Thermo-electrochemical generator: energy harvesting & thermoregulation for liquid cooling applications†
Ali Hussain
Kazim
ab,
A. Sina
Booeshaghi
c,
Sai T.
Stephens
a and
Baratunde A.
Cola
 *ad
*ad
aGeorge W. Woodruff School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA
bDepartment of Mechanical Engineering, University of Engineering and Technology, Lahore, Punjab 54890, Pakistan
cDepartment of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
dSchool of Materials Science and Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA. E-mail: cola@gatech.edu
First published on 8th June 2017
Abstract
Managing big data is a thermodynamics problem; decreasing size and increasing performance of electronic devices necessitate the use of liquid cooling to dissipate massive amounts of heat that are generated as a result. In locations such as data centers, CPU cooling is accomplished through the use of air and liquid methods. Currently, the purpose of existing liquid cooling designs is to provide cooling to these high power CPU's. We have tested and validated a modified liquid cooling design system that supplements the current cooling architecture with the ability to harvest energy from the waste-heat rejected from these heat sources. An electrolyte capable of undergoing a reversible redox reaction is pumped through a macro-channel flow thermo-electrochemical cell (fTEC). The heat energy flow is coupled with electrical energy through the thermoelectric effect allowing cooling and power harvesting to occur in parallel. Our current design generated 88 μW of power with a power density of 0.05 W m−2, achieved a heat transfer coefficient of 450 W (m2 K)−1. This technology can be employed in any location where liquid cooling is used, from CPU's in data centers to battery packs in electric vehicles. With a fTEC, heat mapping for the entire data center would be possible. This would improve thermoregulation and in addition the energy harvested will reduce the overhead cost for running a data center.
1 Introduction
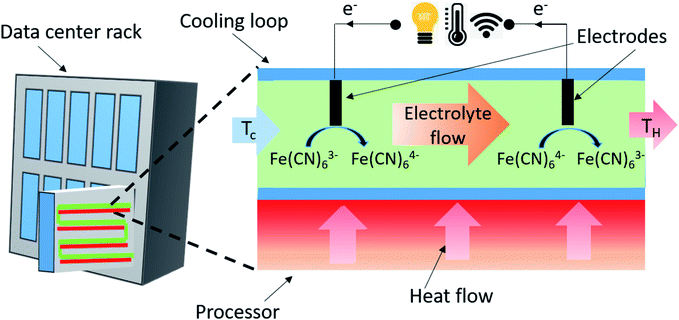
Waste heat is a by-product of all energy conversion mechanisms. Of the various grades, low-grade waste heat (<230 °C) is the most ubiquitous and at the same time most difficult to recover due to challenges such as material limitation, sizing issues and finding end use for recovered heat.1 Sources of low-grade waste-heat include the human body which maintains a temperature of 37 °C that results in a Carnot efficiency of 5.5%, with the environment, and heat loss of 100 W during normal routine activity.2 Oceans provide a tremendous reservoir of thermal energy, forming world's largest source of solar energy collection and storage and can produce 20 times the total electricity consumption in the United States if only 0.1% of its thermal energy is converted to electricity.3 The takeaway is that there are numerous low-grade heat sources where we can employ thermoelectric energy conversion technology in order to unlock a new avenue for energy recycling processes.The focus of this work is to develop and understand a flow thermo-electrochemical cell (fTEC) (Fig. 1) that both converts thermal energy into electrical energy while, in parallel, providing thermoregulation to devices that emit waste-heat, in the form of liquid cooling.
In this paper, we focus on a specific case for this technology: waste-heat and thermoregulation of CPUs in data centers. The United States is home to up to 12 million servers which consume, at an annual rate of 91 billion kW h.4 This is enough to power all of the homes in New York City for two years. It is projected that by 2020, that the number could increase to 140 billion kilowatt-hours annually. Keeping these data centers cool is no simple task; with power densities of up to 578.7 kW m−2 and expelled heat of <230 °C, the waste heat that must be removed consumes massive amounts of electricity. Typically half of the energy consumed in data centers is used to provide cooling to these CPUs in order to operate the current infrastructure in a manner that avoids catastrophic electrical component failure. In an attempt to alleviate the low efficiencies of cooling technologies, liquid cooling has emerged as a viable method.5–7 The need for liquid cooling is ever present if we are to keep up with Moore's law. This technology, coupled with energy generation, will help us improve thermal management and increase power production that will aid in reducing our carbon footprint.8,9
2 Background
The thermoelectric effect, discovered in 1821 by Thomas Johann Seebeck,10 is a link that connects heat and electricity. The effect quantifies how temperature gradient inputs result in voltage outputs. It is employed in a variety of applications from solid-state heat engines to thermocouples.2.1 Thermoelectric effect
The thermoelectric effect, also known as the Seebeck effect, is an observable property of various metals and chemical solutions and is defined in the following way relating the electromotive force Eemf to the Seebeck coefficient, Se, and temperature, T:| Eemf = −Se∇T. | (1) |
Given a static electric field Es = −∇V, the total electromotive force is expressed as a sum of the electric fields E = Es + Eemf. We can then use Ohm's law, J = σE to express current density, J, as a function of E and the material's electrical conductivity, σ:
| J = σ(−∇V − S∇T). | (2) |
The Seebeck coefficient is then defined for the case when J = 0,
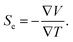 | (3) |
2.2 Thermoelectric cells
There are two major types of thermoelectric cells: solid-state and liquid electrochemical. A liquid thermo-electrochemical cells (TECs) typically consists of a stationary electrolyte (sTEC) sandwiched between two electrodes. The sTEC, Fig. S1,† has a prescribed temperature difference between two electrodes at which the working fluid oxidizes and reduces. The charge carriers transport between the two electrode surfaces by natural convection, migration and diffusion. Contrarily, the fTEC is a device where the only prescribed temperature is at the boundary of the cell. Forced convection in addition to migration and diffusion forces the working fluid from one electrode to the other. This type of liquid thermo-electrochemical cells (TECs) couples the thermoelectric energy generation of traditional solid-state TECs with that of liquid cooling technology.The advantage of liquid thermo-electrochemical cells (TECs) is their high (Se) as high as 10 mV K−1 (ref. 11 and 12) up to 40 times higher than their state-of-the-art solid-state TEC counterparts.13–15 One major drawback of TECs, however, is that they suffer from low current generation capacity due to high ohmic, charge, and mass transfer overpotential, primarily due to slow ionic transport between electrodes.16 Regardless, their merits, such as low cost and flexible geometries, have motivated recent developments in this technology, resulting in power outputs as high as 6.6 W m−2 (ref. 17) and 12 W m−2.18 The introduction of ionic liquids has also addressed the previous limitation of high operating temperatures, well above 100 °C.17,19
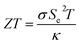 | (4) |
In an effort to maximize the voltage output, ZT, and power output, and to overcome the material constraints, cost-ineffectiveness, and lack of reliability of current thermoelectric cells, new attempts have been made to produce improved alternatives that focus on higher Seebeck coefficients. These new alternatives are sTECs and fTECs.
| Fe(CN)63− + e− ↔ Fe(CN)64− | (5) |
| Fe3+ + e− ↔ Fe2+, E0 = 0.771 V | (6) |
| E0f > 0 | (7) |
| ΔG0 = −nFE0f < 0 | (8) |
| =−74.39 kJ mol−1 | (9) |
Now we must consider the oxidation half reaction of Fe(II) back to Fe(III), where an electron is liberated from the t2g d-orbital and transferred to the external circuit. This is not a favorable reaction under standard conditions since the backwards reaction has a negative reduction potential, E0b. This implies that the reaction is not spontaneous in the backward direction, ΔG0b > 0. But the reaction we are studying is not under standard conditions. If we were to heat up the oxidation half reaction, by raising the temperature of the fluid, then we would be changing the Gibbs free energy:
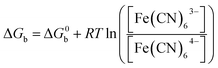 | (10) |
In fact we can raise the temperature just enough to thermally regenerate Fe(CN)63− from Fe(CN)64−, by making the backwards reaction spontaneous, i.e. making ΔG < 0. After the forward reaction occurs then we are left with an overabundance of [Fe(CN)64−] and almost no [Fe(CN)63−]. This is enough to make ΔGb < 0. This allows us to continually oxidize and reduce the Fe ions within the fluid, allowing for continual transport and flow of electrons to an external circuit.
In order to maximize the voltage output we either increase the temperature difference between the anode and the cathode or select a material with a higher Seebeck coefficient 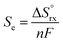 .
.
For our testing we chose a reversible redox system, specifically (Fe(CN)63−/Fe(CN)64−), with a high Seebeck coefficient of ∼1.4–1.6 mV K−1.22–24 This results in a high open circuit voltage.
2.3 Power generation
Power generation requires a high voltage and current output. To achieve the latter the use of expensive metals such as platinum, as an electrode material, was previously required. The use of MWNTs as electrodes has dramatically reduced the cost of TECs. The problem of low current generation due to high mass transfer resistances remains. Our research focused on reducing these mass transfer resistances by designing and testing an fTEC where redox ions are driven from one electrode to another by forced convection in addition to migration and diffusion, Fig. 1.To understand power output must understand both voltage and current output. Current output can be thought of as electron fluxes at the electrodes, both anode and cathode. Fluxes at electrode surfaces result in a buildup of current densities that are determined by the kinetics of the chemical reactions at the electrode i, i ∈ {anode, cathode} given by the Butler–Volmer equation.25
As mentioned previously, mass flow of the electrolyte is beneficial, as it would aid in movement of ions. For a typical sTEC, ion transport from the anode to the cathode is restricted to natural convection, diffusion and migration. However, in the fTEC the forced convection of ions replenishes and removes any already oxidized or reduced ion species at the electrode surface. Unidirectional flow restricts movement of ions backwards and reduces one of the performance-limiting steps in fTECs, that of ion transport, to and from electrode surfaces.
In a TEC the redox ions dictate the electrical conductivity of the solution and the solvent influences the thermal properties. Despite recent progress, a suitable application that combines the aforementioned merits and that minimizes the limitations of TECs has yet to surface.
3 Experimental design
We have developed a device that removes wasted low-grade thermal energy from a system while also utilizing that wasted thermal energy as a driver for producing usable electric energy Fig. 2. We designed and fabricated a physical fTEC and outlined numerous experiments that validated its use as a heat sink and an energy harvester. We utilized an aqueous solution of a (Fe(CN)63−/Fe(CN)64−) system that transfers electrons at electrodes by undergoing a temperature driven chemical reaction Fig. 1. The present configuration of fTECs can harvest heat from 44 electrodes.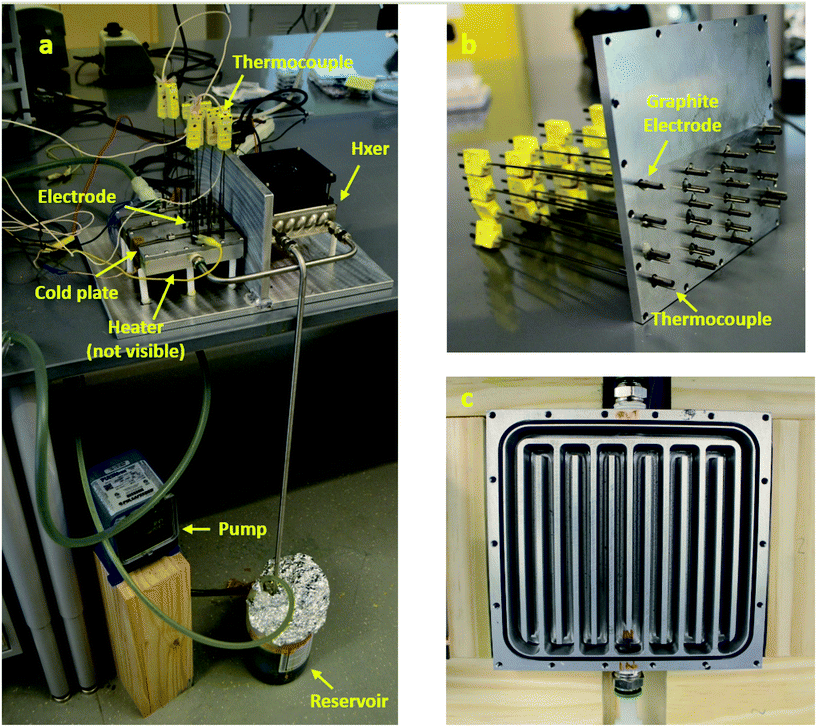 | ||
| Fig. 2 (a) complete experimental setup of the fTEC. (b) Top plate showing the electrode and thermocouple positions (c) Bottom plate showing the path of the electrolyte. | ||
Experimentally, we pumped the redox solution through a macro-channel heat exchanger with constant temperature boundary conditions to test the heat transfer coefficient, voltage, current, and power outputs for a given flow rate. This allowed us to determine the fTEC's ability to convect heat and generate electricity. A schematic can be seen in Fig. 1. Design consideration in more detail can be found in Section four of the ESI.†
4 Results
Flowing the electrolyte function in two ways: (i) to remove heat from the system by convective heat transfer and (ii) to assist in the migration of charge from electrode to electrode. We considered the flow rate and its effects on the thermal and electrodynamics of the system. We found that a lower flow rate will produce a larger path-wise temperature gradient, resulting in a higher electrical output between two path-wise electrodes. Consequently the heat transferred from the system will decrease due to a lower convective heat transfer coefficient. A higher flow rate, however, will increase the amount of heat transferred from the system, but will decrease the temperature gradient between electrodes, thus decreasing the electrical output. In this section we discuss results of our simulations and experiments and perform a comparison of the two.4.1 Simulations
We performed multi-physics simulations with COMSOL to analyze the thermal and electrodynamic components of our system and utilized parameters that were consistent with our experimental setup.26 It is important to note that the working fluid is primarily water and that the addition of the ferri/ferrocyanide salts, at any concentration below the saturation limit, does not result in a perceivable change to the boiling point of the solvent. Given that our Seebeck coefficient is Se = 1.4 [mV K−1], and that the maximum temperature of our fluid (prior to entering a phase change to gas) is Tmax = 373 K with the fluid at room temperature being Tin = 293 K, then approximately the maximum realizable voltage possible is Vmax = 0.11 V.The temperature and velocity distribution of the working fluid, given a constant temperature boundary condition, is shown in Fig. S2a and b.† Importantly, COMSOL simulation shows that the flowing electrolyte is able to produce power (Fig. S2d†). The simulations show that the power output produces a peak, as shown in (Fig. S2d†) at around 0.9 μW. The maximum realizable power output numerically achieved for two electrodes, 1.5 inches apart for our experimental flow rates, was 2 μW, which is close to our experimental results Fig. 3c.
4.2 Experimental
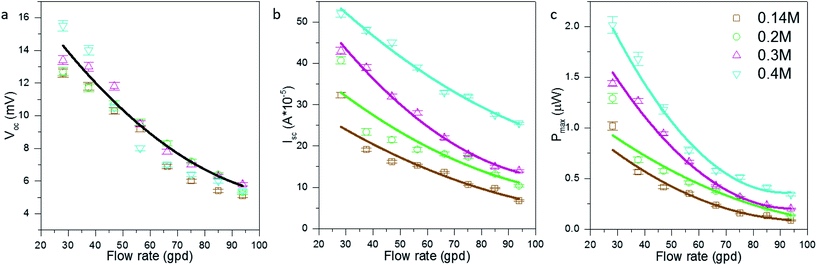
The power, voltage, and current performance between two adjacent electrodes is shown in Fig. 3. The Voc is independent of the concentrations of the electrolyte, for high concentrations near the saturation limit, identified in earlier literature.27–29 However, the increase in concentration has a proportional effect on the measured current (Fig. 3b). The increase in current is due to larger number of redox ions available for reaction at the electrodes, as well as increase in electrical conductivity of the electrolyte. The increase in electrical conductivity reduces mass transfer resistance of ions. The maximum power Pmax was calculated using:16| Pmax = 0.25VocIsc | (11) |
Our results show that the open circuit voltage (Fig. 3a) increases with a decrease in flow rate because a slower flow rate raises the temperature gradient between the two electrodes. The higher temperature difference due to a low flow rate increases the open circuit voltage available at the electrodes. As mentioned previously the Voc is independent of concentration of the electrolyte, this is apparent from the fairly close scatter and the various concentrations are represented by a single trend line. The higher temperature of the down-line electrode also increases the conductivity of ions and consequently the current output. Overall a maximum power output of 2 μW was recorded for a flow rate of 28.16 GPD and redox species concentration of 0.4 M. For the current physical arrangement of 44 pairs of electrodes, a maximum experimental output of 88 μW is achieved.
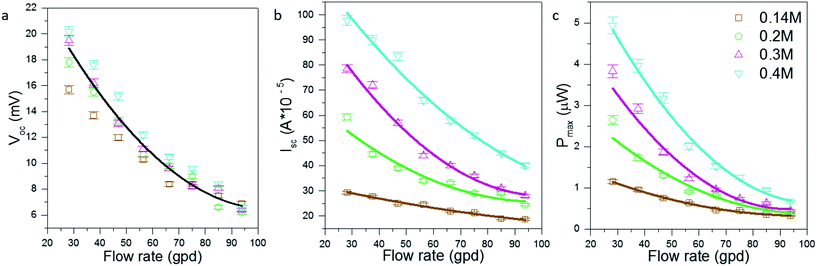
After measuring the power performance of adjacent electrodes, performance of the electrodes furthest apart (33.7′′) was recorded (Fig. 4). For a conventional stationary TEC the power drops as the electrodes are taken further apart because the ohmic (ion mass transfer) resistance increases. For a stationary TEC, if the electrodes are kept at constant temperature and are moved further apart from each other the Voc will remain same as the temperature difference is constant but the current will drop as the internal ohmic resistance of thermocell increases. However for the fTEC, the Voc, Isc and Pmax were observed to be higher than those in the case of near-pair electrodes. This was due to higher temperature difference for the electrodes which are further apart. The higher temperature difference was able to overcome the increase in internal ohmic resistance that was a result of an increase in electrode separation spacing.23,30 The trend for Voc, Isc and Pmax is having a power law relationship with respect to flow rate; the power law trend is more apparent with Pmax as it is a product of two variables: open circuit voltage and short circuit current.
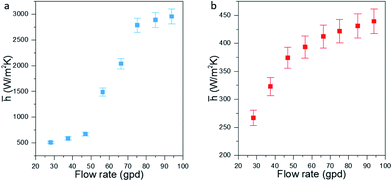
In addition to converting waste heat, the fTEC provided convective cooling. The convective heat transfer coefficient is a major bottleneck in achieving low thermal resistance for cooling. Cooling performance is assessed by calculating the average heat transfer coefficient (Fig. 5) at the entrance and for the entire fTEC. The average heat transfer is calculated using:
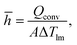 | (12) |
The flow is simultaneously developing the hydrodynamic and thermal boundary layers. This is suitable for maximum heat transfer. To compare the data with existing empirical models, the non-dimensional Nusselt and Reynolds number were calculated for our setup (Fig. 6). The non-dimensional data compared well with the limited literature available for simultaneously developing flow for rectangular32 and circular channels.31,33
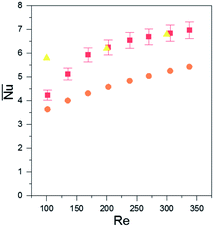 | ||
| Fig. 6 Average Nusselt number calculated for the entire fTEC experimental (red square), rectangular channel literature data (yellow triangle) and circular channel literature data (orange circles). | ||
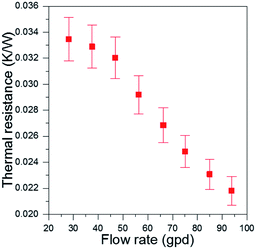
To compare the heat convection capability of our fTEC with that of other liquid cooling architectures, we calculated the thermal resistance R (Fig. 7) using:
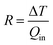 | (13) |
5 Discussion
In our current design of the fTEC all of the electrodes are inline and inserted into the top plate. The electrodes interact with the flowing fluid and are influenced by the temperature of the fluid. They increase in temperature primarily due to the convective heat transfer of heat from the boundary conditions to the flowing fluid. The inline temperature gradient that results is perpendicular to the maximum temperature difference available (i.e. between the top and bottom plate). To take advantage of the maximum temperature gradient available in the perpendicular direction, electrodes should to be located on the top and bottom of the fTEC Fig. S3.†To investigate the proposed configuration, COMSOL simulation was carried out. The geometry and all other boundary conditions were kept constant. The only modification was the position of the electrodes which was changed to being located perpendicular to the direction of the heat flux. Our simulations demonstrated a maximum power output that increased by 7.4 times (Fig. S4†). Given 44 pair of electrodes, a combined power of 651 μW will be available.
Further investigation of electrode materials could yield a fTEC that overcomes its low current generation abilities. MWNTs have been shown to be an inexpensive substitute to other expensive metals for their use as electrodes in sTECs with reported high power conversion efficiencies 1.4% and 2.63% of the Carnot efficiency.29,34–36 The use of carbon nanotubes has helped researchers look beyond the cost of fTEC development and focus on performance parameters26,27,37–43 and practical applications.44–46
Future development of fTECs should focus on a microchannel heat exchanger that has a channel width less than 1 mm that would enhance the heat transfer coefficient and resultant cooling capability47 of the fTEC. For our current setup the highest Reynolds number was about 340 (laminar, Re < 2300) corresponding to a flow rate of 93.87 GPD. Turbulent flow will also increase the heat transfer coefficient and reduce thermal resistance.
Presently in data centers, low-power temperature sensors are used in key locations to monitor temperature and forward the information via relay nodes to a central monitoring client.48,49 By employing the thermoelectric effect, our fTEC can be used to monitor temperature without the need for these sensors since the voltage output will be proportional to the temperature of the device temperature. As a result, real-time monitoring of temperature throughout places such as data centers at any instance will be possible. Knowledge of the complete temperature profile of data centers will greatly reduce chances of hot spot occurrence and device damage.48 By employing a fTEC, we can have a battery-less thermocouple-less temperature monitoring system. This application can be extended to wherever liquid cooling is employed. One such example is in battery packs of electric vehicles. Liquid cooling provides greater safety, performance, and longevity of battery life compared to traditional cooling methods.50,51 The novel fTEC design can complement existing safety features in batteries by providing a complete temperature profile Fig. S5.† With the current unoptimized design both in terms of electrode configuration and their material, we have a power density of 0.05 W m−2. In future work, we plan to achieve beyond the current largest power density of 12 W m−2. In doing so, a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ft2 data center will be able to produce 65 kW of recycled power. This can be utilized to provide power for overhead energy in data centers or for 50 households.
000 ft2 data center will be able to produce 65 kW of recycled power. This can be utilized to provide power for overhead energy in data centers or for 50 households.
6 Conclusion
The fTEC has been shown to provide cooling and harvest electrical energy from waste-heat source, in parallel. Its development can be extended to all applications employing liquid cooling. The current design is yet to be optimized both in terms of electrode material and electrode position. In the present unoptimized design both in terms of electrode configuration and their material, we can harvest 2 μW of power per electrode pair, up to 88 μW of total power, at low flow rates. This energy can be useful for powering low-powered devices such as wireless sensors used in sending information such as temperature profiles to a central monitoring client. We also showed a cooling performance in terms of the heat transfer coefficient of 450 W (m2 K)−1 which is an order of magnitude higher than that of free air convection and two-fold higher than that of forced air convection.7 Materials preparation
The solution of potassium ferri/ferro cyanide was made using potassium ferricyanide(III) and potassium hexacyanoferrate(II) trihydrate, which were purchased from Sigma Aldrich having a molecular weight of 329.264 g mol−1 and 422.39 g mol−1, respectively. A high volume of about 5000 mL was prepared. Due to the high volume the concentration was increased in increments to ensure complete solubility.A fine extruded graphite rod having diameter 0.125′′ was purchased from Graphtek LLC. According to product specifications the particle size is 0.02032 cm, density is 1.76 g cm−3, thermal conductivity is 65 W (m K)−1, flexure strength is 34.5 MPa and compressive strength is 66.2 MPa. Heat shrink tubing was used to isolate the graphite electrode from the top plate of the fTEC to prevent short-circuiting.
The main parameters of interest in the system are the temperature of the fluid at the electrodes, and the voltage/current output at the electrodes for a given electrode position. To collect electrolyte temperature, in-line K-type thermocouples were utilized at the electrode locations. Open circuit voltage and short-circuit current was recorded for 5 minutes using CH instruments model 660E potentiostat. The average value of the measurement over this time interval was taken for further study. Electric resistance heaters were used to maintain a constant bottom plate temperature. When temperature drops below the setting temperature, the heaters are turned ON. When the set temperature is attained the heaters are turned OFF; however the temperature of the bottom plate keeps rising few degrees above the set temperatures. This cycle of maintaining a temperature at a set temperature typically took around 2.5 minutes. The voltage as expected changed proportional to the set temperature cycle. So in order to capture the complete temperature (voltage) cycle Voc and Isc were measured for 5 minutes. The measurements were repeated at least three times.
Acknowledgements
We are grateful for the financial support of the National Science Foundation Award No. CBET 1055479 and the MIT Martin Luther King Jr. Visiting Scholars Fellowship Program. A. H. Kazim acknowledges support from a Fulbright Fellowship.References
- Combined heat and power, waste heat, and district energy, United states department of energy technical report, 2011.
- T. Starner, IBM Syst. J., 1996, 35, 618–629 CrossRef.
- Ocean Thermal Energy Conversion: An Overview, Nrel technical report, 1989.
- America's Data Centers Are Wasting Huge Amounts of Energy, Natural resources defense council technical report, 2014.
- G. I. Meijer, Science, 2010, 328, 318–319 CrossRef CAS PubMed.
- H. Zhang, S. Shao, H. Xu, H. Zou and C. Tian, Renewable Sustainable Energy Rev., 2014, 35, 171–182 CrossRef CAS.
- S. Alkharabsheh, J. Fernandes, B. Gebrehiwot, D. Agonafer, K. Ghose, A. Ortega, Y. Joshi and B. Sammakia, J. Electron. Packag., 2015, 137, 040801 CrossRef.
- I. Stark, International Workshop on Wearable and Implantable Body Sensor Networks (BSN’06), 2006, pp. 19–22 Search PubMed.
- E. Barbier, Renewable Sustainable Energy Rev., 2002, 6, 3–65 CrossRef.
- T. J. Seebeck, Ann. Phys., 1826, 82, 253–286 CrossRef.
- M. Bonetti, S. Nakamae, M. Roger and P. Guenoun, J. Chem. Phys., 2011, 134, 1–9 CrossRef PubMed.
- T. J. Abraham, D. R. MacFarlane and J. M. Pringle, Energy Environ. Sci., 2013, 6, 2639 CAS.
- J.-S. Rhyee, K. H. Lee, S. M. Lee, E. Cho, S. I. Kim, E. Lee, Y. S. Kwon, J. H. Shim and G. Kotliar, Nature, 2009, 459, 965–968 CrossRef CAS PubMed.
- H. Ohta, S. Kim, Y. Mune, T. Mizoguchi, K. Nomura, S. Ohta, T. Nomura, Y. Nakanishi, Y. Ikuhara and M. Hirano, et al. , Nat. Mater., 2007, 6, 129–134 CrossRef CAS PubMed.
- A. I. Boukai, Y. Bunimovich, J. Tahir-Kheli, J.-K. Yu, W. A. Goddard and J. R. Heath, Nature, 2008, 451, 168–171 CrossRef CAS PubMed.
- T. Quickenden and C. Vernon, Sol. Energy, 1986, 36, 63–72 CrossRef CAS.
- H. Im, T. Kim, H. Song, J. Choi, J. S. Park, R. Ovalle-Robles, H. D. Yang, K. D. Kihm, R. H. Baughman and H. H. Lee, et al. , Nat. Commun., 2016, 7, 10600 CrossRef CAS PubMed.
- L. Zhang, T. Kim, N. Li, T. J. Kang, J. Chen, J. M. Pringle, M. Zhang, A. H. Kazim, S. Fang and C. Haines, et al. , Adv. Mater., 2017, 1605652 CrossRef PubMed.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232 CAS.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105–114 CrossRef CAS PubMed.
- B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich, B. Yu, X. Yan, D. Wang, A. Muto and D. Vashaee, et al. , Science, 2008, 320, 634–638 CrossRef CAS PubMed.
- T. Hirai, K. Shindo and T. Ogata, J. Electrochem. Soc., 1996, 143, 1305–1313 CrossRef CAS.
- Y. Mua and T. I. Quickenden, J. Electrochem. Soc., 1996, 143, 2558 CrossRef CAS.
- K. B. Koller and F. M. Hawkridge, J. Am. Chem. Soc., 1985, 107, 7412–7417 CrossRef CAS.
- A. J. Bard, L. R. Faulkner, J. Leddy and C. G. Zoski, Electrochemical methods: fundamentals and applications, Wiley New York, 1980, vol. 2 Search PubMed.
- P. F. Salazar, S. Kumar and B. A. Cola, J. Appl. Electrochem., 2014, 44, 325–336 CrossRef CAS.
- T. J. Kang, S. Fang, M. E. Kozlov, C. S. Haines, N. Li, Y. H. Kim, Y. Chen and R. H. Baughman, Adv. Funct. Mater., 2012, 22, 477–489 CrossRef CAS.
- Y. Kuzminskii, V. Zasukha and G. Kuzminskaya, J. Power Sources, 1994, 52, 231–242 CrossRef CAS.
- P. F. Salazar, S. Kumar and B. A. Cola, J. Electrochem. Soc., 2012, 159, B483 CrossRef CAS.
- T. I. Quickenden, J. Electrochem. Soc., 1995, 142, 3985 CrossRef CAS.
- T. L. Bergman, F. P. Incropera, D. P. DeWitt and A. S. Lavine, Fundamentals of Heat and Mass Transfer, John Wiley & Sons, 2011 Search PubMed.
- M. V. D. V. J. D. K. S. Garimella and W. J. Dowling, Heat Transfer Eng., 2001, 22, 12–25 CrossRef.
- E. N. Sieder and G. E. Tate, Ind. Eng. Chem., 1936, 28, 1429–1435 CrossRef CAS.
- R. Hu, B. A. Cola, N. Haram, J. N. Barisci, S. Lee, S. Stoughton, G. Wallace, C. Too, M. Thomas, A. Gestos, M. E. Dela Cruz, J. P. Ferraris, A. A. Zakhidov and R. H. Baughman, Nano Lett., 2010, 10, 838–846 CrossRef CAS PubMed.
- H. Im, T. Kim, H. Song, J. Choi, J. S. Park, R. Ovalle-Robles, H. D. Yang, K. D. Kihm, R. H. Baughman, H. H. Lee, T. J. Kang and Y. H. Kim, Nat. Commun., 2016, 7, 10600 CrossRef CAS PubMed.
- M. S. Romano, N. Li, D. Antiohos, J. M. Razal, A. Nattestad, S. Beirne, S. Fang, Y. Chen, R. Jalili and G. G. Wallace, et al. , Adv. Mater., 2013, 25, 6602–6606 CrossRef CAS PubMed.
- A. H. Kazim and B. A. Cola, J. Electrochem. Soc., 2016, 163, F867–F871 CrossRef CAS.
- W. Qian, M. Li, L. Chen, J. Zhang and C. Dong, RSC Adv., 2015, 5, 97982–97987 RSC.
- A. Gunawan, H. Li, C.-H. Lin, D. A. Buttry, V. Mujica, R. A. Taylor, R. S. Prasher and P. E. Phelan, Int. J. Heat Mass Transfer, 2014, 78, 423–434 CrossRef CAS.
- P. F. Salazar, S. T. Stephens, A. H. Kazim, J. Pringle and B. A. Cola, J. Mater. Chem. A, 2014, 1–7 Search PubMed.
- T. J. Abraham, D. R. MacFarlane, R. H. Baughman, N. Li, Y. Chen and J. M. Pringle, MRS Proc., 2013, 1575 Search PubMed.
- M. S. Romano, S. Gambhir, J. M. Razal, A. Gestos, G. G. Wallace and J. Chen, J. Therm. Anal. Calorim., 2012, 109, 1229–1235 CrossRef CAS.
- H. Im, T. J. Kang, D. W. Kim and Y. H. Kim, Journal of the Korean Society for Aeronautical & Space Sciences, 2012, 40, 1010–1015 Search PubMed.
- A. Gunawan, N. W. Fette and P. E. Phelan, ASME 2015 Power Conference collocated with the ASME 2015 9th International Conference on Energy Sustainability, the ASME 2015 13th International Conference on Fuel Cell Science, Engineering and Technology, and the ASME 2015 Nuclear Forum, 2015, p. V001T11A002 Search PubMed.
- S. Uhl, E. Laux, T. Journot, L. Jeandupeux, J. Charmet and H. Keppner, J. Electron. Mater., 2014, 43, 3758–3764 CrossRef CAS.
- H. Im, H. G. Moon, J. S. Lee, I. Y. Chung, T. J. Kang and Y. H. Kim, Nano Res., 2014, 7, 1–10 CrossRef.
- F. Zhou, E. M. Dede and S. N. Joshi, Thermal Measurement, Modeling & Management Symposium (SEMI-THERM), 2015 31st, vol. 2015, pp. 60–67 Search PubMed.
- B. Weiss, H. L. Truong, W. Schott, T. Scherer, C. Lombriser and P. Chevillat, IBM RZ, 2011, 3807 Search PubMed.
- J. Polastre, R. Szewczyk and D. Culler, IPSN 2005. Fourth International Symposium on Information Processing in Sensor Networks, 2005, vol. 2005, pp. 364–369 Search PubMed.
- G. Berdichevsky, K. Kelty, J. Straubel and E. Toomre, The Tesla Roadster Battery System, Tesla Motors Inc, 2007, pp. 1–5 Search PubMed.
- T. Kimura, S. Hamada, Y. Ogata, T. Yamauchi and T. Sekimori, Battery pack, US Pat. 6,569,561, 2003.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00161d |
| This journal is © The Royal Society of Chemistry 2017 |