Enhanced catalytic activity and stability of Pt nanoparticles by surface coating of nanosized graphene oxide for hydrogen production from hydrolysis of ammonia–borane†
Wanyue
Ye
 ,
Yuzhen
Ge
,
Zhanming
Gao
,
Rongwen
Lu
* and
Shufen
Zhang
,
Yuzhen
Ge
,
Zhanming
Gao
,
Rongwen
Lu
* and
Shufen
Zhang

State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, People's Republic of China. E-mail: lurw@dlut.edu.cn
First published on 18th September 2017
Abstract
We report the electronic modification of silica supported Pt nanoparticles (SiO2@Pt) by coating a 1 nm thin layer of nanosized graphene oxide (NGO). The resulting SiO2@Pt@NGO showed much enhanced catalytic activity and stability for hydrogen production from hydrolysis of ammonia–borane compared with SiO2@Pt and graphene supported Pt nanoparticles, with an impressive initial TOF value reaching 324.6 molH2 molPt−1 min−1. Detailed characterization by means of HRTEM and EDS elemental mapping proved the structural correctness of SiO2@Pt@NGO. The XPS results showed that the binding energy of Pt0 4f7/2 in SiO2@Pt@NGO was 71.12 eV slightly higher than 70.84 eV of Pt0 4f7/2 in SiO2@Pt, indicating more electron-deficient Pt atoms after the interaction with NGO, which may be responsible for the enhanced catalytic performance.
Introduction
Hydrogen is regarded as one of the most ideal energy carriers for future fuel applications. It has attracted much attention and been considered to be a promising candidate for fulfilling the ever-growing demand for clean and sustainable energy sources.1 Besides the advantages like high energy density and green combustion products in air, the efficiently controlled storage and release of hydrogen under ambient conditions are still challenging to fully realize “the hydrogen economy”.2–4 Recently, ammonia–borane (AB) has garnered increased attention as a potential solid hydrogen storage material, due to its high hydrogen weight density (19.6 wt%, which is much higher than the US Department of Energy 2020 targets for gravimetric hydrogen densities of 5.5 wt%),5 ease of transportation, and impressive chemical and thermal stabilities.2 Under ideal conditions and in the presence of suitable catalysts, the hydrolysis of one mole of AB can generate three equivalents of H2 according to the following equation:6| NH3BH3 + 2H2O → NH4+ + BO2− + 3H2 | (1) |
The synthesis of an efficient catalyst for catalytic hydrolysis of AB is a highly vital area of research. Noble metals such as Pt,7 Ru,8 Rh9, Pd10etc. have been reported to be the most active elements for the catalytic hydrolysis of AB, and some of the recently reported noble metal based catalysts even achieved turnover frequency (TOF) values of >1000 molH2 molcat−1 min−1 but usually had poor durability in several cycles.9,11 Besides the active components, supports also play an important role in determining the final performance of catalysts, which has been a long-standing issue in the field of catalysis. Carbon materials such as carbon nanotubes (CNTs), porous carbons, metal–organic frameworks (MOFs) and graphene have been widely investigated as the most attractive supports of catalysts for this reaction on account of their excellent chemical and physical properties.5 Especially, graphene has been exploited as an excellent support benefited from its synergistic effect with metallic catalysts. It acts as an electron acceptor due to the fact that the integration between graphene and metallic catalysts can markedly enhance the catalytic activity.12–14 For example, Xu et al. reported monodispersed Pt nanoparticles supported on reduced graphene oxide which showed high catalytic activity toward hydrogen generation from hydrolysis of AB with the initial TOF value reaching 284 molH2 molPt−1 min−1.15 Lu et al. reported highly dispersed Pt–CeO2 hybrids arched on reduced graphene oxide achieving the initial TOF value of 93.8 molH2 molPt−1 min−1.7 Nonetheless, graphene as a catalyst support has some demerits such as difficulty in recycling, higher cost, etc. Also, the mechanism of the improved performance of graphene supported catalysts still needs further study.16
Herein, we report the preparation of nanosized graphene oxide (NGO) coated SiO2@Pt nanocatalysts (SiO2@Pt@NGO) which showed much enhanced activity for hydrogen production from hydrolysis of AB compared with SiO2@Pt and graphene supported Pt nanoparticles. The HRTEM images of SiO2@Pt@NGO showed the tight interaction between the uniformly dispersed Pt nanoparticles, SiO2 and graphene. The XPS results showed that the binding energy of Pt0 4f7/2 in SiO2@Pt@NGO was slightly higher than that in SiO2@Pt, indicating more electron-deficient Pt atoms, which were favourable for the catalysis of the hydrolysis reaction. The support of SiO2 benefited the dispersion of Pt nanoparticles and the recycling of catalysts after the reaction, and the protection of SiO2 and NGO prevented the aggregation of Pt nanoparticles, which showed better durability of catalysts during the recycling.
Results and discussion
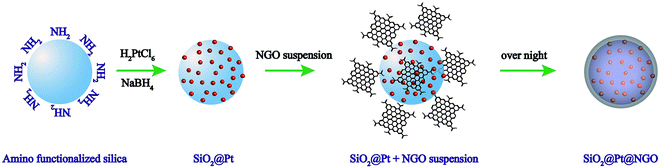
The procedure for the preparation of SiO2@Pt@NGO is presented in Scheme 1 (see the ESI for the experimental details†). Amino-functionalized silica nanoparticles (AFSN) with an average size of 60 nm were first synthesized by a reverse-microemulsion method. After the redispersion of AFSN in an aqueous solution of H2PtCl6, the Pt4+ ions coordinated with the surface amino-groups and then reduced by freshly prepared NaBH4 aqueous solution, generating uniformly supported Pt nanoparticles (SiO2@Pt). Nanosized graphene oxide (NGO) was prepared by a two-step oxidation process.17 Large-sized graphene oxide (LGO, micrometer scale) was synthesized by a modified Hummers method,18,19 and then nanosized graphene oxide (NGO) was prepared by the further oxidation of LGO. For the preparation of SiO2@Pt@NGO, the as-prepared NGO solution was directly added dropwise into the suspension of SiO2@Pt, and the product was separated by centrifugation after stirring overnight and washed with water several times to remove the acid.SiO2@Pt with Pt loadings of 1 wt%, 2 wt%, 5 wt% and 10 wt% was synthesized by using different concentrations of H2PtCl6 aqueous solutions, and the corresponding TEM images are shown in Fig. S1.† Pt nanoparticles were uniformly dispersed on the surface of AFSN but some aggregates emerged as the loading of Pt increased to 5 wt% and 10 wt%. The corresponding XRD patterns are presented in Fig. S2,† and the four characteristic peaks at (2θ =) 39.8°, 46.2°, 67.5° and 81.28° can be indexed to the (111), (200), (220) and (311) planes of face-centered cubic Pt (JCPDS #65-2868), and also the intensity of diffraction peaks increased with the increase in Pt loading. The catalytic activity of SiO2@Pt for the hydrogen production from hydrolysis of AB was tested, and the normalized hydrogen evolution curves are shown in Fig. S3.† With the increase of Pt loading, the activity first increased and then decreased. SiO2@Pt with a Pt loading of 2 wt% showed the best activity and only half the time was needed for the complete hydrogen generation from hydrolysis of AB compared with SiO2@Pt with a Pt loading of 10 wt%. This phenomenon can be attributed to the electronic properties and geometric properties of Pt nanoparticles with different sizes.20 Thus, we chose SiO2@Pt with a Pt loading of 2 wt% as the material for the following study, and the content of Pt in SiO2@Pt@NGO with different loadings was analyzed by ICP-AES (Table S1†).
In the early reports, graphene supported metallic catalysts showed excellent activity for hydrogen production from hydrolysis of AB.12 However, the preparation of graphene directly supported Pt nanoparticles for this reaction has been rarely reported and the reason for the activity improvement by using graphene as the support of catalysts is still not clear. In a recent report, Sun et al. prepared a graphene supported single atom Pt catalyst, which showed outstanding activity for the hydrogen evolution reaction in water splitting. They ascribed the better performance of the catalyst to the partially unoccupied density of states of the Pt atoms by combining the X-ray absorption fine structure and density functional theory analysis.21 Ma et al. also reported that electron-deficient Pt nanoparticles usually exhibit better catalytic performance in many important reactions like water-gas shift reactions.22 In this report, we tried to coat the as-prepared SiO2@Pt nanoparticles with very thin graphene oxide sheets and studied the effect of the graphene coating on the electronic structure changes of the Pt nanoparticles and the resulting catalytic performance.
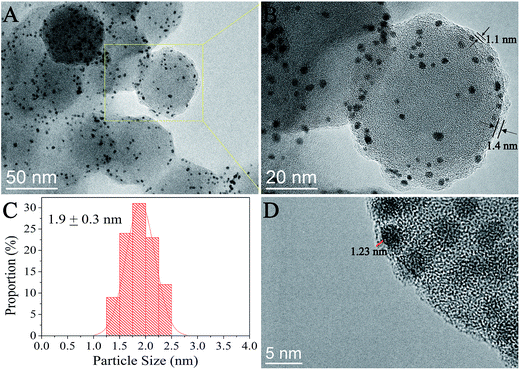
The structure of the as-prepared NGO was first characterized by TEM and AFM and the results are shown in Fig. S4.† The TEM images indicated most of the NGO with a lateral size of ∼100 nm which was consistent with the AFM results, and the AFM results also showed that the thickness of NGO was 0.7 nm and 1.3 nm corresponding to 1–2 layers of graphene.23–25Fig. 1 shows the HRTEM images of the as-prepared SiO2@Pt@NGO with a NGO loading of 2 wt%; the Pt nanoparticles with an average size of 1.9 nm were uniformly dispersed on the surface of AFSN after the coating of NGO. The thickness of the coating was about ∼1 nm from the HRTEM image (Fig. 1B and D), corresponding to 1–2 layers of NGO. Meanwhile, the Pt nanoparticles were well restricted between AFSN and graphene oxide, making these small Pt particles more stable. The loading and thickness of the NGO layer could be easily controlled by adding different volumes of the as-prepared NGO solution in a certain concentration.
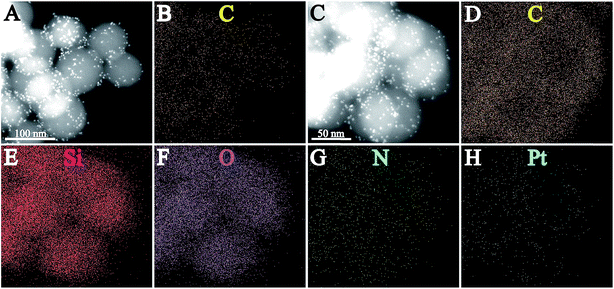
Fig. 2 shows the high-angle annular dark field (HAADF) STEM images and EDX elemental mapping of the as-prepared SiO2@Pt and SiO2@Pt@NGO. The bright dots in Fig. 2A represent the generated Pt nanoparticles on the surface of SiO2@Pt, and the corresponding elemental mapping result (Fig. 2B) indicates well distributed element carbon in the whole sample region which can be attributed to the signal of carbon in aminopropyl groups. Fig. 2C–H show the HAADF-STEM image and elemental distribution of C, Si, O, N, and Pt in SiO2@Pt@NGO; signals for all elements match well with the selected sample region. In particular, the image of element Pt indicates highly dispersed Pt nanoparticles. Meanwhile, the signal intensity for element C in SiO2@Pt@NGO was much stronger than that in SiO2@Pt, implying the higher content of carbon on the surface of SiO2@Pt@NGO and the successful coating of NGO on the surface of SiO2@Pt, which was consistent with the Raman spectra of the comparison between GO and SiO2@Pt@NGO (Fig. S6†).
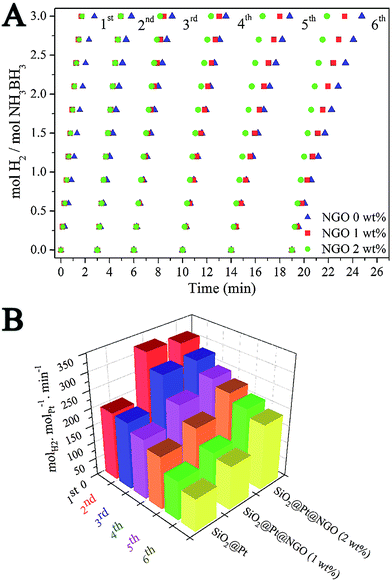
The catalytic activity of SiO2@Pt and SiO2@Pt@NGO for hydrogen production from hydrolysis of AB was tested and the results are shown in Fig. 3. The complete hydrolysis time of AB for SiO2@Pt was 2.75 min with the TOF value of 196.9 molH2 molPt−1 min−1. After coating with NGO, the activity of SiO2@Pt@NGO was markedly enhanced even with the NGO coating amount as low as 0.5 wt%, and the reaction can be completed in 1.5 min with the TOF value reaching 324.6 molH2 molPt−1 min−1, which is much higher than most of the recently reported Pt based catalysts (Table S2†). The activity was maintained as the coating amount of NGO reaching 1.0 wt% and 2.0 wt%, but decreased when the coating amount of NGO reaching 5 wt%, which may be attributed to the prevention of the diffusion of AB molecules to hydrolyze on the surface of the Pt nanoparticles by the thick NGO shell. For comparison, Pt/LGO, in which Pt nanoparticles are directly supported on graphene oxide with the same loading of Pt, was also synthesized and evaluated its catalytic activity. The TEM images of Pt/LGO are shown in Fig. S7;† the average sizes of the Pt nanoparticles were 2.6 ± 0.4 nm, which were slightly larger than those in SiO2@Pt@NGO without the distribution effect of amino groups. The complete hydrolysis of AB by Pt/LGO was achieved in 4 min, with the TOF value of 129.8 molH2 molPt−1 min−1 which was much lower than those of SiO2@Pt and SiO2@Pt@NGO, indicating the synergistic effect between AFSN, Pt nanoparticles and the surface graphene coating.
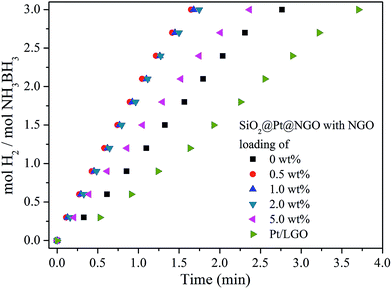 | ||
| Fig. 3 The hydrogen evolution curves for catalytic hydrolysis of AB by SiO2@Pt@NGO with different NGO loadings and Pt/LGO. | ||
To explain the synergistic effect between SiO2@Pt and the NGO coating and the influence of the NGO coating on the electronic structure changes of Pt nanoparticles, XPS was used to further study the surface elemental composition and the corresponding chemical status of SiO2@Pt and SiO2@Pt@NGO with different coating amounts of NGO, and the results are shown in Fig. 4. As shown in Fig. 4A, the spectrum of SiO2@Pt displays two distinct peaks for element Pt with binding energies of 70.84 eV and 74.17 eV, which can be assigned to Pt 4f7/2 and Pt 4f5/2 of metallic Pt, respectively. With the increase of the NGO coating amount, the binding energies of Pt 4f7/2 and Pt 4f5/2 for Pt0 were slightly shifted to 71.12 eV and 74.45 eV, implying the presence of more electron-deficient Pt atoms after the coating of NGO. The tiny changes in the electronic status of Pt atoms benefited the facilitation of the hydrolysis of AB. Besides the existence of Pt0, a minor amount of Pt2+ can also be detected for all samples with the binding energy of 72.37 eV and 75.70 eV for Pt 4f7/2 and Pt 4f5/2. Fig. 4B shows the corresponding XPS spectra for element C; the binding energy of 286.25 eV can be ascribed to the C in –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O as there were a certain number of functional groups like –C
O as there were a certain number of functional groups like –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH in NGO prepared by the modified Hummers method. Meanwhile, the signal intensity increased with the increasing coating amount of NGO on the surface of SiO2@Pt, which also proved the successful preparation of SiO2@Pt@NGO.
O and –COOH in NGO prepared by the modified Hummers method. Meanwhile, the signal intensity increased with the increasing coating amount of NGO on the surface of SiO2@Pt, which also proved the successful preparation of SiO2@Pt@NGO.
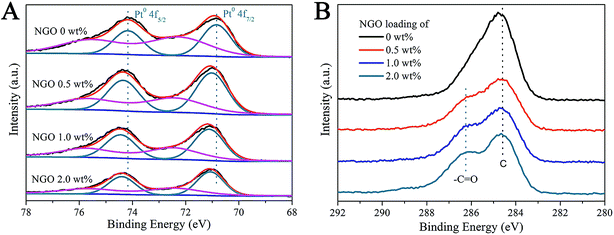 | ||
| Fig. 4 XPS spectra of (A) element Pt and (B) element C in SiO2@Pt@NGO with different coating amounts of NGO. | ||
In the previous reports, most of the graphene oxides used for the support of metallic catalysts were in micrometer scales,26–28 and the preparation of a nanosized graphene oxide supported catalyst has been rarely reported. The main reasons lie in the complexity of the preparation of NGO and the difficulty of the separation of catalyst/NGO from the preparation solutions and reaction solutions. While, spreading nanosized graphene oxide sheets on the silica nanosphere can not only availably prevent crimping and folding during the catalytic process, but can also make them easily collected by centrifugation. For comparison, large scale graphene oxide (in micrometer scale) coated SiO2@Pt (SiO2@Pt@LGO) was prepared by the same method and tested the corresponding catalytic activity for hydrolysis of AB. The TEM images of the as-prepared SiO2@Pt@NGO and SiO2@Pt@LGO are shown in Fig. S8A and B.† Different from the uniformly dispersed SiO2@Pt@NGO nanoparticles, the as-prepared SiO2@Pt@LGO nanoparticles were cross-linked with each other by LGO. Due to the tension of LGO, the Pt nanoparticles were dissociated from the surface of silica and wrapped by LGO. Fig. S8C† shows the hydrogen evolution curves for the same amount of SiO2@Pt@NGO and SiO2@Pt@LGO catalyzing hydrogen production from AB, and the result indicated that the completion of reaction for SiO2@Pt@LGO was achieved in 4 min, which was much longer than that of SiO2@Pt@NGO. The main reasons for such phenomena could be ascribed to the aggregated Pt nanoparticles, which decreased the number of active sites and the wrinkled LGO, which covered the surface of the Pt nanoparticles preventing the adsorption and activation of AB molecules.
The durability of catalysts plays an important role in determining their practical applications. Fig. 5A shows the hydrogen evolution curves of SiO2@Pt and SiO2@Pt@NGO in six runs for catalytic hydrolysis of AB. Fig. 5B shows the corresponding TOF values, which were calculated from the curves in Fig. 5A. The detailed recycling procedure could be found in the Experimental section (ESI†). The completion of reaction for SiO2@Pt took 2.75 min in the first run and 5.75 min in the sixth run, with the corresponding TOF value decreasing from 196.9 molH2 molPt−1 min−1 to 94.6 molH2 molPt−1 min−1. After the coating of 1 wt% NGO, the initial TOF value of SiO2@Pt@NGO was markedly increased to 324.6 molH2 molPt−1 min−1 in the first run and decreased to 126 molH2 molPt−1 min−1 in the sixth run. By increasing the coating amount of NGO to 2 wt%, the initial TOF value of SiO2@Pt@NGO was maintained as 311.6 molH2 molPt−1 min−1 in the first run and decreased to 189.7 molH2 molPt−1 min−1 in the sixth run, which is almost the same as the initial TOF value of SiO2@Pt, indicating that the coating of NGO can efficiently enhance the stability of the catalyst. The improvement of the stability and activity of catalysts was benefited from the confinement of Pt nanoparticles by NGO and AFSN preventing the aggregation and loss of Pt nanoparticles during the recycling test which can be proved by the TEM images of the catalysts after the durability test (Fig. S11†).
Conclusions
In summary, silica supported uniformly dispersed Pt nanoparticles were successfully coated by very few layers of nanosized graphene oxide. The as-prepared SiO2@Pt@NGO showed much enhanced activity and stability for hydrogen production from hydrolysis of ammonia–borane compared with SiO2@Pt. The initial TOF value of SiO2@Pt@NGO can reach 324.6 molH2 molPt−1 min−1 even with the NGO loading as low as 0.5 wt%. The enhanced catalytic performance of SiO2@Pt@NGO could be attributed to the synergistic effect among NGO, Pt nanoparticles and AFSN, especially the modified electronic structure of Pt nanoparticles by NGO coating, which has been proved by XPS. Finally, the coating of NGO prevented the loss and aggregation of Pt nanoparticles during the durability test. After six times of recycling, the TOF value of SiO2@Pt@NGO can still be maintained at 189.7 molH2 molPt−1 min−1 when the NGO loading was 2 wt%. We hope that our new findings may provide an alternative method for the future design of highly efficient catalysts and extend the use of graphene as an electron modifier and also as a protector of metallic catalysts.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is supported by the Joint Funds of the National Natural Science Foundation of China (U1608223), the National Natural Science Foundation of China (21576044, 21536002), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (21421005), and the Dalian University of Technology Innovation Team (DUT2016TB12).References
- F.-Z. Song, Q.-L. Zhu and Q. Xu, Monodispersed PtNi nanoparticles deposited on diamine-alkalized graphene for highly efficient dehydrogenation of hydrous hydrazine at room temperature, J. Mater. Chem. A, 2015, 3(46), 23090–23094 CAS.
- K. Feng, J. Zhong, B. Zhao, H. Zhang, L. Xu, X. Sun and S. T. Lee, CuxCo1-xO nanoparticles on graphene oxide as a synergistic catalyst for high-efficiency hydrolysis of ammonia–borane, Angew. Chem., Int. Ed., 2016, 55(39), 11950–11954 CrossRef CAS PubMed.
- L. Barreto, A. Makihira and K. Riahi, The hydrogen economy in the 21st century: A sustainable development scenario, Int. J. Hydrogen Energy, 2003, 28(3), 267–284 CrossRef CAS.
- Y. Ge, Z. H. Shah, X.-J. Lin, R. Lu, Z. Liao and S. Zhang, Highly efficient Pt decorated CoCu bimetallic nanoparticles protected in silica for hydrogen production from ammonia–borane, ACS Sustainable Chem. Eng., 2016, 5(2), 1675–1684 CrossRef.
- W.-W. Zhan, Q.-L. Zhu and Q. Xu, Dehydrogenation of ammonia borane by metal nanoparticle catalysts, ACS Catal., 2016, 6(10), 6892–6905 CrossRef CAS.
- Q. Xu and M. Chandra, A portable hydrogen generation system: catalytic hydrolysis of ammonia–borane, J. Alloys Compd., 2007, 446–447, 729–732 CrossRef CAS.
- Q. Yao, Y. Shi, X. Zhang, X. Chen and Z. H. Lu, Facile synthesis of platinum-cerium(IV) oxide hybrids arched on reduced graphene oxide catalyst in reverse micelles with high activity and durability for hydrolysis of ammonia borane, Chem.–Asian J., 2016, 11(22), 3251–3257 CrossRef CAS PubMed.
- C. Du, Q. Ao, N. Cao, L. Yang, W. Luo and G. Cheng, Facile synthesis of monodisperse ruthenium nanoparticles supported on graphene for hydrogen generation from hydrolysis of ammonia borane, Int. J. Hydrogen Energy, 2015, 40(18), 6180–6187 CrossRef CAS.
- S. Akbayrak, Y. Tonbul and S. Özkar, Ceria supported rhodium nanoparticles: superb catalytic activity in hydrogen generation from the hydrolysis of ammonia borane, Appl. Catal., B, 2016, 198, 162–170 CrossRef CAS.
- S. Akbayrak, M. Kaya, M. Volkan and S. Özkar, Palladium(0) nanoparticles supported on silica-coated cobalt ferrite: a highly active, magnetically isolable and reusable catalyst for hydrolytic dehydrogenation of ammonia borane, Appl. Catal., B, 2014, 147, 387–393 CrossRef CAS.
- Y. Ge, W. Ye, Z. H. Shah, X. Lin, R. Lu and S. Zhang, PtNi/NiO clusters coated by hollow silica: novel design for highly efficient hydrogen production from ammonia-borane, ACS Appl. Mater. Interfaces, 2017, 9(4), 3749–3756 CAS.
- J. Wang, Y.-L. Qin, X. Liu and X.-B. Zhang, In situ synthesis of magnetically recyclable graphene-supported Pd@Co core–shell nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane, J. Mater. Chem., 2012, 22(25), 12468 RSC.
- P. D. Tran, S. K. Batabyal, S. S. Pramana, J. Barber, L. H. Wong and S. C. Loo, A cuprous oxide-reduced graphene oxide (Cu2O-rGO) composite photocatalyst for hydrogen generation: employing rGO as an electron acceptor to enhance the photocatalytic activity and stability of Cu2O, Nanoscale, 2012, 4(13), 3875–3878 RSC.
- K. Zhou, Y. Zhu, X. Yang, X. Jiang and C. Li, Preparation of graphene–TiO2 composites with enhanced photocatalytic activity, New J. Chem., 2011, 35(2), 353–359 RSC.
- Y. Chen, X. Yang, M. Kitta and Q. Xu, Monodispersed Pt nanoparticles on reduced graphene oxide by a non-noble metal sacrificial approach for hydrolytic dehydrogenation of ammonia borane, Nano Res., 2017 DOI:10.1007/s12274-017-1593-4.
- A. Morais, C. Longo, J. R. Araujo, M. Barroso, J. R. Durrant and A. F. Nogueira, Nanocrystalline anatase TiO2/reduced graphene oxide composite films as photoanodes for photoelectrochemical water splitting studies: the role of reduced graphene oxide, Phys. Chem. Chem. Phys., 2016, 18(4), 2608–2616 RSC.
- H.-i. Kim, G.-h. Moon, D. Monllor-Satoca, Y. Park and W. Choi, Solar photoconversion using graphene/TiO2 composites: nanographene shell on TiO2 core versus TiO2 nanoparticles on graphene sheet, J. Phys. Chem. C, 2012, 116(1), 1535–1543 CAS.
- W. S. Hummers Jr and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80(6), 1339 CrossRef.
- S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner, A chemical route to graphene for device applications, Nano Lett., 2007, 7(11), 3394–3398 CrossRef CAS PubMed.
- W. Chen, J. Ji, X. Feng, X. Duan, G. Qian, P. Li, X. Zhou, D. Chen and W. Yuan, Mechanistic insight into size-dependent activity and durability in Pt/CNT catalyzed hydrolytic dehydrogenation of ammonia borane, J. Am. Chem. Soc., 2014, 136(48), 16736–16739 CrossRef CAS PubMed.
- N. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. Xiao, R. Li, T. K. Sham, L. M. Liu, G. A. Botton and X. Sun, Platinum single-atom and cluster catalysis of the hydrogen evolution reaction, Nat. Commun., 2016, 7, 13638 CrossRef CAS PubMed.
- L. Lin, W. Zhou, R. Gao, S. Yao, X. Zhang, W. Xu, S. Zheng, Z. Jiang, Q. Yu, Y. W. Li, C. Shi, X. D. Wen and D. Ma, Low-temperature hydrogen production from water and methanol using Pt/alpha-MoC catalysts, Nature, 2017, 544(7648), 80–83 CrossRef CAS PubMed.
- C. Chen, Q.-H. Yang, Y. Yang, W. Lv, Y. Wen, P.-X. Hou, M. Wang and H.-M. Cheng, Self-assembled free-standing graphite oxide membrane, Adv. Mater., 2009, 21(29), 3007–3011 CrossRef CAS.
- Z. H. Ni, H. M. Wang, J. Kasim, H. M. Fan, T. Yu, Y. H. Wu, Y. P. Feng and Z. X. Shen, Graphene thickness determination using reflection and contrast spectroscopy, Nano Lett., 2007, 7(9), 2758–2763 CrossRef CAS PubMed.
- I. Jung, M. Vaupel, M. Pelton, R. Piner, D. A. Dikin, S. Stankovich, J. An and R. S. Ruoff, Characterization of thermally reduced graphene oxide by imaging ellipsometry, J. Phys. Chem. C, 2008, 112(23), 8499–8506 CAS.
- S. M. Choi, M. H. Seo, H. J. Kim and W. B. Kim, Synthesis of surface-functionalized graphene nanosheets with high Pt-loadings and their applications to methanol electrooxidation, Carbon, 2011, 49(3), 904–909 CrossRef CAS.
- S. Guo, S. Dong and E. Wang, Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation, ACS Nano, 2010, 4(1), 547–555 CrossRef CAS PubMed.
- S. Wu, J. Liu, Z. Tian, Y. Cai, Y. Ye, Q. Yuan and C. Liang, Highly dispersed ultrafine Pt nanoparticles on reduced graphene oxide nanosheets: in situ sacrificial template synthesis and superior electrocatalytic performance for methanol oxidation, ACS Appl. Mater. Interfaces, 2015, 7(41), 22935–22940 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00384f |
| This journal is © The Royal Society of Chemistry 2017 |