Molybdenum diboride nanoparticles as a highly efficient electrocatalyst for the hydrogen evolution reaction†
Palani R.
Jothi‡
 ac,
Yuemei
Zhang‡
ac,
Yuemei
Zhang‡
 ac,
Jan P.
Scheifers
ac,
Jan P.
Scheifers
 a,
Hyounmyung
Park
a,
Hyounmyung
Park
 abc and
Boniface P. T.
Fokwa
abc and
Boniface P. T.
Fokwa
 *abc
*abc
aDepartment of Chemistry, University of California, Riverside, CA 92521, USA. E-mail: bfokwa@ucr.edu; Web: http://www.fokwalab.ucr.edu
bDepartment of Chemical and Environmental Engineering, University of California, Riverside, CA 92521, USA
cCenter for Catalysis, University of California, Riverside, CA 92521, USA
First published on 7th September 2017
Abstract
Non-noble metal nanomaterials (molybdenum sulfides, phosphides, carbides, and nitrides) have recently emerged as highly active electrocatalysts for the hydrogen evolution reaction (HER). Molybdenum borides in contrast have not been studied for their HER activity at the nanoscale, however, they were recently shown to be already efficient HER catalysts in the bulk (microscale). In this study, we report on the first nanocrystalline molybdenum boride (MoB2) synthesized by a simple, one-step, relatively low temperature (650 °C) and environmentally benign redox-assisted solid state metathesis (SSM) reaction. The obtained MoB2 nanospheres exhibit a low onset overpotential of 154 mV at 10 mA cm−2, a Tafel slope of 49 mV dec−1 and high stability. Furthermore, density functional theory (DFT) calculations show that several surfaces are active and that the optimum evolution of H2 occurs at a hydrogen coverage between 75% and 100% on the B-terminated {001} surface. These experimental and theoretical results open new avenues to design new architectures of inexpensive and highly efficient boron-based HER catalysts, such as boride nanospheres (with maximum active sites) or materials with B-terminated surfaces (e.g. {001} nanosheets of AlB2-type borides or even the recently discovered borophene and related 2D compounds).
Introduction
The development of sustainable, clean energy sources for fuel production and utilization is in urgent demand and a global challenge. Hydrogen (H2) could be one of the cleanest fuels, but it is still mostly produced from fossil fuels. Instead, research has been focused on the production of hydrogen through water electrolysis,1–3via the hydrogen evolution reaction (HER).4,5 However, the main challenge in this electrochemical process remains the replacement of expensive, non-abundant but highly efficient noble metal-based HER catalysts6,7 by earth-abundant and inexpensive ones. During the past decade, a great number of earth-abundant metal catalyst systems8 have been studied both at the bulk and at the nanoscale.8–12Molybdenum (Mo) based inorganic materials such as chalcogenides,13–15 nitrides,16 carbides16–20 and phosphides21–26 have attracted much attention for electrochemical energy systems due to their low-cost, facile nanoscale synthesis, high chemical stability and corrosion resistance. Metal borides have also been considered as potential electrocatalysts for the HER because of their high stability in acidic and basic media.27–32 Recently, Hu et al. reported commercially available polycrystalline molybdenum boride (α-MoB) as an efficient electrocatalyst for the HER.18 Our group has lately synthesized several binary bulk molybdenum borides and discovered that β-MoB and MoB2 are further examples of efficient electrocatalysts for the HER, whereas Mo2B is not as efficient.33 Furthermore, a clear boron-dependency of the HER activity was found in the Mo–B system, and MoB2 was identified as the most efficient catalyst.33 These discoveries hinted at intensifying research in this class of understudied boride materials for their HER activity, especially at the nanoscale. In fact, borides still have not attracted as much attention as the above-mentioned classes of materials because of the difficulties encountered during their synthesis, especially at the nanoscale.34–36
Herein we report a simple, low-temperature synthesis of nanocrystalline molybdenum diboride (MoB2 – containing graphene-like boron layers) and the study of its HER activity in acidic medium. In addition, density functional theory (DFT) calculations were carried out, to explore the distinct HER activities of different surfaces.
Results and discussion
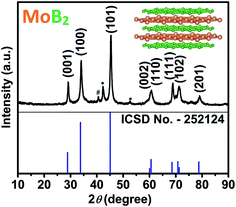
Transition metal borides are refractory materials with melting points usually above 2000 °C, the syntheses of which have traditionally been carried out at extremely high temperatures (e.g. arc melting above 2500 °C), leading exclusively to bulk phases. Kaner et al. have reported an elegant solid-state metathesis (SSM) reaction for the synthesis of a variety of polycrystalline materials including borides.37,38 Most attempts to synthesize transition metal borides at temperatures below 800 °C using several methods (self-propagating synthesis, mechanochemical synthesis, and solution synthesis) have generally been followed by annealing between 900 °C and 1400 °C.35,39–42 Given the high temperatures used for annealing, mainly microscale polycrystalline products are obtained. Therefore, synthesizing these materials at the nanoscale is even more challenging, because the temperature required for such syntheses should typically be below the above-mentioned temperatures. Nevertheless, many synthetic methods have been attempted recently, such as ionothermal flux synthesis, hydrolysis of NaBH4, metal-hydrolysis-assisted synthesis, etc.35,41,43–45 A very recent attempt to synthesize nanocrystalline MoB2 using metal-hydrolysis-assisted synthesis from MoO3 has resulted in elemental molybdenum as the major phase including small amounts of MoB2.45 In this work, a single step solid-state metathesis (SSM) process was successfully applied toward the synthesis of nanocrystalline MoB2 with particle size below 60 nm. High purity powders of anhydrous MoCl5 and MgB2 were mixed in an inert atmosphere and pressed into a pellet. The pellet was placed in a quartz tube, which was then sealed and heated at 650 °C for 24 hours. After cooling to room temperature, the resulting MgCl2 was dissolved in HCl. More details about the synthesis procedure are given in the ESI.† The crystallinity and phase analysis of the remaining product was performed by powder X-ray diffraction (PXRD). As shown in Fig. 1, the diffraction pattern contains high intensity peaks indicating the highly crystalline nature of the synthesized sample. The main peak positions and intensities matched well with the standard positions of hexagonal MoB2 (space group P6/mmm, ICSD no. 252124). The synthesized sample contains the impurity phases of Mo and β-MoB (Fig. 1). The refined lattice parameters of MoB2 are a = 3.044(1) Å and c = 3.071(1) Å and are in excellent agreement with those reported (a = 3.040 Å, and c = 3.072 Å).46,47
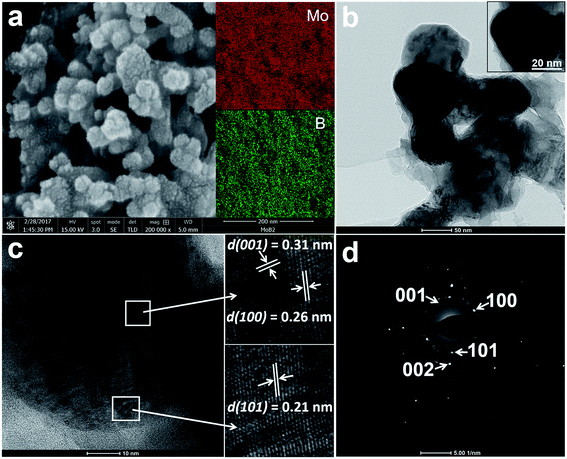
Fig. 2a shows a high-resolution SEM (HRSEM) image of the MoB2 sample. The observed surface morphology reveals the aggregated nanospheres of MoB2. The size of the nanoparticles ranges from 30 nm up to 60 nm. A closer look at the nanoparticle's surface reveals tiny grains (1–3 nm). This is the first observation of such features for nanoscale borides. Recently, a similar pomegranate-like morphology was found in the composite material N, P-doped Mo2C@C (nanospheres), whose high HER activity was attributed to this unique morphology.48 The BET surface area of the MoB2 nanoparticles was measured by N2 adsorption–desorption studies, the result of which shows a surface area of only 14 m2 g−1, suggesting a high degree of aggregation of the nanoparticles. The BET isotherm curve is shown in the ESI (Fig. S1†). The elemental composition and homogeneity of the sample were measured by energy dispersive X-ray spectroscopy (EDS) and inductively coupled plasma optical emission spectrometry (ICP-OES) analysis. The EDS mapping results show homogeneous distribution of the two elements (Fig. 2a inset), and the ICP-OES analysis determined the Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B atomic ratio to be 1
B atomic ratio to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.1, which is in good agreement with the composition of the starting mixture (see the ESI†). This result suggests that an excess of amorphous boron is also present. This is, however, not surprising given the fact that more boron than the stoichiometric value was needed for this synthesis to achieve high yield. This is common for the synthesis of boride nanomaterials as was reported by Portehault et al.43 for the ionothermal synthesis of pure nanocrystalline boride phases; for example, to obtain pure NbB2, an initial Nb/B ratio of 1
5.1, which is in good agreement with the composition of the starting mixture (see the ESI†). This result suggests that an excess of amorphous boron is also present. This is, however, not surprising given the fact that more boron than the stoichiometric value was needed for this synthesis to achieve high yield. This is common for the synthesis of boride nanomaterials as was reported by Portehault et al.43 for the ionothermal synthesis of pure nanocrystalline boride phases; for example, to obtain pure NbB2, an initial Nb/B ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was needed.
4 was needed.
The HRTEM images of the MoB2 sample shown in Fig. 2b–d also confirm the formation of aggregated spherical nanoparticles composed of tiny grains (Fig. 2b). The average size of an individual nanosphere is about 50 nm (Fig. 2b inset). A HRTEM image showing several lattice fringes on a single MoB2 nanosphere is given in Fig. 2c and shows interplanar d-spacings of 0.21 nm, 0.26 nm and 0.31 nm; these d values correspond to {101}, {100} and {001} crystal planes of the hexagonal MoB2 structure (ICSD no. 252124), respectively. The selected area electron diffraction (SAED) pattern, obtained from the MoB2 nanosphere, shows rings composed of diffraction spots (Fig. 2d). This SAED pattern also confirms the polycrystallinity (presence of tiny grains) of the MoB2 nanospheres. These TEM results are consistent with the powder XRD results. High angle annular dark field scanning transmission electron microscopy (HAADF-STEM) images with EDS maps of the MoB2 nanospheres were also successfully collected, and are given in the ESI (Fig. S2†).
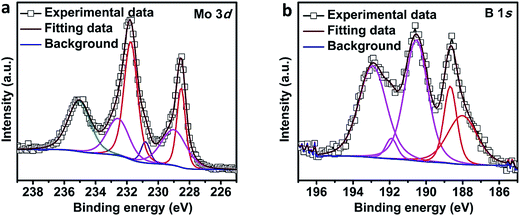
To further characterize the synthesized MoB2, XPS analysis was performed. The full survey spectrum, given in the ESI (Fig. S3†), shows Mo 3d and B 1s peaks but also O 1s and C 1s impurity peaks which are typical features usually observed in XPS surveys of metal borides, carbides and phosphides.18,22,49 The deconvoluted spectra of Mo 3d and B 1s for MoB2 are shown in Fig. 3. The fraction of molybdenum (Mo) metal peaks in the sample was estimated by deconvoluting the Mo 3d spectra (Fig. 3a) to metal Mo0 (228.5 eV & 231.7 eV) and several molybdenum oxides (228.9 eV, 230.8 eV, 232.5 eV and 235.0 eV). The resolved peaks for the B 1s spectra (Fig. 3b) evidence two different boron environments. The lower binding energy values of 188.0 eV and 188.6 eV confirm the B0 state of metal borides and the higher binding energy values (190.5 eV, 191.8 eV and 192.9 eV) were assigned to boron oxide. The higher binding energy peaks in both Mo 3d and B 1s spectra imply surface oxidation of the sample, which is in accordance with those reported for molybdenum based materials including metal borides.18,22,49–51 The details of peak values are given in the ESI (Table S1†).
The HER performance of the MoB2 nanoparticles was investigated in a 0.5 M H2SO4 electrolyte using a standard three electrode configuration (using a graphite rod as the counter electrode). Fig. 4a shows the polarization curves of MoB2 at a scan rate of 1 mV s−1 together with those of commercial 5% Pt/C, molybdenum (Mo) metal, amorphous boron (B) and carbon sheet (CS) shown for comparison. The pure carbon sheets show almost negligible HER activity, while molybdenum and boron are just slightly active, the former being more active than the latter. In contrast, MoB2 shows high activity with a low overpotential of ∼154 mV at 10 mA cm−2versus the reversible hydrogen electrode (RHE). This onset overpotential of MoB2 is not only the smallest recorded for any boride compound to date but also smaller than those reported for pure carbide and nitride nanomaterials under the same conditions.16,18 The observed overpotential has been improved by ca. 150 mV compared with that found for HER active bulk-MoB2 under the same conditions, indicating the importance of achieving nanoscale materials.33 This large activity improvement can be explained by the large electrochemically active surface area, which was obtained from electrochemical capacitive measurements using the cyclic voltammetry (CV) method, through the electrochemical double layer capacitance (Cdl) determination.52 Fig. S4a (see the ESI†) shows the CV curves of MoB2 in the range 0.10–0.20 V vs. the RHE at different scan rates. The measured capacitive currents ΔJ (Ja–Jc) at 0.15 V vs. the RHE were plotted against the scan rate, as shown in Fig. S4b (see the ESI†), and the resulting straight line was fitted, yielding a Cdl value of 27.8 mF cm−2. This value is in the same range as those found for other similar nanomaterials (carbides, sulfides and phosphides).17,52,53 Also, it is drastically higher than that obtained for bulk-MoB2 (0.1 mF cm−2),33 indicating that the spherical nature of the particles is also as suitable for generating active sites as particles with edges and flat faces, as it was recently found for the highly HER active N, P-doped Mo2C@C nanospheres.48 This high activity was achieved for agglomerated nanoparticles having a small BET surface area, and thus the HER activity of this material can be further increased if agglomeration can be prevented, for example through carbon encapsulation of nanoparticles, as it was successfully done for the N, P-doped Mo2C@C nanospheres recently.48 Such synthetic studies are planned.
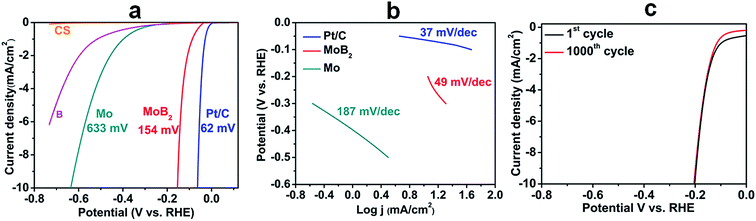 | ||
| Fig. 4 (a) Polarization curves of MoB2, amorphous B, Mo, Pt/C and carbon sheet (CS) measured at a scan rate of 1 mV s−1 in 0.5 M H2SO4 (b) Tafel plots of MoB2, Mo and Pt/C, and (c) electrochemical stability curves of MoB2 nanoparticles for 1000 CV cycles at a scan rate of 100 mV s−1. The IR-drop was corrected (see the ESI†). | ||
The HER reaction mechanism of the catalyst was probed by a Tafel analysis through the estimation of the rate-determining step (RDS). In general, three reactions are used as the RDS of the HER in acidic solutions, namely the Volmer reaction (with a Tafel slope of ∼120 mV dec−1), the Heyrovsky reaction (with a Tafel slope of ∼40 mV dec−1) and the Tafel reaction (with a Tafel slope of ∼30 mV dec−1). The Tafel slope (Fig. 4b) was estimated from the relationship between the potential (V vs. the RHE) and the logarithmic current density (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j), yielding a much smaller value of 49 mV dec−1 compared with those of Mo (187 mV dec−1) and all other Mo-based bulk borides recorded under similar experimental conditions,33 thus contributing to the high HER activity of nanoscale MoB2. The HER reaction mechanism for MoB2 has not been studied yet, but the measured Tafel slope is very close to the values reported for MoP22 and Mo2C samples.18 In these two studies, it was suggested that the HER process could operate via a quick Volmer reaction, followed by a rate-determining Heyrovsky reaction. We think that this mechanism may also apply to our MoB2 sample. The HER performance values of nano-MoB2 are comparable to those found for the best pure Mo-based materials (phosphides, nitrides, carbides, and selenides) reported to date (see the ESI, Table S2†). The electrochemical stability of the MoB2 electrode was also examined using the cyclic voltammograms (CV) obtained at a scan rate of 100 mV s−1 for continuous 1000 cycles, as shown in Fig. 4c. Only a 7% current density drop is observed at an overpotential of 0.2 V after 1000 cycles, thus confirming the high HER stability of the synthesized nano-MoB2 catalyst under acidic conditions.
j), yielding a much smaller value of 49 mV dec−1 compared with those of Mo (187 mV dec−1) and all other Mo-based bulk borides recorded under similar experimental conditions,33 thus contributing to the high HER activity of nanoscale MoB2. The HER reaction mechanism for MoB2 has not been studied yet, but the measured Tafel slope is very close to the values reported for MoP22 and Mo2C samples.18 In these two studies, it was suggested that the HER process could operate via a quick Volmer reaction, followed by a rate-determining Heyrovsky reaction. We think that this mechanism may also apply to our MoB2 sample. The HER performance values of nano-MoB2 are comparable to those found for the best pure Mo-based materials (phosphides, nitrides, carbides, and selenides) reported to date (see the ESI, Table S2†). The electrochemical stability of the MoB2 electrode was also examined using the cyclic voltammograms (CV) obtained at a scan rate of 100 mV s−1 for continuous 1000 cycles, as shown in Fig. 4c. Only a 7% current density drop is observed at an overpotential of 0.2 V after 1000 cycles, thus confirming the high HER stability of the synthesized nano-MoB2 catalyst under acidic conditions.
DFT calculations were applied to explore the active sites and the distinct HER activities of different surfaces. The Gibbs free energy (ΔGH) for atomic hydrogen (H) adsorption on a catalyst surface is commonly used as a descriptor of the HER activity.22,54–57 An optimal HER activity can be achieved at a ΔGH value close to zero, and under these conditions the overall reaction of both H adsorption and H2 desorption has the maximum rate.54 ΔGH was calculated using the equation ΔGH = ΔEH + ΔEZPE − TΔS, where ΔEH is the H-surface binding energy, and ΔEZPE is the zero-point energy difference between adsorbed H and free H2 (ΔEZPE is usually very small, from 0.01 eV to 0.05 eV, hence neglected here).55,56TΔS is obtained by TΔS ≈ −1/2TS0(H2), where S0(H2) = 130.7 J mol−1 K−1 is the entropy of H2 in the gas phase under standard conditions.57 Therefore, ΔGH ≈ ΔEH + 0.20. ΔEH can be calculated from the equation ΔEH = E[surface + nH] − E[surface + (n − 1)H] − 1/2E[H2], where E[H2] is the total energy of a gas phase H2 molecule, and E[surface + nH] and E[surface + (n − 1)H] represent the total energy of n and (n − 1) hydrogen atoms adsorbed on the surface, respectively.
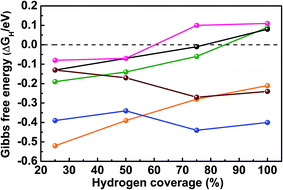
As mentioned above, SEM reveals the spherical morphology of MoB2, an indication of multiple surface terminations. The TEM measurement confirms the polycrystalline nature of the nanospheres (containing tiny grains on the surface) through the identification of several surfaces on a single nanosphere: {001}, {100} and {101}. Therefore, the {001}, {100}, {101} and {110} surfaces of MoB2 were chosen based on the TEM measurements as well as by the semi-empirical Bravais–Friedel–Donnay–Harker (BFDH) method58 to examine the HER activity of MoB2. Overall, ΔEH and ΔGH were calculated for eight different surfaces [Pt {111}, Mo {110}, Mo- and B- terminated MoB2 {001}, Mo- and B- terminated MoB2 {100}, mixed Mo/B MoB2 {101} and mixed Mo/B MoB2 {110} surfaces] at 25% hydrogen coverage (H coverage) to determine the preferred adsorption sites on each surface (see the ESI, Tables S3 and S5†). Three types of adsorption sites were considered: on top of a metal or B atom (T), on a metal–metal or boron–boron bridge site (Bg) and on a hollow (Ho) site (see the ESI, Fig. S5 and S7†). The Gibbs free energy was then examined at different H coverages for the preferred adsorption site of each surface. We have also calculated ΔGH for the Ho Pt {111} site at different H coverages, thus enabling a comparison of our results with the reported ones.55 The complete results of these calculations are given in the ESI (Tables S3–S6 and Fig. S5–S7†) and plotted in Fig. 5.
Fig. 5 shows that the calculated ΔGH value for the Ho Pt {111} surface is −0.13 eV at 25% H coverage, and ΔGH increases with increasing H coverage reaching zero between 75% and 100%, in agreement with previously calculated results.55 As shown in Tables S3 and S5 (see the ESI†) for 25% H coverage, the Ho site on the Mo {110} surface, the Ho site on the Mo-terminated MoB2 {001} surface, the Bg site on the B-terminated MoB2 {001} surface, the Bg2 site on the Mo-terminated MoB2 {100} surface, the T1 site on the B-terminated MoB2 {100} surface, the T2 site on the MoB2 {101} surface and the Ho site on the MoB2 {110} surface are the preferred adsorption sites because of their optimal surface binding energy (large and negative ΔEH). Increasing the H coverage up to 100%, ΔGH values of the B layer, the Mo/B mixed layer and the Pt layer behave similarly, whereas the three Mo-based layers show different behaviors (Fig. 5). In fact, at 25% H coverage on the Mo {110} surface of elemental Mo the hydrogen atoms bond strongly (ΔEH = −0.59 eV), but ΔGH does not vary much as the coverage increases from 25% to 100% (Fig. 5), in accordance with the poor catalytic HER activity of molybdenum. For 25% H coverage on the Mo-terminated MoB2 {001} surface, the hydrogen atoms bind even stronger (ΔEH = −0.72 eV) than on the elemental Mo {110} surface, however ΔGH increases significantly in this case from −0.52 eV at 25% H coverage to −0.21 eV at 100% H coverage, indicating better catalytic activity than that of the elemental Mo {110} surface. The binding energy of 25% H on the Mo-terminated MoB2 {100} surface is similar to that on the Pt {111} surface, but ΔGH decreases as the H coverage increases. Additionally, the ΔGHvs. H coverage behavior on this surface is similar to that of the Mo {110} surface but with smaller ΔGH values (Fig. 5), indicating better catalytic activity than that of the elemental Mo {110} surface. Interestingly, for 25% H coverage ΔEH calculated (−0.39 eV) for the B-terminated MoB2 {001} surface is very close to that of the Ho Pt {111} surface (−0.33 eV). Moreover, as the H coverage increases, ΔGH for the B-terminated MoB2 {001} surface increases similarly to that of the Ho Pt {111} surface (Fig. 5 and ESI Table S4†), reaching zero between 75% and 100% H coverage in both cases. Another highly active site is the Ho site of the MoB2 {110} surface, as its ΔGH stays close to zero at 25% and 50% H coverages and reaches zero between 50% and 75% H coverage (Fig. 5). ΔGH for the T2 site of the MoB2 {101} surface also stays close to zero at 25% and 50% H coverages, but with positive values, indicating good HER activity but lower than that of the MoB2 {110} and the B-terminated MoB2 {001} surfaces (see the ESI, Table S6†).
In general, the B-terminated MoB2 {001} surface (B layer) is the most HER active surface. Furthermore, the B layer and the Mo/B mixed layers (MoB2 {110} and {101} surfaces) are more active than the three Mo layers (Mo {110}, Mo-terminated MoB2 {100} and {001} surfaces). This finding suggests that boron plays a prominent role in the catalytic activity of this compound.
This theoretical investigation shows that several surfaces in MoB2 are HER active; thus the more exposed active surfaces, the better, which helps understand why a spherical morphology may lead to high activity. Nevertheless, the B-terminated {001} surface and the Mo/B mixed {110} surface are the most active, indicating that growing nanosheets of MoB2 with a B-terminated {001} surface or MoB {110} surface may lead to even higher activity, whereas nanorods grown along the [001] direction may be less active.
In summary, nanocrystalline molybdenum diboride (MoB2) has been successfully synthesized for the first time using a simple, low temperature, environmentally benign synthesis method, and studied as an electrocatalyst for the HER in acidic medium. The obtained MoB2 nanoparticles exhibit the best HER performance in terms of onset overpotential, current density and electrochemical stability for any boride studied to date and are comparable to the best pure nanomaterials reported (carbides, nitrides, sulfides and phosphides). This study suggests that this synthesis method can be extended to other nanoscale transition metal borides. In addition, the DFT calculations indicate that several surfaces are active in MoB2, but that the B–B bridge site on the B-terminated MoB2 {001} surface (graphene-like boron layer) is the most active site, and it exhibits a zero Gibbs free energy (ΔGH) at H coverage between 75% and 100%, similar to the Pt {111} surface.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank UC Riverside (startup fund to BPTF) for financial support. We thank the San Diego Supercomputer Center (SDSC) and the High-Performance Computing Center (HPCC) at UC Riverside for providing computing resources. The XPS data were collected with an instrument acquired through the NSF MRI program (DMR-0958796). We are grateful to the Conley group (UC Riverside) for the BET measurements and to Prof. Krassimir Bozhilov (Director of CFAMM at UC Riverside) for the TEM measurements and insightful discussion.References
- M. H. Miles, J. Electroanal. Chem., 1975, 60, 89–96 CrossRef CAS.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS PubMed.
- P. C. K. Vesborg, B. Seger and I. Chorkendorff, J. Phys. Chem. Lett., 2015, 6, 951–957 CrossRef CAS PubMed.
- J. Greeley and N. M. Markovic, Energy Environ. Sci., 2012, 5, 9246–9256 CAS.
- J. B. Goodenough, J. Solid State Electrochem., 2012, 16, 2019–2029 CrossRef CAS.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- M. Wang, L. Chen and L. C. Sun, Energy Environ. Sci., 2012, 5, 6763–6778 CAS.
- M. S. Faber and S. Jin, Energy Environ. Sci., 2014, 7, 3519–3542 CAS.
- J. M. McEnaney, J. C. Crompton, J. F. Callejas, E. J. Popczun, C. G. Read, N. S. Lewis and R. E. Schaak, Chem. Commun., 2014, 50, 11026–11028 RSC.
- E. J. Popczun, C. W. Roske, C. G. Read, J. C. Crompton, J. M. McEnaney, J. F. Callejas, N. S. Lewis and R. E. Schaak, J. Mater. Chem. A, 2015, 3, 5420–5425 CAS.
- H. Li, C. Tsai, A. L. Koh, L. Cai, A. W. Contryman, A. H. Fragapane, J. Zhao, H. S. Han, H. C. Manoharan and F. Abild-Pedersen, Nat. Mater., 2016, 15, 48–53 CrossRef CAS PubMed.
- J. Hu, B. L. Huang, C. X. Zhang, Z. L. Wang, Y. M. An, D. Zhou, H. Lin, M. K. H. Leung and S. H. Yang, Energy Environ. Sci., 2017, 10, 593–603 CAS.
- F. H. Saadi, A. I. Carim, J. M. Velazquez, J. H. Baricuatro, C. C. L. McCrory, M. P. Soriaga and N. S. Lewis, ACS Catal., 2014, 4, 2866–2873 CrossRef CAS.
- W. F. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896–8909 RSC.
- L. Liao, S. N. Wang, J. J. Xiao, X. J. Bian, Y. H. Zhang, M. D. Scanlon, X. L. Hu, Y. Tang, B. H. Liu and H. H. Girault, Energy Environ. Sci., 2014, 7, 387–392 CAS.
- H. Vrubel and X. L. Hu, Angew. Chem., Int. Ed., 2012, 51, 12703–12706 CrossRef CAS PubMed.
- C. Wan, Y. N. Regmi and B. M. Leonard, Angew. Chem., Int. Ed., 2014, 53, 6407–6410 CrossRef CAS PubMed.
- Z. Xing, Q. Liu, A. M. Asiri and X. Sun, Adv. Mater., 2014, 26, 5702–5707 CrossRef CAS PubMed.
- X. Chen, D. Wang, Z. Wang, P. Zhou, Z. Wu and F. Jiang, Chem. Commun., 2014, 50, 11683–11685 RSC.
- P. Xiao, M. A. Sk, L. Thia, X. M. Ge, R. J. Lim, J. Y. Wang, K. H. Lim and X. Wang, Energy Environ. Sci., 2014, 7, 2624–2629 CAS.
- J. F. Callejas, C. G. Read, C. W. Roske, N. S. Lewis and R. E. Schaak, Chem. Mater., 2016, 28, 6017–6044 CrossRef CAS.
- J. M. McEnaney, J. C. Crompton, J. F. Callejas, E. J. Popczun, A. J. Biacchi, N. S. Lewis and R. E. Schaak, Chem. Mater., 2014, 26, 4826–4831 CrossRef CAS.
- W. Cui, Q. Liu, Z. Xing, A. M. Asiri, K. A. Alamry and X. Sun, Appl. Catal., B, 2015, 164, 144–150 CrossRef CAS.
- W. Cui, N. Cheng, Q. Liu, C. Ge, A. M. Asiri and X. Sun, ACS Catal., 2014, 4, 2658–2661 CrossRef CAS.
- M. D. Scanlon, X. J. Bian, H. Vrubel, V. Amstutz, K. Schenk, X. L. Hu, B. H. Liu and H. H. Girault, Phys. Chem. Chem. Phys., 2013, 15, 2847–2857 RSC.
- S. Gupta, N. Patel, A. Miotello and D. C. Kothari, J. Power Sources, 2015, 279, 620–625 CrossRef CAS.
- P. Los and A. Lasia, J. Electroanal. Chem., 1992, 333, 115–125 CrossRef CAS.
- M. Zeng, H. Wang, C. Zhao, J. Wei, K. Qi, W. Wang and X. Bai, ChemCatChem, 2016, 8, 708–712 CrossRef CAS.
- S. J. Sitler, K. S. Raja and I. Charit, J. Electrochem. Soc., 2016, 163, H1069–H1075 CrossRef CAS.
- J. Masa, P. Weide, D. Peeters, I. Sinev, W. Xia, Z. Sun, C. Somsen, M. Muhler and W. Schuhmann, Adv. Energy Mater., 2016, 6, 1502313 CrossRef.
- H. Park, A. Encinas, J. P. Scheifers, Y. Zhang and B. P. T. Fokwa, Angew. Chem., Int. Ed., 2017, 56, 5575–5578 CrossRef CAS PubMed.
- B. Albert and H. Hillebrecht, Angew. Chem., Int. Ed., 2009, 48, 8640–8668 CrossRef CAS PubMed.
- S. Carenco, D. Portehault, C. Boissiere, N. Mezailles and C. Sanchez, Chem. Rev., 2013, 113, 7981–8065 CrossRef CAS PubMed.
- B. P. T. Fokwa, Borides: Solid State Chemistry. Encyclopedia of Inorganic and Bioinorganic Chemistry, ed. R. A. Scott, John Wiley & Sons, Ltd, Chichester, U.K., 2014 Search PubMed.
- J. B. Wiley and R. B. Kaner, Science, 1992, 255, 1093–1097 CAS.
- L. Rao, E. G. Gillan and R. B. Kaner, J. Mater. Res., 1995, 10, 353–361 CrossRef CAS.
- M. Jha, K. V. Ramanujachary, S. E. Lofland, G. Gupta and A. K. Ganguli, Dalton Trans., 2011, 40, 7879–7888 RSC.
- M. Jha, R. Patra, S. Ghosh and A. K. Ganguli, J. Mater. Chem., 2012, 22, 6356–6366 RSC.
- C. Kapfenberger, K. Hofmann and B. Albert, Solid State Sci., 2003, 5, 925–930 CrossRef CAS.
- C. Petit and M. P. Pileni, J. Magn. Magn. Mater., 1997, 166, 82–90 CrossRef CAS.
- D. Portehault, S. Devi, P. Beaunier, C. Gervais, C. Giordano, C. Sanchez and M. Antonietti, Angew. Chem., Int. Ed., 2011, 50, 3262–3265 CrossRef CAS PubMed.
- G. Gouget, P. Beaunier, D. Portehault and C. Sanchez, Faraday Discuss., 2016, 191, 511–525 RSC.
- L. Zhou, L. Yang, L. Shao, B. Chen, F. Meng, Y. Qian and L. Xu, Inorg. Chem., 2017, 56, 2440–2447 CrossRef CAS PubMed.
- E. Storms and B. Mueller, J. Phys. Chem., 1977, 81, 318–324 CrossRef CAS.
- Q. Tao, X. Zhao, Y. Chen, J. Li, Q. Li, Y. Ma, J. Li, T. Cui, P. Zhu and X. Wang, RSC Adv., 2013, 3, 18317–18322 RSC.
- Y.-Y. Chen, Y. Zhang, W.-J. Jiang, X. Zhang, Z. Dai, L.-J. Wan and J.-S. Hu, ACS Nano, 2016, 10, 8851–8860 CrossRef CAS PubMed.
- G. Mavel, J. Escard, P. Costa and J. Castaing, Surf. Sci., 1973, 35, 109–116 CrossRef CAS.
- B. Feng, J. Zhang, Q. Zhong, W. Li, S. Li, H. Li, P. Cheng, S. Meng, L. Chen and K. Wu, Nat. Chem., 2016, 8, 563–568 CrossRef CAS PubMed.
- T. T. Xu, J. G. Zheng, N. Q. Wu, A. W. Nicholls, J. R. Roth, D. A. Dikin and R. S. Ruoff, Nano Lett., 2004, 4, 963–968 CrossRef CAS.
- M. A. Lukowski, A. S. Daniel, F. Meng, A. Forticaux, L. Li and S. Jin, J. Am. Chem. Soc., 2013, 135, 10274–10277 CrossRef CAS PubMed.
- Y.-Y. Ma, C.-X. Wu, X.-J. Feng, H.-Q. Tan, L.-K. Yan, Y. Liu, Z.-H. Kang, E.-B. Wang and Y.-G. Li, Energy Environ. Sci., 2017, 10, 788–798 CAS.
- Y. Zheng, Y. Jiao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2015, 54, 52–65 CrossRef CAS PubMed.
- E. Skúlason, V. Tripkovic, M. E. Björketun, S. Gudmundsdottir, G. Karlberg, J. Rossmeisl, T. Bligaard, H. Jónsson and J. K. Nørskov, J. Phys. Chem. C, 2010, 114, 18182–18197 Search PubMed.
- Q. Tang and D.-e. Jiang, ACS Catal., 2016, 6, 4953–4961 CrossRef CAS.
- P. W. Atkins, Physical Chemistry, Freeman and Company, New York, 3rd edn, 1985 Search PubMed.
- R. Docherty, G. Clydesdale, K. J. Roberts and P. Bennema, J. Phys. D: Appl. Phys., 1991, 24, 89–99 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00397h |
| ‡ P. R. J. and Y. Z. contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |