Facile synthesis of bio-fuel from glycerol over zinc aluminium phosphate nanoplates†
Nagabhatla
Viswanadham
 *,
Sandeep K.
Saxena
and
P.
Sreenivasulu
*,
Sandeep K.
Saxena
and
P.
Sreenivasulu

Academy CSIR, Conversion and Catalysis Division, CSIR – Indian Institute of Petroleum, Dehradun-248005, India. E-mail: nvish@iip.res.in; Fax: +91-135-2525702; Tel: +91-135-2525856
First published on 17th May 2017
Abstract
Herein, nanoplates of crystalline zinc aluminum phosphate exhibiting hexagonal and square planar morphology were successfully synthesized by adopting a novel and simple synthesis method; in this method, a single template, TPABr, was used in minute amounts and a shorter synthesis time of 24 h was required. The materials were characterized by X-ray diffraction, FTIR, SEM, XPS, TEM, thermal analysis, ICP, and ammonia TPD. The synthesis temperature governed the morphology of the nanoplates; however, irrespective of the shape of the nanoplates, the materials exhibited promising catalytic activity towards the production of bio-fuel solketal (2,2-dimethyl-1,3-dioxolane-4-methanol), a valuable bio-fuel, via acetalization of biodiesel waste glycerol under solvent-free reaction conditions.
Introduction
The concept of scaling down the particle size to nanometer level for achieving enhancement in the properties of a material has been practically realized in recent years. This has led to the development of various synthesis routes for the production of nano size materials and their significant impact in many fields of science for widespread applications. Especially, the synthesis methods allowed the production of nanoparticles (NPs) of various shapes and compositions, making it easy to explore these NPs for selectively functionalized applications.1,2 Materials with nanoscale particle sizes were observed to exhibit novel properties that were not found in macroscopic materials and have recently offered an attractive alternative to conventional catalysts.3 Especially, for catalytic applications, where the material should possess high surface area and easy accessibility of active sites for reactants, nanomaterials have emerged as suitable alternatives to traditional materials of micro level particle sizes. The smaller size of the particles means high surface area, and the active components present on nanoparticles are highly accessible to facilitate an effective contact between the reactants and catalyst.4,5Nanocatalysis is thus aimed at designing catalysts with excellent activity, greater selectivity, and high stability. These characteristics can be achieved by tailoring the shape, morphology, size, composition, electronic structure, and chemical and thermal stability of particular nanomaterials.6
Many efforts have been made in recent years to obtain nanoparticles with the desired morphology, size, and crystallinity through the optimization of the synthetic procedure, where special emphasis has been given to the stabilization of the nanoparticles in suspension and decreasing the production price to make the materials suitable for commercial applications.7–9 Typical synthesis methods for nanosized materials involve the use of different solvents (water, methanol, ethanol, etc.) and excess organic additives with a specified composition to control the formation of materials in the nanometer range. The crystal size has been successfully reduced in most of the cases via significantly increasing the concentration of the organic template, replacing water with other solvents, decreasing the synthesis temperature, and extending the crystallization process.10 However, these conditions make the synthesis of the nanocrystalline materials complicated and economically non-viable, due to the large consumption of the expensive organic templates and the increased consumption of energy.11 Hence, a simple, economic, and environmentally benign method for the synthesis of nanomaterials is highly desirable and challenging.
A wide variety of metal AlPO4 (MAlPO4) exhibiting characteristics of zeolites and additional novel properties have been synthesized and successfully employed for acid-catalyzed reactions, photoluminescence, magnetism, and ion-exchange applications.12,13 The materials are commonly synthesized under hydrothermal conditions, where the concentration and composition of the gel used for the synthesis, nature and amount of the structure directing agents, and synthesis temperature and reaction time greatly influence the morphology and textural properties of the nanomaterials. A common problem observed in the synthesis of MAlPO4 is the structural instability of AlPO4 that occurs when high amounts of metal is loaded. However, recently, thermally stable MAlPO4 materials have been successfully obtained using novel structure directing agents (SDAs), where the protonated organic SDA and H2O play a charge-balancing role as per the host–guest charge–density-matching principle proposed by Bu and Feng et al.14,15 These facts strongly demand simple and efficient synthesis routes for obtaining high quality MAlPO4 materials that can exhibit high catalytic activity towards the desired product in a stable manner.
One of the recently emerging processes that demand highly efficient catalysts is the value addition of huge quantities (10 wt%) of glycerol produced as a by-product during the biodiesel production process.16 Among the various chemical conversions, acetalization is particularly beneficial for the production of ketals having excellent fuel blending applications that can improve the quality as well as the volume of the biodiesel.17 The oxygen-bearing cyclic alcohol solketal is known for its excellent blending properties towards gasoline, diesel, and biodiesel fuels, as well as its ability to decrease particulate, hydrocarbon, formaldehyde, and carbon monoxide emissions while improving the cold-flow, flash-point, and viscosity-related properties.18–27
The present study aims at the synthesis of zinc-containing catalyst nanoplates for the conversion of bio-waste glycerol into valuable oxygenate fuel, solketal, through an acetalization reaction. The catalyst exhibited excellent properties in terms of oxygenate yields under solvent-free reaction conditions and consistency in the catalytic activity up to the several reaction cycles, required for industrial applications.
Experimental
In a typical synthesis, 0.1 g tetrapropylammonium bromide solution was slowly added to 10 g of ammonium orthophosphate (99%) solution under constant stirring; then, 10 g of aluminium isopropoxide and 0.85 g of zinc nitrate powders were dispersed in the solution mixture under vigorous stirring at room temperature. For the uniformly mixed solution, 0.5 g of hydrofluoric acid (about 48% HF) was added, and the resultant mixture was allowed to stir for one hour to obtain a homogeneous gel. The resultant gel was used as a source material for the hydrothermal synthesis of materials at two different temperatures, i.e., 100 °C and 150 °C, in Teflon-lined stainless steel autoclaves for 24 h. The synthesized samples obtained by the hydrothermal treatment at 100 °C and 150 °C were further calcined at 500 °C for 4 h to remove the template, and the resultant materials were designated as ZAPNP-1 and ZAPNP-2, respectively. The physicochemical properties were analysed via various methods and have been described in detail to understand the properties of the catalysts (Text 1.1†).Catalyst performance studies of the materials were conducted in the present study towards the acetalization of glycerol. In a typical reaction procedure, 0.25 g of catalyst (5% glycerol by weight) was placed in a round-bottom flask and 18.9 g of acetone and 5 g of glycerol with a glycerol to acetone molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 was added to it followed by refluxing at 60 °C for various reaction times; at the end of the reaction, the catalyst (solid particles) was recovered from the reaction mixture via centrifugation, thoroughly washed with ethanol, and reused for multiple cycles. The products obtained at different reaction intervals were filtered and analyzed by GC equipped with a DB wax column and flame ionization detector (FID).
6 was added to it followed by refluxing at 60 °C for various reaction times; at the end of the reaction, the catalyst (solid particles) was recovered from the reaction mixture via centrifugation, thoroughly washed with ethanol, and reused for multiple cycles. The products obtained at different reaction intervals were filtered and analyzed by GC equipped with a DB wax column and flame ionization detector (FID).
Results and discussion
The structure of the crystalline zinc aluminophosphate (ZAPNP) materials was identified via the X-ray diffraction patterns of the samples, and the peaks at 2θ = 23°, 27°, 31°, and 36° were observed (Fig. 1a). Further, the structure of the samples was confirmed by FTIR spectra (Fig. 1b). Both ZAPNP samples exhibited characteristic vibrational bands related to the [PO4]3− unit (1127, 504 cm−1) and pseudo lattice Al (748 cm−1).28,29 The two bands obtained at 1009 and 990 cm−1 are related to the bond vibrations of the Zn–O–P structure, which supports the involvement of Zn in the crystalline framework structure.30 The XPS survey spectra of the samples reveal the presence of Zn, Al, P, and O in both samples (Fig. S12a and S12b†). There were two symmetric peaks observed in the Zn 2p region (Fig. S12c and S12d†). The peak centred at around 1012 eV corresponds to Zn 2p3/2, whereas other peak centred at 1035 eV corresponds to Zn 2p1/2. The spin–orbit splitting of Zn 2p observed between 3/2 and 1/2 spins is about 23 eV, which is consistent with the corresponding value for pure ZnO, indicating the normal oxidation state of Zn2+ in the ZAPNP-1 and ZAPNP-2 samples synthesized in the present study.31,32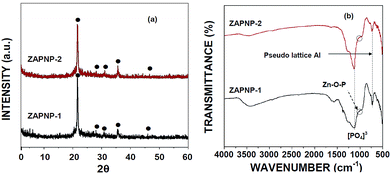 | ||
| Fig. 1 Morphological properties of the ZAPNP-1 and ZAPNP-2 samples: (a) XRD patterns and (b) FTIR patterns. | ||
The crystal morphology of the samples obtained by SEM is shown in Fig. 2. It was found that the hydrothermal synthesis temperature influenced the crystal morphology. The sample synthesized using the lower temperature hydrothermal treatment exhibited bunches of hexagonal plate-like structures (Fig. 2a), whereas the high temperature hydrothermally synthesized sample exhibited well-defined square planar morphology (Fig. 2c). The morphology of both samples studied by SEM after calcinations at 500 °C (Fig. 2b and d for the samples ZAPNP-1 and ZAPNP-2, respectively) indicated that their structural morphology was retained even after the heat treatment that was applied to remove the template. SEM EDAX analysis also revealed the presence of Zn, Al, P, and O atoms in the samples (Fig. S2†). The chemical composition of the samples was further confirmed by ICP analysis (Table S1†).
 | ||
| Fig. 2 SEM images of the ZAPNP-1 and ZAPNP-2 samples: (a and c) as-synthesized and (b and d) the respective calcined samples. | ||
The structure of both ZAPNP samples was further analyzed by TEM (Fig. 3), where thin bunches of nanoplates of ∼200 nm size arranged in a stacked manner were observed. The ZAPNP-1 sample synthesized at low temperature (images in Fig. 3a and b) exhibited clear-cut hexagonal plate morphology, whereas the ZAPNP-2 sample synthesized at high temperature (images in Fig. 3c and d) exhibited square planar morphology. The selected area electron diffraction (SAED) patterns of both samples (Fig. S3†) showed bright spots that revealed the typical crystalline nature of the ZnAlPO4 materials.
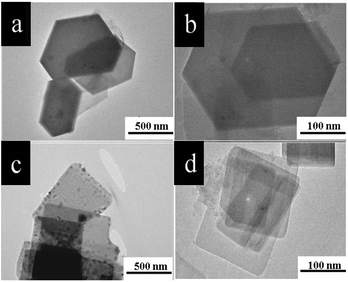 | ||
| Fig. 3 TEM images of the ZAPNP-1 and ZAPNP-2 samples: (a and c) as-synthesized (b and d) the respective calcined samples. | ||
Overall, the SEM and TEM results indicated the presence of nanoplates in both the samples (ZAPNP-1 and ZAPNP-2) synthesized under two different conditions. Further, the TEM images indicated that the samples retained their structural morphology even after calcinations at 500 °C, revealing their thermal stability. This aspect was further confirmed from the thermal analysis patterns measured via TGA/DTA (Fig. S4a and S4b†). The samples showed two areas of weight loss: (1) at 80–150 °C related to the desorption of physically/chemically absorbed water and (2) a high temperature weight loss at up to 450 °C related to the decomposition of the organic template. Except for these losses, there was no observed weight loss related to the structural destruction of the crystalline sample in the thermal analysis.
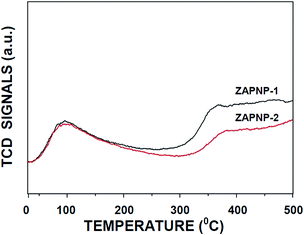
The TPD-NH3 measurements conducted on the samples indeed indicated the acidic property of the samples (Fig. 4). The samples exhibited two desorption peaks: the low-temperature desorption peak, representing weak acidity, and the high temperature desorption peak, representing strong acidity. The acidity patterns of both samples (ZAPNP-1 and ZAPNP-2) are comparable with a slight difference in the strong acidity region that may be due to the difference in the hydrothermal treatment temperatures adopted during the synthesis.
The detailed characterization data shed light on the formation of crystalline ZnAlPO4 material, possessing acidity and porosity suitable for catalytic applications and involving bulky molecular transformations.
The glycerol to solketal reaction involves the acetalization of glycerol molecules, which requires acid sites and sufficient space for the bulky molecule formation and diffusion. The ZAPNP samples possessing acid and metal functions are expected to act as efficient bi-functional catalysts for organic transformations. The data given in Table 1 indeed indicate very high conversions of glycerol facilitated under solvent-free reaction conditions on both catalysts. On the ZAPNP-1 catalyst, the glycerol conversion was low (77 wt%) during the first 30 min of reaction time, but it quickly increased to 98 wt% (steady state) within 60 min of reaction time (entries 1–4). The ZAPNP-2 catalyst also exhibited a similar trend with 72 wt% glycerol conversion at 30 min reaction time, which was increased to 98 wt% conversion within 60 min reaction time (entries 6–9). The selectivity to solketal was always 100% and no side product, except water, was obtained during the reaction, which made the overall solketal yield reach 98 wt%. The results suggest that irrespective of the morphology, the ZAPNP materials have similar chemical nature that is responsible for their comparable catalytic activity. The much higher performance of the present catalyst systems can be ascribed to the easy accessibility of the active sites (acid sites and metal sites), which are arranged on the nanoplates to provide high reactivity to the molecules and facile diffusion of the bulky solketal product.
| Entry | Catalyst | Reaction time (min) | Glycerol conversion (wt%) | Solketal selectivity (%) |
|---|---|---|---|---|
a 0.25 g of catalyst (5% of glycerol weight) was taken in a round-bottom flask and 18.9 g of acetone and 5 g of glycerol with glycerol to acetone molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 was added to it and refluxed at 60 °C.
b The data obtained after five consecutive reaction cycles over the catalyst mentioned in entry 2 under similar reaction conditions mentioned in entry 2.
c The data obtained after five consecutive cycles over the catalyst mentioned in entry 7 under similar reaction conditions mentioned in entry 7. 6 was added to it and refluxed at 60 °C.
b The data obtained after five consecutive reaction cycles over the catalyst mentioned in entry 2 under similar reaction conditions mentioned in entry 2.
c The data obtained after five consecutive cycles over the catalyst mentioned in entry 7 under similar reaction conditions mentioned in entry 7.
|
||||
| 1 | ZAPNP-1 | 30 | 77 | 100 |
| 2 | 60 | 98 | 100 | |
| 3 | 120 | 98 | 100 | |
| 4 | 240 | 98 | 100 | |
| 5b | 60 | 98 | 100 | |
| 6 | ZAPNP-2 | 30 | 72 | 100 |
| 7 | 60 | 96 | 100 | |
| 8 | 120 | 98 | 100 | |
| 9 | 240 | 98 | 100 | |
| 10c | 60 | 92 | 100 | |
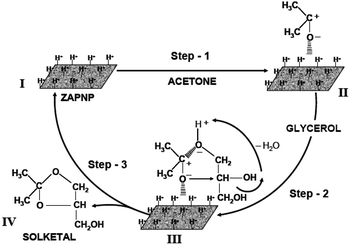
A possible reaction pathway for the formation of solketal through the acetalization of glycerol is given in the scheme (Fig. 5). Herein, polarizability of the reactants and dehydration to produce the glycerol acetone adduct are the two reactions required for the successful formation of the solketal product. The acid sites created by Zn–Al–P (structure I) can activate the acetone molecule to form a C–O bond-polarized adsorbed molecule on the catalyst (structure II); then, glycerol is added to form the glycerol–acetone adduct (structure III), which upon dehydration can produce solketal (structure IV). The product desorbed from the catalyst can create the active site that will be available for the adsorption of the fresh reactant molecule (structure I). The presence of zinc in the catalyst structure may also help in the polarization of the acetone molecule. The nanoplate structure of the catalyst provides facile interaction of the reactants with the active species on the catalyst and facilitate the facile diffusion of the bulky molecules produced.
The chemically bonded Zn within the ZAPNP is also expected to provide stability in terms of activity of the catalyst (unlike the metal-supported bi-functional catalysts), reaction time, and regeneration cycles. We used the hot filtration method to check the catalyst stability, where the catalyst was separated from the reaction medium after 30 minutes of reaction time through filtration and the reaction mixture was allowed to react until 120 minutes. As shown in Fig. S7,† after the removal of the catalysts from the reaction media, almost constant conversion values (2–3% increase) were obtained, whereas in the presence of the catalysts, increase in the conversion up to 98% was achieved. Further, the ICP analysis data of the filtrate (Table S1, ESI†) indicates the negligible amounts of catalyst components in the filtrate (0.41% of the Zn and 0.03 to 0.06% of P originally present in the catalyst). The liquid obtained after the reaction (Table S1 ESI†) did not show the presence of any considerable amount of catalyst chemical moiety, supporting the stability of the elements bonded in the crystalline structure of Zn–Al–P. This is further supported by the stability in the activity of the catalyst for up to several reaction cycles (Table 1). These studies indicate excellent stability in activity of the catalyst in terms of glycerol conversion as well as solketal selectivity in the studied course of 5 reaction cycles (entries 5 and 10). The fresh and spent catalyst materials also exhibited comparable morphology and chemical composition, as measured by SEM, TEM, and ICP analysis (Fig. S5 and S6 and Table S1†).
The nanoparticulate crystalline zinc aluminophosphate obtained by a simple hydrothermal treatment method in the present study possesses excellent material properties suitable for catalytic applications with stability in activity, which is a desired aspect for industrial applications.
Conclusion
Square planar and hexagonal nanoplates of crystalline zinc aluminophosphate were synthesized by a simple hydrothermal synthesis at low temperatures. Irrespective of the type of morphology, the chemical nature of the synthesized samples is comparable in terms of their properties measured by XRD, XPS, FTIR, SEM, TEM, ICP, and ammonia TPD; moreover, as high as 98 wt% solketal was produced under solvent-free reaction conditions. The simple synthesis method of the nanoplates and their significant catalytic activity in the production of the biofuel solketal from bio-waste glycerol make these catalysts promising for industrial applications. The catalyst materials also exhibited high thermal stability (450 °C) and stability in activity under reaction conditions and are suitable for other high temperature applications.References
- G. A. Somorjai, H. Frei and J. Y. Park, J. Am. Chem. Soc., 2009, 131, 16589–16605 CrossRef CAS PubMed.
- Z. Y. Zhou, N. Tian, J. T. Li, I. Broadwell and S. G. Sun, Chem. Soc. Rev., 2011, 40, 4167–4185 RSC.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayad, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- P. Sreenivasulu, D. Nandan, B. Sreedhar and N. Viswanadham, RSC Adv., 2013, 3, 13651–13654 RSC.
- S. Mintova, N. H. Olson, V. Valtchev and T. Bein, Science, 1999, 283, 958–960 CrossRef CAS PubMed.
- B. A. Holmberg, H. Wang, J. M. Norbeck and Y. Yan, Microporous Mesoporous Mater., 2003, 59, 13–28 CrossRef CAS.
- O. Larlus, S. Mintova and T. Bein, Microporous Mesoporous Mater., 2006, 96, 405–412 CrossRef CAS.
- S. C. Larsen, J. Phys. Chem. C, 2007, 111, 18464–18474 CAS.
- H. V. Heyden, S. Mintova and T. Bein, J. Mater. Chem., 2006, 16, 514–518 RSC.
- S. T. Wilson, B. M. Lok, C. A. Messina, R. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc., 1982, 104, 1146–1147 CrossRef CAS.
- S. Zhang, Z. Zhao and Y. Ao, Appl. Catal., A, 2015, 496, 32–39 CrossRef CAS.
- X. Bu, P. Feng and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 11204–11205 CrossRef CAS.
- P. Feng, X. Bu and G. D. Stucky, Nature, 1997, 388, 735 CrossRef CAS.
- N. Viswanadham and S. K. Saxena, Fuel, 2013, 103, 980–986 CrossRef CAS.
- L. Chen, B. Nohair and S. Kaliaguine, Appl. Catal., A, 2016, 509, 143–152 CrossRef CAS.
- M. R. Nanda, Z. Yuan, W. Qin, H. S. Ghaziaskar, M. A. Poirier and C. Xu, Fuel, 2014, 128, 113–119 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- M. R. Nanda, Y. Zhang, Z. Yuan, W. Qin, H. S. Ghaziaskar and C. Xu, Renewable Sustainable Energy Rev., 2016, 56, 1022–1031 CrossRef CAS.
- L. Li, T. I. Koranyi, B. F. Sels and P. P. Pescarmona, Green Chem., 2012, 14, 1611–1619 RSC.
- S. S. Priya, P. R. Selvakannan, K. V. R. Chary, M. L. Kantam and S. K. Bhargava, Mol. Catal., 2017, 434, 184–193 CrossRef.
- J. K. Kus, A. Held, M. Frankowski and K. Nowinska, J. Mol. Catal. A: Chem., 2017, 426, 205–212 CrossRef.
- M. N. Timofeeva, V. N. Panchenko, V. V. Krupskaya, A. Gil and M. A. Vicente, Catal. Commun., 2017, 90, 65–69 CrossRef CAS.
- T. E. Davies, S. A. Kondrat, E. Nowicka, J. J. Graham, D. C. Apperley, S. H. Taylor and A. E. Graham, ACS Sustainable Chem. Eng., 2016, 4, 835–843 CrossRef CAS.
- E. Alptekin, Energy, 2017, 119, 44–52 CrossRef CAS.
- P. Manjunathan, S. P. Maradur, A. B. Halgeri and G. V. Shanbhag, J. Mol. Catal. A: Chem., 2015, 396, 47–54 CrossRef CAS.
- Y. Wang, L. Shao, Y. Li, X. Li, J. Li, J. Yu and R. Xu, Dalton Trans., 2012, 41, 6855–6860 RSC.
- K. Mtalsi, T. Jei, M. Montes and S. Tayane, J. Chem. Technol. Biotechnol., 2001, 76, 128–138 CrossRef CAS.
- O. Pawlig and R. Trettin, Chem. Mater., 2000, 12, 1279–1287 CrossRef CAS.
- D. Li, Y. Zhang, D. Liu, S. Yao, F. Liu, B. Wang, P. Sun, Y. Gao, X. Chuai and G. Lu, CrystEngComm, 2016, 18, 8101–8107 RSC.
- R. O. Moussodia, L. Balan, C. Merlin, C. Mustin and R. Schneider, J. Mater. Chem., 2010, 20, 1147–1155 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, SAED, EDX, TGA, SEM, TEM of spent catalyst and ICP. See DOI: 10.1039/c7se00225d |
| This journal is © The Royal Society of Chemistry 2017 |