (line 1 in Fig. 10b) gives higher slopes than the single-exponential data and correspondingly a higher activation energy of about 0.655 eV was obtained which is close to the experimental value attributed to the β relaxation process (0.62 eV).38
IV. Conclusions
With the aim to show the effect of a proper consideration of specific intermolecular interactions like hydrogen bonding on the realistic assessment of the thermal and dielectric properties of bulk polymers, MD simulations of the aliphatic polyamide Nylon 6 in a wide temperature range were performed with the use of the AMBER99sb force field. Two sets of the partial atomic charges (AM1-BCC and HF/6-31G* (RESP)), acceptable for use in the AMBER99sb force field, were examined. In this work, we have employed three different estimations of the glass transition temperature from the temperature dependence of structural characteristics such as density, electrostatic energy and dielectric constant (inverse value). It was observed that using HF/6-31G* (RESP) partial atomic charges leads to reasonable results for the calculation of density, glass transition temperature and coefficient of thermal expansion of Nylon 6 samples. Moreover, the study of the dielectric properties demonstrated a good agreement between the values of the static dielectric constant estimated from our simulations with the HF/6-31G* (RESP) atomic charges and the experimental one.The relaxation processes in the simulated system of Nylon 6 were evaluated in terms of the time decay of dipole moment autocorrelation functions (DACF). The DACF were analysed by using KWW functions and the CONTIN approach. Because the simulations were executed at relatively slow cooling rates (1.5 × 1010), two secondary relaxation processes were identified: not only the γ relaxation associated with the dynamics of single monomer units, but also the β relaxation which usually relates to the motions involving several monomer units influenced by hydrogen bonded amide groups. Comparison of the obtained results and the experimental data demonstrated very close values of the activation energies of the relaxation processes observed in our simulations and experimentally identified as γ and β relaxation processes.
Thus, the reasonable agreement between our simulations and experiments suggests that the AMBER 99sb force field with the HF-6-31g* (RESP) partial atomic charges provides an adequate representation of the bulk structure and dynamics of Nylon 6. Armed with this agreement, we will continue our research by studying composites of Nylon 6 with different fillers. Moreover, we are sure that the AMBER 99sb force field with the partial atomic charges calculated in the present study for Nylon 6 by using the HF-6-31g* (RESP) approach can be applied for any aliphatic Nylon. This conclusion is based on the fact that these Nylons differ in terms of the length of the –(CH2)n– sequences between amide groups from n = 4 in Nylon 6,6 to n = 11 in Nylon 12. These sequence length changes (in comparison with Nylon 6) cannot have any significant impact on the electron density distribution in amide groups.
Acknowledgements
The calculations were performed using the resources of the Lomonosov supercomputer at Moscow State University and Center of collective use “Complex of modeling and data research mega-class facilities” NRC “Kurchatov Institute” (unique identifier RFMEFI62114X0006). This study was supported by the Russian Ministry of Education and Science within State Contract 14.Z50.31.0002 (megagrant).References
- S. V. Lyulin, A. A. Gurtovenko, S. V. Larin, V. M. Nazarychev and A. V. Lyulin, Macromolecules, 2013, 46(15), 6357–6363 CrossRef CAS.
- Fibrous and Composite Materials for Civil Engineering Applications, ed. R. Fangueiro, Elsevier, Cambridge, 1st edn, 2011 Search PubMed.
- Dudley's Handbook of Practical Gear Design and Manufacture, ed. S. P. Radzevich, CRC Press, Miami, 2nd edn, 2012 Search PubMed.
- V. Hornak, R. Abel, A. Okur, B. Strockbine, A. Roitberg and C. Simmerl, Proteins, 2006, 65, 712–725 CrossRef CAS PubMed.
- O. F. Lange, D. Spoel and B. L. Groo, Biophys. J., 2010, 99, 647–655 CrossRef CAS PubMed.
- A. Jakalian, D. B. Jack and C. I. Bally, J. Comput. Chem., 2002, 23, 1623–1641 CrossRef CAS PubMed.
- A. Jakalian, B. L. Bush, D. B. Jack and C. I. Bally, J. Comput. Chem., 2000, 21, 132–146 CrossRef CAS.
- Y. Li and W. A. Goddard, Macromolecules, 2002, 35, 8440–8455 CrossRef CAS.
- J. Yang, H. Shin, H. Kim and H. K. Lee, Appl. Phys. Lett., 2014, 104, 101901 CrossRef.
- N. Tahreen and A. K. M. Masud, International Journal of Manufacturing, Materials, and Mechanical Engineering, 2013, 3, 62–73 CrossRef.
- J.-S. Yang, C. L. Yang, M.-S. Wang, B.-D. Chen and X. Guang, RSC Adv., 2012, 2, 2836–2841 RSC.
- Y.-F. Mo, C.-L. Yang, Y.-F. Xing, M.-S. Wang and X. G. Ma, e-Polym., 2014, 14, 169–176 CAS.
- D. R. Katti, K. S. Katti, M. Raviprasad and C. Gu, J. Nanomater., 2012, 341056, DOI:10.1155/2012/341056.
- M. J. Abraham, T. Murtola, R. Schulz, S. Ráll, J. C. Smith, B. Hess and E. Lindahl, SoftwareX, 2015, 1–2, 19–25 CrossRef.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089–10092 CrossRef CAS.
- U. Essmann, L. Perera, M. L. Berkowitz, T. Darden, H. Lee and L. G. Pedersen, J. Chem. Phys., 1995, 103, 8577–8593 CrossRef CAS.
- B. Hess, H. Bekker, H. J. C. Berendsen and J. G. E. M. Fraaije, J. Comput. Chem., 1997, 18, 1463–1472 CrossRef CAS.
- S. V. Lyulin, S. V. Larin, A. A. Gurtovenko, N. V. Lukasheva, V. E. Yudin, V. M. Svetlichnyi and A. V. Lyulin, Polym. Sci., Ser. A, 2012, 54, 631–643 CrossRef CAS.
- Introduction to Nanofiber Materials, ed. F. K. Ko and Y. Wan, Cambridge University Press, Cambridge, 2014 Search PubMed.
- T. D. Fornes and D. R. Paul, Polímeros: Ciência e Tecnologia, 2003, 13, 212–217 CrossRef CAS.
- M. Petukhov, G. Rychkov, L. Firsov and L. Serrando, Protein Sci., 2004, 13, 2120–2129 CrossRef CAS PubMed.
- S. V. Lyulin, S. V. Larin, A. A. Gurtovenko, V. M. Nazarychev, S. G. Falkovich, V. E. Yudin, V. M. Svetlichnyi, I. V. Gofman and A. V. Lyulin, Soft Matter, 2014, 10, 1224–1232 RSC.
- Thermal Characterization of Polymeric Materials, ed. A. Turi, Academic Pr, New York, 1st edn, 1981 Search PubMed.
- E. Mukouyama and A. Takegawa, High-molecular chemistry (Jap.), 1956, 136(13), 323–330 Search PubMed.
- Y. P. Khanna, W. P. Kuhn and W. J. Sichina, Macromolecules, 1996, 28, 2644–2646 CrossRef.
- J. Buchholz, W. Paul, F. Varnik and K. Binder, Chem. Phys., 2002, 117, 7364–7373 CAS.
- A. V. Lyulin, N. K. Balabaev and M. A. J. Michels, Macromolecules, 2002, 35, 9595–9604 CrossRef CAS.
- V. M. Nazarychev, S. V. Larin, A. V. Yakimansky, N. V. Lukasheva, A. A. Gurtovenko, I. V. Gofman, V. E. Yudin, V. M. Svetlichnyi, J. M. Kenny and S. V. Lyulin, J. Polym. Sci., Part B: Polym. Phys., 2015, 53, 912–923 CrossRef CAS.
- I. Perepechko, Low Temperature Properties of Polymers, MIR Publishers, Moscow, 1980 Search PubMed.
- Comprehensive Nanoscience and Technology, ed. D. Andrews, G. Scholes and G. Wiederrecht, Elsevier, 2010, vol. 5 Search PubMed.
- R. C. Baljon, M. H. M. Van Weert, R. B. DeGraaff and R. Khare, Macromolecules, 2005, 38, 2391–2399 CrossRef.
- CRC Handbook of Chemistry and Physics, ed. R. C. Weast, CRC Press, Boca Raton, 67th edn, 1968 Search PubMed.
- J. Gao and X. Xia, Science, 1992, 258, 631–639 CAS.
- Ch. M. Baker, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2015, 5, 241–254 CrossRef CAS.
- O. Gereben and L. Pusztai, Chem. Phys. Lett., 2011, 507, 80–83 CrossRef CAS.
- E. Laredo, M. Grimau, F. Sánchez and A. Bello, Macromolecules, 2003, 36, 9840–9850 CrossRef CAS.
- E. N. Lushin and R. A. Castro, St. Petersburg State Polytechnical University Journal, Physics and Mathematics, 2013, 182, 90–92 Search PubMed.
- N. Noda, Y.-H. Lee, A. J. Bur, V. M. Prabhu, C. R. Snyder, S. C. Roth and M. McBrearty, Polymer, 2005, 46, 7201–7217 CrossRef CAS.
- M. Turky, A. M. Ghoneim, A. Kyritsis, K. Raftopoulos and M. A. Moussa, J. Appl. Polym. Sci., 2011, 122, 2039–2046 CrossRef.
- G. Williams and D. C. Watts, Trans. Faraday Soc., 1970, 66, 80–85 RSC.
- C. P. Lindsey and G. D. Patterson, J. Chem. Phys., 1980, 73(7), 3348–3357 CrossRef CAS.
- S. W. Provencher, Comput. Phys. Commun., 1982, 27(3), 229–242 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |






