Effects of polymeric coatings on the service life of bismuth telluride-based thermoelectric materials
Witold
Brostow
 a,
I Kang
Chen
a and
John B.
White
ab
a,
I Kang
Chen
a and
John B.
White
ab
aLaboratory of Advanced Polymers and Optimized Materials (LAPOM), Department of Materials Science and Engineering, University of North Texas Denton, 3940 North Elm Street, Denton, TX 76207, USA. E-mail: wkbrostow@gmail.com; Web: htpps://www.lapom.unt.edu
bMarlow Industries, Inc., Dallas, Texas 75238, USA
First published on 23rd June 2017
Abstract
Thermoelectric (TE) devices have one main disadvantage: short service lifetime. These devices undergo degradation by sublimation, oxidation, and reactions in corrosive environments. To prevent the degradation, we have applied two high temperature polymers (HTPs) as coatings for Bi2Te3-based TE materials. Sintering temperatures were from 250 °C to 400 °C. EDS and SEM results show that the coatings prevent the oxidation and sublimation of TE materials. We also shorten the curing cycle time and lower the energy costs. Electrical resistivity values show that Bi2Te3 materials can perform well up to 300 °C with our HTPs.
1. Introduction
There are two kinds of thermoelectric (TE) devices:1–5 TE generators (TEGs) of electricity and TE coolers (TECs). TEGs are based on the Seebeck effect discovered in 1821 (ref. 3 and 5): a temperature difference ΔT between two different TE materials generates electricity. TECs are based on the twin Peltier effect discovered in 1834 (ref. 3 and 5): an electric circuit involving two TE materials in contact can cool one of these materials while heating the other.Large ΔT appears for instance between the ambient temperature and that of car exhausts. However, hybrid cars based on the Seebeck effect do not exist. Refrigerators have liquid coolants which after service go into the air and gradually destroy the ozone layer of the Earth. Solid-state refrigerators based on the Peltier effect are not popular either. This while potential applications of TEGs and TECs cover a very wide range. TEGs and TECs have small sizes, high reliability, no moving components, and do not create any noise.
Given the importance of TE materials, there is an extensive work in this area. Thus, techniques for the deposition of bismuth telluride as thin films such as ion beam sputtering are being developed.6 The situation is somewhat complicated by the existence of several phases of bismuth telluride—seen in the X-ray results of Walachová and her colleagues.7 A different line of activity consists in adding other metals such as antimony, creating bismuth antimony telluride.8,9 The progress is not always fast. Poudel and his colleagues soberly state that “The dimensionless thermoelectric figure of merit (ZT) in bismuth antimony telluride (BiSbTe) bulk alloys has remained around 1 for more than 50 years”. They actually succeeded in clearly increasing that figure.
In fact, most of the efforts are focused on increasing the figure of merit. We focus here on a different issue. Given the applications, TE materials undergo cyclic temperature changes over large ΔT intervals. During thermal cycling Bi undergoes oxidation while Te escapes by sublimation. Addition of other metals does not improve this situation in a significant way.
We have proposed a different approach:10 encapsulating TE devices in polymeric coatings. While there is a variety of polymeric coatings used for a variety of purposes, ordinary polymers are not usable for our purpose. Polyethylene has melting temperatures between 70 °C and 135 °C. However, we have high temperature polymers (HTPs) which should survive much higher temperatures. In this letter we report preservation—or otherwise—of Bi and Te at elevated temperatures without, respectively, oxidation or sublimation.
2. Experimental
2A. Materials
We have used finely grained Bi2Te3 Micro-Alloyed Materials from Marlow Industries; the samples were diced to needed dimensions from 3 × 3 × 6 cuboids. We have developed several HTPs and procedures for using them as coatings; two called HTP2 and HTP9 have been selected for this project. HTP2 is made from a polymeric acid solution with 78 wt% solvent. HTP9 is also based on an advanced polymeric acid, and contains 78% of the same solvent, plus a curing additive.2B. Encapsulation of Bi2Te3 materials
The surface sides of Bi2Te3 materials were dip coated; both HTP2 and HTP9 have solution viscosity values close to 100 Pa s; thus, this is a convenient technique from the point of view of manufacturing. After coating, the samples were heated to 200 °C and kept at that temperature for 10 minutes to vaporize the solvent. Samples coated with either HTP2 or HTP9 then underwent one of three heating scenarios, up to 250 °C, or to 300 °C, or to 400 °C. In each case, the sample after reaching the final temperature was kept at that temperature for 10 minutes, resulting in the formation of a cured insoluble material with good heat resistance.2C. Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed on a Perkin Elmer TG-7 apparatus (Waltham, MA). This technique is well described by Gedde.11 7–10 mg of each dry sample was heated from 50 °C to 650 °C at 10 °C min−1.2D. Electrical resistivity
A four-point Jandel RM3000 resistivity meter (Heights, AZ) was used. The probes came into contact with four side surfaces (not including top and bottom sides) of a cuboid; averages from several locations are reported.2E. Energy-dispersive spectroscopy (EDS)
A FEI Quanta ESEM (Hillsboro, OR) apparatus configured with EDS was used. In ‘normal’ scanning electron microscopy (SEM) one works with dry samples, often coated with a metallic layer for better contrast. An environmental SEM (ESEM) is capable of providing results for uncoated samples, even wet ones; the observation was also performed in the presence of air or some other gases. We have in EDS a beam of X-rays focused onto the sample. Some electrons in the inner shell of the atomic nucleus are excited to higher energy levels, leaving behind holes. Then electrons from the higher shells fill some of these holes; the differences in energy between the higher and lower shells might be emitted as X-rays. An energy-dispersive spectrometer measures the number and energies of the X-rays so emitted. These energies are characteristic of the emitting elements. Thus, the chemical composition (elemental analysis) of the sample is determined.3. Thermal stability of HTPs
We used TGA to determine the temperatures of various decomposition processes. Factors that can affect the thermal stability of HTPs include pre-baking time temperature ramp, post-baking time, and sintering temperature.Table 1 shows weight loss in % determined by TGA of samples subjected to different curing cycles.
| Samples | Curing cyclesb | Weight loss (%) | ||
|---|---|---|---|---|
| 200 °C | 400 °C | 600 °C | ||
| a Samples were selected for four-point probe resistivity testing. b The temperature ramp for each curing cycle is 1 °C min−1. | ||||
| HTP2-1a | 10 min at 250 °C | 2.54 | 10.28 | 16.02 |
| HTP2-2 | 60 min at 250 °C | 1.86 | 6.62 | 9.87 |
| HTP2-3 | 120 min at 250 °C | 1.54 | 4.36 | 9.13 |
| HTP2-4 | 180 min at 250 °C | 1.19 | 4.20 | 5.86 |
| HTP2-5a | 10 min at 300 °C | 1.48 | 2.77 | 11.86 |
| HTP2-6 | 60 min at 300 °C | 1.49 | 2.80 | 7.40 |
| HTP2-7 | 120 min at 300 °C | 1.77 | 2.21 | 8.69 |
| HTP2-8 | 10 min at 350 °C | 1.84 | 2.71 | 10.18 |
| HTP2-9a | 10 min at 400 °C | 1.58 | 1.96 | 9.70 |
| HTP9-1a | 10 min at 250 °C | 2.20 | 6.33 | 10.20 |
| HTP9-2 | 60 min at 250 °C | 1.03 | 3.57 | 9.03 |
| HTP9-3 | 120 min at 250 °C | 0.95 | 2.95 | 7.37 |
| HTP9-4 | 180 min at 250 °C | 0.58 | 2.42 | 7.55 |
| HTP9-5a | 10 min at 300 °C | 1.40 | 3.90 | 8.71 |
| HTP9-6 | 60 min at 300 °C | 0.70 | 1.95 | 5.97 |
| HTP9-7 | 120 min at 300 °C | 0.75 | 1.87 | 6.68 |
| HTP9-8 | 10 min at 350 °C | 1.37 | 2.35 | 7.41 |
| HTP9-9a | 10 min at 400 °C | 0.74 | 0.50 | 4.55 |
We see that a longer post-baking time and higher sintering temperature enhance the thermal stability of HTPs. For HTP2 a stronger effect of post-baking time is seen, as demonstrated by weight loss at 600 °C for samples HTP2-1 to HTP2-4; the weight loss goes down from 16.0% to 5.9%. On the other hand, the weight loss goes down from 10.2 to 4.6 when we look at samples HTP9-1, 5, 8, and 9. Apparently for HTP9 the sintering temperature is the strongest factor affecting the thermal stability.
Overall, HTP9 shows better thermal stability than HTP2. However, HTP2-4 kept at 250 °C for 180 minutes is promising when we are concerned with energy consumption at the elevated temperature.
4. Electrical resistivity
After investigating curing cycles for HTP2 and HTP9, we encapsulated both p-type and n-type Bi2Te3 based samples in the HTPs and subjected them to different curing cycles. The samples were kept at 250 °C, 300 °C and 400 °C reached with 1 °C min−1 temperature ramp and held for 10 minutes at the final temperature. The bulk resistivity was determined at 25 °C; the results are shown in Fig. 1.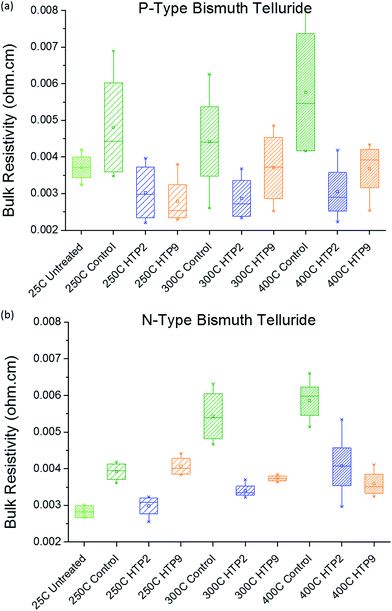 | ||
| Fig. 1 (a) Electrical resistivity of p-type Bi2Te3 at different sintering temperatures; (b) electrical resistivity of n-type Bi2Te3 at different sintering temperatures. | ||
As expected, the resistivity values increase with increasing sintering temperature in control samples (uncoated) for both p-type and n-type Bi2Te3 based samples. Compared with control samples at different sintering temperatures, the bulk resistivities of coated n-type Bi2Te3 based samples are lower in all cases except for the HTP9 coated sample kept at 250 °C. The exception might be due to insufficient time of curing.
The coated p-type Bi2Te3 based samples have comparable or lower bulk resistivity than the green sample (untreated). A possible explanation for the lower resistivity is a change in bulk material properties that increases the carrier concentration during the heating process so that extra carriers move from the valence band to the conduction band.12,13
5. Sublimation and oxidation from EDS
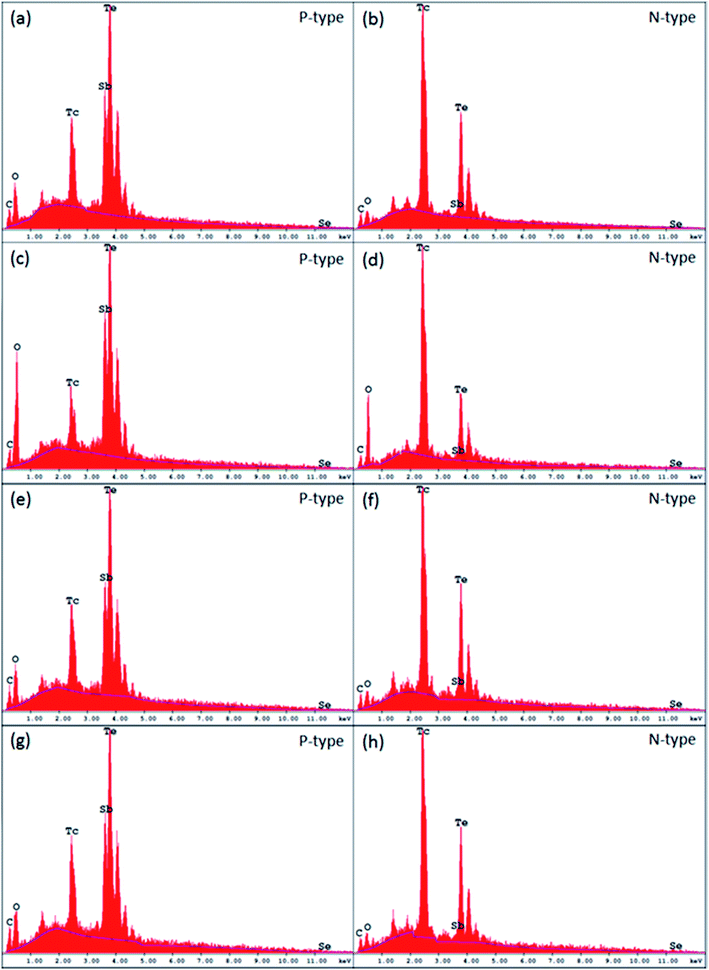
To evaluate the sublimation and oxidation effects, we have applied EDS to both p-type and n-type Bi2Te3 materials before and after 400 °C curing cycles. The results are shown in Fig. 2.Evidently samples of both p- and n-type without polymeric coatings have much higher levels of oxidation. For the p-type uncoated Bi2Te3 material after a 400 °C curing cycle, the level of oxidation is three times higher than for coated and submitted to 400 °C or for untreated (control at 25 °C) samples. The results are similar to those for n-type Bi2Te3 materials. The degree of oxidation for the n-type uncoated sample is seven times higher than for coated or untreated samples.
Clearly polymeric coatings also prevent the sublimation of the samples, especially in n-type Bi2Te3 materials. For example, the weight loss of tellurium in n-type coated HTP2 samples is between 0.21 and 1.1%. Without a polymeric coating, the n-type Bi2Te3 material which underwent a 400 °C curing cycle had lost 10.4% of tellurium.
6. Effects of heating on morphology
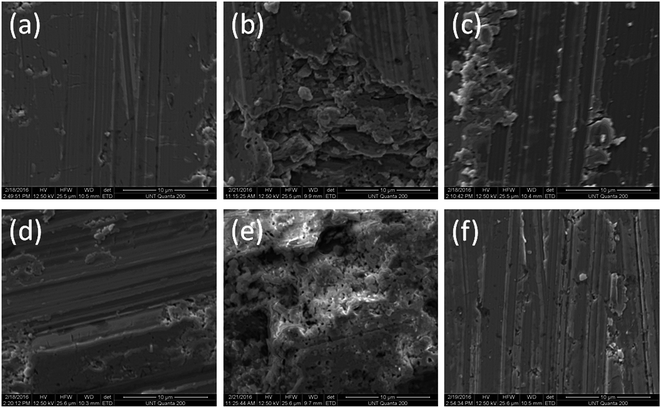
We have studied the morphology of the samples by using environmental scanning electron microscopy (ESEM). The SEM technique is well explained by Gedde11 and by Michler and Balta-Calleja.14Our results are shown in Fig. 3.
We see hollow microstructures in Fig. 3(b) and (e) – apparently a consequence of the sublimation of tellurium.
We also observe that the surfaces of both p-type and n-type materials without polymeric coatings have significant cracks. Thus, our HTP coatings prevent the oxidation of bismuth, prevent the sublimation of tellurium, and also mitigate crack formation.
Since HTPs typically undergo curing, the viscosity of uncured HTPs is a factor in the encapsulation of TE devices. We have applied a series of fillers in uncured HTPs and determined the viscosity as a function of temperature.15 For some fillers significant lowering of viscosity by the fillers was achieved.
Acknowledgements
Bi2Te3 material sample support from Marlow Industries, Dallas, TX, is gratefully acknowledged.References
- G. S. Nolas, J. Sharp and H. J. Goldsmid, Thermoelectrics Basic Principles and New Materials Developments, Springer Series in Materials Science, Heidelberg, 2001, vol. 45 Search PubMed.
- L. E. Bell, Science, 2008, 321, 1457 CrossRef CAS PubMed.
- W. Brostow, G. Granowski, N. Hnatchuk, J. Sharp and J. B. White, J. Mater. Educ., 2014, 36, 175 Search PubMed.
- H. J. Goldsmid, Materials, 2014, 7, 2577 CrossRef CAS.
- W. Brostow and H. E. Hagg Lobland, Materials: Introduction and Applications, John Wiley & Sons, New York, 2017, ch. 20 Search PubMed.
- Z. H. Zheng, P. Fan, T. B. Chen, Z. K. Cai, P. J. Liu, G. X. Liang, D. P. Zhang and X. M. Cai, Thin Solid Films, 2012, 520, 5245 CrossRef CAS.
- J. Walachová, R. Zeipl, V. Malina, M. Pavelka, M. Jelinek, V. Studnicka and P. Losták, Appl. Phys. Lett., 2005, 87, 081902 CrossRef.
- S. Miura, Y. Sato, K. Fukuda, K. Nishimura and K. Ikeda, Mater. Sci. Eng., A, 2000, 277, 244 CrossRef.
- B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich, B. Yu, X. Yan, D. Wang, A. Muto, D. Vashaee, X. Chen, J. Liu, M. S. Dresselhaus, G. Chen and Z. Ren, Science, 2008, 320, 634 CrossRef CAS PubMed.
- W. Brostow, T. Datashvili, H. E. Hagg Lobland, T. Hilbig, L. Su, C. Vinado and J. B. White, J. Mater. Res., 2012, 27, 2930 CrossRef CAS.
- U. W. Gedde, Polymer Physics, Springer-Kluver, Boston-Dordrecht, 2001 Search PubMed.
- H. Beyer, J. Nurnus, H. Bottner, A. Lambrecht, E. Wagner and G. Bauer, Phys. E, 2002, 13, 965 CrossRef CAS.
- R. E. Hummel, Electronic Properties of Materials, Springer, Lewiston, NY, 4th edn, 2011, ch. 7 Search PubMed.
- G. H. Michler and F. J. Balta-Calleja, Nano- and Micromechanics of Polymers: Structure Modification and Improvement of Properties, Hanser, Munich – Cincinnati, 2012 Search PubMed.
- W. Brostow, J. Chang, H. E. Hagg Lobland, J. M. Perez, S. Shipley, J. Wahrmund and J. B. White, J. Nanosci. Nanotechnol., 2015, 15, 6604 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |