Hydrothermal co-liquefaction of biomasses – quantitative analysis of bio-crude and aqueous phase composition†
René B.
Madsen
,
Rikke Z. K.
Bernberg
,
Patrick
Biller
,
Jacob
Becker
,
Bo B.
Iversen
 and
Marianne
Glasius
and
Marianne
Glasius
 *
*
Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C, Denmark. E-mail: glasius@chem.au.dk
First published on 12th April 2017
Abstract
Hydrothermal liquefaction (HTL) is a promising technology for conversion of wet biomasses to liquid fuels, but considerable amounts of oxygen and nitrogen remain in the bio-crude, while large amounts of water-soluble organics are displaced to the aqueous phase (AqP). In this study the bio-crude and AqP from HTL of 11 different feedstocks of lignocellulosics, residues, macroalgae, microalgae, and their mixtures were analyzed for elemental composition, total acid number, total organic carbon (TOC), total nitrogen, and pH. Quantitative analysis of major compound classes present in both bio-crudes and AqPs was achieved using gas chromatography coupled to mass spectrometry employing prior derivatization of authentic standards. A wide range of biochemical content was obtained through mixing of biomasses and quantitative analysis showed particular interaction between carbohydrates and proteins with extended effect on lipids. The ability of ammonia and amines to form Schiff bases was the key factor affecting elemental distribution and the direction of reaction pathways involved in the formation of cyclic oxygenates, hydroxypyridines, oxygenated aromatics, diols, and fatty acids in bio-crudes. Similarly, Schiff base formation accounts for increased formation of nitrogen-containing compounds in the AqP, leading to a decrease in TOC and total nitrogen in products from HTL of mixed biomasses. This work highlights the quantitative differences in bio-crude and AqP composition from HTL of varying biomasses and provides new knowledge of the effect of mixing biomasses on elemental distribution and composition of product fractions. The results provide valuable information for optimizing the feedstocks used for HTL based on biochemical composition.
1. Introduction
As concern for the environmental impact of fossil fuels is increasing, governmental policies aim at becoming less dependent on fossil fuels, and the pursuit for sustainable bio-fuels and chemicals is receiving more attention, a variety of biomasses and residues available in high volumes are being researched with the aim of providing drop-in bio-fuels. Biomasses and residues have high natural moisture contents, making wet processing methods desirable in order to avoid the energy penalty of drying. Hydrothermal liquefaction (HTL) is carried out in aqueous media at medium temperature (e.g. 350 °C) and high pressure (e.g. 250 bar) with typical solid loadings of 5–30 wt%, converting biopolymers to a bio-crude which can be further upgraded to a drop-in fuel comparable to gasoline, diesel, and kerosene.1–3Since any organic feedstock is amenable to HTL, a vast number of feedstocks have been investigated for their conversion efficiency in HTL. This includes lignocellulosics, macroalgae, microalgae, and a variety of residues.4–7 The HTL process generates four different phases; a desired bio-crude, an aqueous phase (AqP) as well as minor fractions of solid residue and gas. Yields of 5–10% gas, 20–40% bio-crude, 30–60% AqP, and 5–25% solid residue are typically reported for batch experiments.8 These values depend on reactor type, biochemical composition of the biomass, process conditions, and product workup. The gas phase consists predominantly of carbon dioxide with minor contributions of hydrogen and volatile organic compounds.9,10 The substantial amount of organics lost to the AqP has been recognized as an important aspect for making HTL economically feasible especially when processing lignocellulosics.11 The bio-crude has predominantly been further processed through hydrotreatment to a potential drop-in fuel while a few attempts have been made at blending with conventional fossil based fuels.2,12
Bio-crude from HTL has been extensively analyzed for general characteristics such as total acid number (TAN), elemental distribution (CHNS–O), higher heating value, water content, viscosity, and boiling point distribution. More advanced techniques such as nuclear magnetic resonance (NMR) and gas chromatography coupled to mass spectrometry (GC-MS) has been employed to obtain molecular information.4,13–15 However, validated quantitative GC-MS analyses applying authentic standards are seldom reported, and interpretations are typically based on relative peak areas, which are highly dependent on sample type, relative response of compounds, sample preparation, instrumental settings, and data processing. The use of relative peak areas thus only allows meaningful comparison between samples from a single feedstock, processed in the same HTL system and analyzed with the same instrumental settings and data processing methods, which makes comparison of literature values difficult at best.
The vast majority of compounds reported from HTL studies contain one or more heteroatoms. Especially the presence of nitrogen is problematic due to the formation of NOx gases upon combustion and difficulties with hydro-denitrogenation during upgrading.16 The presence of oxygen has several negative effects as well, such as lower heating values, decreased stability of the bio-crude, and higher hydrogen demand during upgrading. Being able to relate elemental distribution to bio-crude composition could provide increased knowledge of the pathways for formation of oxygenated and nitrogenated compounds.
Limited analytical focus has been applied to the AqP and measurements typically include total organic carbon (TOC), total nitrogen (TN), total ammonia, total phosphate, mineral content, and pH.17 Recently a few studies have developed methods for quantitative GC-MS and GCxGC-MS analysis of the AqP.18,19 The complexity of the product fractions imply that micro-emulsions are likely occurring in both bio-crude and aqueous phase.20 Hence, relating compositional information of these very related product fractions could lead to added knowledge of both reaction pathways and dispersion of compounds.
Recently, the effect of mixing different strains of microalgae feedstocks for HTL was investigated showing that the nitrogen distribution could be estimated from the protein content.21 Another recent study investigated the effect of mixing microalgae, wood, and sugar beet feedstocks showing that the nitrogen content of the bio-crude increased when microalgae was mixed.22 Other studies on co-liquefaction of biomass have related the elemental distribution to the composition of bio-crude using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) and GC-MS based on relative peak areas.23,24
In this work we applied one set of processing conditions to a range of 11 feedstocks and eight mixtures of them spanning biochemical composition from high carbohydrate to high lipid or protein, as well as lignin. The biomasses included lignocellulosic (softwood, hardwood, and perennial grasses), aquatic (microalgae and macroalgae), and residues (household waste and DDGS). The study is limited by the use of batch reactors requiring a solvent-based work up, which may alter the composition of especially the bio-crude.
Bio-crude and AqP were characterized with quantitative analytical methods to ensure comparable results. The bio-crude was characterized with CHNS–O, TAN, and GC-MS while the AqP was characterized with TOC, TN, pH, and GC-MS. The analytical GC-MS methods were in both cases validated prior to use and included authentic standards. The link between elemental distribution and specific compound classes was studied through measured and calculated values for mixtures of biomass. However, it should be noticed that oxygen content of bio-crudes was measured by difference leaving a degree of uncertainty in evaluating elemental distribution. A network of reaction pathways was subsequently constructed based on presented results.
2. Materials and methods
2.1 Chemicals and reagents
Potassium carbonate, chloroform (HPLC grade), dichloromethane (HPLC grade), methyl chloroformate (MCF), N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA), and pyridine (HPLC grade) were purchased from SigmaAldrich. Standards were obtained from SigmaAldrich, Acros Organics, or Fluka (see Table 2 and Table S3† for a full list). Household waste was obtained in 12 wt% aqueous solution. Miscanthus x giganteus (M. x giganteus), willow wood, and poplar wood was supplied by Department of Agroecology, Aarhus University (Denmark). Timothy white clover (Clover) was harvested in Scotland. Dried Distillers Grains with Solubles (DDGS) was delivered from Lantmännen Agroetanol AB, Norrköping, Sweden. Microalgae included Spirulina and Chlorella vulgaris (C. vulgaris) acquired from commercial sources, and Nannochloropsis gaditana (N. gaditana) from Lgem, The Netherlands, macroalgae included Laminaria hyperborea (L. hyperborea) from the University of Leeds (UK), and Laminaria digitata (L. digitata) from the Danish Technological Institute, Denmark.2.2 Hydrothermal liquefaction
Biomass slurries were prepared by mixing 10 wt% biomass, 2 wt% potassium carbonate, and 88 wt% demineralized water. Feedstocks consisted of 11 biomasses and eight mixtures; poplar, spirulina, and C. vulgaris were mixed in binary and ternary mixtures. DDGS, M. x giganteus, and N. gaditana were also prepared as separate binary and ternary mixtures. The biochemical composition of biomasses and mixtures is presented in Table 1 on dry and ash free basis. HTL experiments were performed in 20 mL batch reactors from Swagelok. Experiments were initiated by loading 10 mL of 10 wt% biomass slurry into the reactor. Reactors were sealed and lowered into an Omega Engineering FSB-4 fluidized sand bath (FSB) preheated to 350 °C. A reaction time of 20 min was applied, after which the reactors were cooled to ambient temperature in a water bath. The reactors were vented and the AqP was decanted into a centrifuge tube which was centrifuged at 6500 rpm for 5 min before the AqP was transferred with a glass pipette to a preparative glass. The AqP was then stored at 5 °C until further analysis. The centrifuge tube was washed with 2 mL of dichloromethane and the reactor was extracted with 4 × 3 mL of dichloromethane which were combined. The dichloromethane phase was vacuum filtrated, and the residue washed with dichloromethane until the filtrate appeared clear. Dichloromethane was evaporated under a stream of nitrogen until constant weight. Each experiment was performed in duplicate and average values are reported. These conditions have been used during several experiments and the repeatability in recovering both the bio-crude and AqP has previously been presented.25,26 Standard deviations are provided, which are therefore based on the entire HTL process and analytical uncertainty including sampling of the highly viscous bio-crude.| C | H | N | S | Oa | Carbohydrate | Protein | Lipid | Lignin | |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated by difference. b Calculated by difference. | |||||||||
| M. x giganteus | 46.69 | 6.24 | 0.49 | 0.09 | 43.73 | 73.8b | 3.0 | 5.4 | 17.8 |
| Willow | 47.20 | 6.28 | 0.22 | 0.04 | 45.67 | 75.5b | 1.4 | 4.5 | 18.5 |
| Poplar | 47.65 | 6.27 | 0.22 | 0.01 | 45.06 | 80.9b | 1.3 | 5.2 | 12.7 |
| Timothy white clover | 47.61 | 6.32 | 1.41 | 0.07 | 38.23 | 74.5b | 8.8 | 8.2 | 8.5 |
| L. digitate | 35.71 | 5.89 | 0.74 | 0.46 | 43.20 | 91.4b | 4.6 | 3.2 | 0.9 |
| L. hyperborea | 31.29 | 5.10 | 1.62 | 0.82 | 34.30 | 82.7b | 10.1 | 6.0 | 1.3 |
| C. vulgaris | 47.86 | 6.94 | 7.85 | 0.51 | 25.44 | 26.5 | 49.1 | 16.3 | 0.0 |
| Spirulina | 48.09 | 6.51 | 10.91 | 0.89 | 15.02 | 14.0 | 68.2 | 20.0 | 0.0 |
| N. gaditana | 49.95 | 7.36 | 7.40 | 0.55 | 26.18 | 14.4 | 46.3 | 37.6 | 0.0 |
| DDGS | 44.98 | 6.92 | 5.23 | 0.85 | 36.59 | 35.0 | 32.7 | 22.4 | 2.8 |
| House hold waste | 42.98 | 5.59 | 2.10 | 0.27 | 31.48 | 42.2 | 13.1 | 40.0 | 3.2 |
| Nanno/DDGS | 47.47 | 7.14 | 6.32 | 0.70 | 31.39 | 24.7 | 39.5 | 30.0 | 1.4 |
| DDGS/Misc | 45.84 | 6.58 | 2.86 | 0.47 | 40.16 | 54.4 | 17.9 | 13.9 | 10.3 |
| Misc/Nanno | 48.32 | 6.80 | 3.95 | 0.32 | 34.96 | 44.1 | 24.7 | 21.5 | 8.9 |
| Misc/Nanno/DDGS | 47.21 | 6.84 | 4.37 | 0.50 | 35.50 | 41.1 | 27.3 | 21.8 | 6.9 |
| Pop/Vulg | 47.76 | 6.61 | 4.04 | 0.26 | 35.25 | 53.7 | 25.2 | 10.8 | 6.4 |
| Spir/Vulg | 47.98 | 6.73 | 9.38 | 0.70 | 20.23 | 20.3 | 58.7 | 18.2 | 0.0 |
| Spir/Pop | 47.87 | 6.39 | 5.57 | 0.45 | 30.04 | 47.5 | 34.8 | 12.6 | 6.4 |
| Spir/Pop/Vulg | 47.87 | 6.57 | 6.33 | 0.47 | 28.51 | 40.5 | 39.5 | 13.8 | 4.2 |
2.3 Analytical methods
Quantitative GC-MS analysis of bio-crude was performed with multicomponent standard solutions prepared in dichloromethane. Samples and standard solutions were mixed with 100 μl of MSTFA and made up to a total volume of 1.00 mL with dichloromethane containing internal standard (p-bromotoluene 20 μg mL−1). Dichloromethane contained additional internal standards (bromoacetic acid and 4-bromophenol) used to monitor the silylation efficiency. Samples were vortexed and placed on a shaker board for one hour. Analysis was performed using an Agilent 7890B GC coupled to a quadrupole mass filter MS (Agilent, 5977A). The GC injection port was operated at 280 °C in 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split mode with the carrier gas, helium, at 1 mL min−1, and the injection volume of 1 μl. The column was a VF-5 ms (60 m × 0.25 mm × 0.25 μm, 5 m EZ-guard, Agilent) with a 5% phenyl, 95% dimethylpolysiloxane stationary phase. The column oven program started at 40 °C which was held for 5 min, progressing at 10 °C min−1 to 100 °C, at 4 °C min−1 to 280 °C (hold time 3 min), and at 10 °C min−1 to 320 °C (hold time 4 min) giving a total run time of 64 min.
1 split mode with the carrier gas, helium, at 1 mL min−1, and the injection volume of 1 μl. The column was a VF-5 ms (60 m × 0.25 mm × 0.25 μm, 5 m EZ-guard, Agilent) with a 5% phenyl, 95% dimethylpolysiloxane stationary phase. The column oven program started at 40 °C which was held for 5 min, progressing at 10 °C min−1 to 100 °C, at 4 °C min−1 to 280 °C (hold time 3 min), and at 10 °C min−1 to 320 °C (hold time 4 min) giving a total run time of 64 min.
Quantitative GC-MS analysis of AqP was performed with multicomponent standard solutions prepared in either water or methanol. Samples and standard solutions were derivatized with methyl chloroformate (MCF) as described in detail elsewhere.25 The same GC-MS and column used for bio-crude analysis was used for AqP analysis. The GC injection port was operated at 280 °C in 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split mode with the carrier gas, helium, at 1 mL min−1, and the injection volume of 1 μl. The column oven program started at 60 °C which was held for 2 min, progressing at 5 °C min−1 to 200 °C, and at 20 °C min−1 to 320 °C (hold time 3 min), giving a total run time of 39 min.
1 split mode with the carrier gas, helium, at 1 mL min−1, and the injection volume of 1 μl. The column oven program started at 60 °C which was held for 2 min, progressing at 5 °C min−1 to 200 °C, and at 20 °C min−1 to 320 °C (hold time 3 min), giving a total run time of 39 min.
In both cases ionization was performed using an electron impact (EI) source in positive ion mode with electron energy of 70 eV. Data was acquired in scan mode (35–500 m/z). The MS was tuned using perfluorotributylamine. Data acquisition was performed using Masshunter software. Analyte responses were normalized with the response of the internal standard before construction of calibration curves. Replicate batch experiments showed only minor differences and the average concentration determined for each experiment is reported and was used for chemometric analysis. Analytes for which a standard was not available were quantified with the calibration curve of an analyte with a similar mass spectrum.
TOC and TN were determined with a Hach Lange DR2800 spectrophotometer with TOC-kit LCK387 and TN-kit LCK338, respectively.
Total acid number was determined by dissolving approximately 20 mg of bio-crude in 50 mL of acetone along with thymol blue (thymolsulphonephthalein). The solution was titrated with 0.1 M potassium hydroxide until persistent color change. The total acid number was calculated as the amount of potassium hydroxide in mg needed to neutralize one gram of bio-crude.
Elemental composition of biomass and bio-crude was determined using a CHNS–O Elementar Vario MACRO cube analyzer (Elementar Analysensysteme). Oxygen content was determined by difference.
Total carbohydrate content was determined using the reaction of phenol and sulfuric acid with carbohydrate followed by colorimetric determination at 420 nm.27 Protein content was calculated by applying a factor of 6.25 to the nitrogen content determined from CHNS. Total lipid content was determined gravimetrically after extraction in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform and methanol aided by sonication. The total lignin content was determined with the acetyl bromide method upon extensive washing to remove proteins.28 The biochemical composition of feedstocks, on dry and ash free basis is presented in Table 1 (mixtures were calculated from the pure feedstocks). The methods for determination of carbohydrate, protein, and lipid contents were adapted from analysis of microalgae6 and some degree of matrix effects are expected, especially regarding carbohydrate determination of lignocellulosics and macroalgae which were overestimated and underestimated, respectively. Therefore, carbohydrate contents of lignocellulosics and macroalgae were determined by difference.
1 chloroform and methanol aided by sonication. The total lignin content was determined with the acetyl bromide method upon extensive washing to remove proteins.28 The biochemical composition of feedstocks, on dry and ash free basis is presented in Table 1 (mixtures were calculated from the pure feedstocks). The methods for determination of carbohydrate, protein, and lipid contents were adapted from analysis of microalgae6 and some degree of matrix effects are expected, especially regarding carbohydrate determination of lignocellulosics and macroalgae which were overestimated and underestimated, respectively. Therefore, carbohydrate contents of lignocellulosics and macroalgae were determined by difference.
2.4 Principal component analysis
Principal component analysis (PCA) is often used as an exploratory method to gain an overview of multivariable data in the form of either groupings, correlations, or outliers.29 The data is transformed into a set of scores and loadings along with residuals, to maximize the covariation between samples. This leads to a set of principal components (PC) situated along the maximum variation of the data. The scores represent the projection of each sample onto each PC while the loadings represent the weight of each variable on each PC. Hence, similarity or dissimilarity of samples can be evaluated based on score plots, and the weight of each variable can be assessed through loading plots.Fold changes are often observed for raw data and without scaling samples with high concentrations will tend to present the largest weight and dominate the data analysis. Discrete data, such as quantitative results, are typically autoscaled, this means that for each sample the mean value of each variable is subtracted and it is divided by the standard deviation for each variable. Evaluating PCA after scaling means that samples with high scores on a given PC do not necessarily contain high concentrations of a variable. However, it will have a higher concentration than other samples with a lower score.
3. Results and discussion
3.1 Analysis of bio-crudes
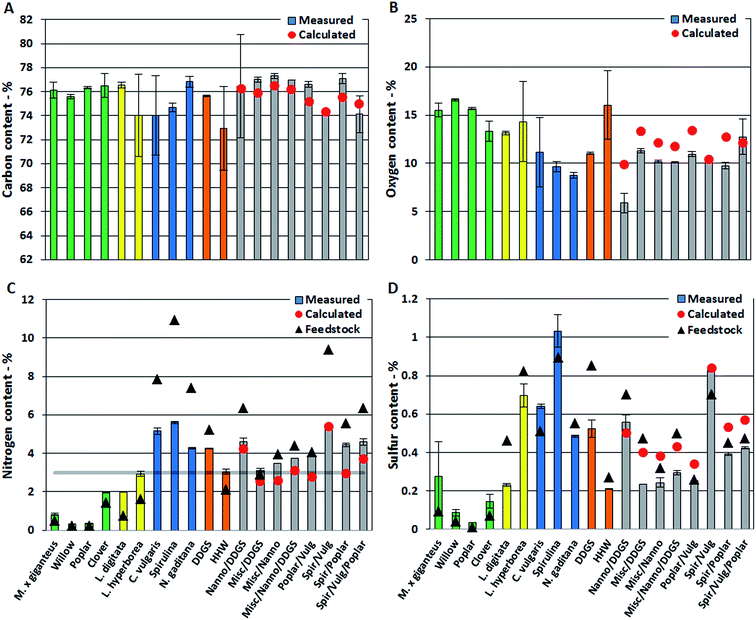
In this section we present quantitative analysis of elemental composition, TAN, and single compounds from different compound classes detected in the bio-crudes. Measured values are provided for bio-crudes of biomasses and mixtures of biomasses. Expected values for bio-crudes of mixtures of biomasses are calculated from the average values measured from bio-crude of neat biomasses. Effects from mixing biomasses are explained by comparing measured and calculated values. First observations from elemental analysis and TAN are presented which are then explained from GC-MS analysis.The oxygen content of bio-crudes varied from 6–17%, which is significantly lower than the biomasses which contained from 15% for Spirulina and up to 46% for willow (Fig. 1B). The oxygen content in bio-crudes followed the order of lignocellulosics > macroalgae > microalgae, while residues showed oxygen contents in between. Generally, measured oxygen content of bio-crudes from mixtures was lower than calculated oxygen content. The effect on carbon and oxygen content in bio-crude when mixing biomasses is proposed to be caused by a change in reaction pathway explained in Section 3.1.2.
The nitrogen content of bio-crudes ranged from 0.3–5.6% (Fig. 1C). Generally it was observed that the nitrogen content is increased in bio-crudes from neat biomasses and mixtures with <3% feedstock nitrogen while it is decreased in bio-crudes made from biomasses with >3% feedstock nitrogen, with a few exceptions. Bio-crudes from neat lignocellulosics, macroalgae and household waste (<3% feedstock nitrogen) had twice the nitrogen content of the feedstock, while the nitrogen content in bio-crudes from microalgae, DDGS, and mixtures (>3% feedstock nitrogen) decreased, dependent on the nitrogen content of the feedstock. It is especially noteworthy that the nitrogen in bio-crudes from mixtures of lignocellulosics and microalgae (which exhibit a combined high protein and carbohydrate content) were comparable to those in bio-crudes made from microalgae with significantly higher protein contents. Furthermore, the measured values of nitrogen content in these mixtures were generally higher than the calculated values. Thus, the reaction between degradation products from carbohydrates and proteins lead to formation of products soluble in the bio-crude retaining more of the nitrogen. To further investigate this trend, mixtures of model compounds (cellulose, soy protein, and rapeseed oil) were subjected to HTL. The nitrogen content of bio-crude from pure protein was 5.8%, when mixing protein with rapeseed oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) it was 2.7, while it was 5.6 when mixing 1
1) it was 2.7, while it was 5.6 when mixing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with carbohydrate (ESI I†).
1 with carbohydrate (ESI I†).
The sulfur content of the bio-crudes was between 0.04% and 1.03% (Fig. 1D). The sulfur content increased slightly for bio-crudes of lignocellulosics, Spirulina, and C. vulgaris while it decreased in all other bio-crudes. The significant amounts of nitrogen and sulfur will lead to formation of NOx and SOx emission during combustion and further upgrading of the bio-crude is necessary to obtain a cleaner bio-fuel.
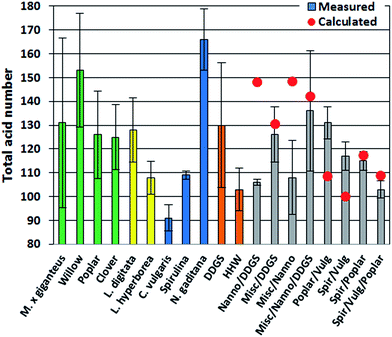
The TAN is an important indicator of potential corrosion effects by the bio-crude. In the petroleum industry high TANs are associated with naphthenic acids and corrosion is generally higher for crude oils with TAN values higher than 0.5.30 However, TAN only indicates the potential for corrosion since the identity of the acids is equally important. In bio-crudes the TAN varied from 91 mg g−1 for C. vulgaris to 166 mg g−1 for N. gaditana. Bio-crudes of lignocellulosics, macroalgae, Spirulina, and household waste generally had TANs of 100–130 mg g−1. No obvious trend for bio-crudes of mixtures could be observed and no apparent correlation to GC-MS analysis could be made (Fig. 2).
 | ||
| Fig. 3 Biplots of scores and loadings from analysis of bio-crudes – (A) PC1 and PC2, (B) PC1 and PC3. | ||
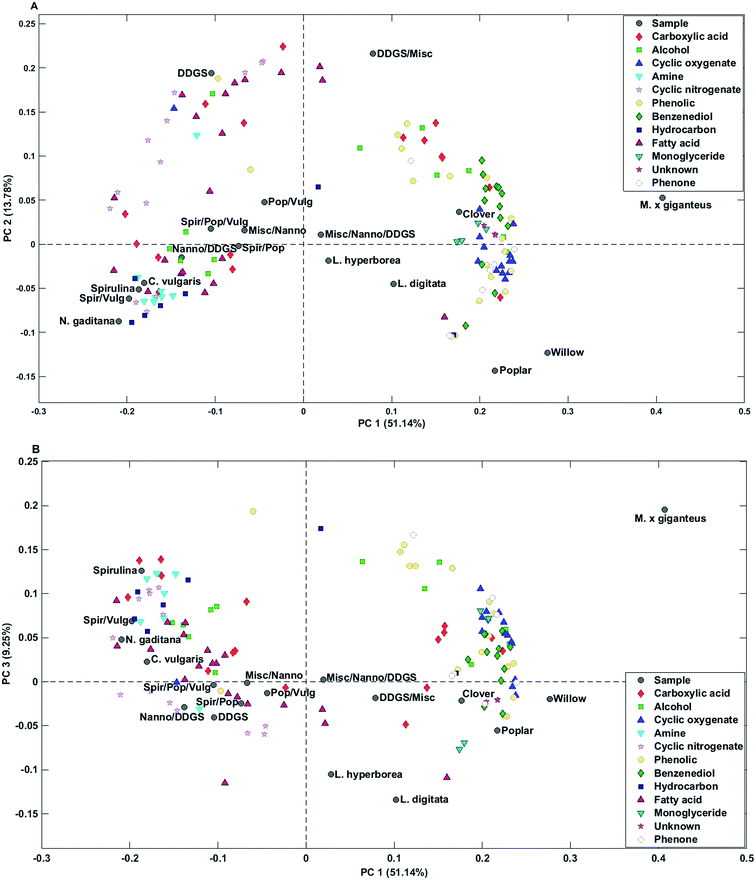
Bio-crude from household waste was distinctively different from the remaining samples and was not included in any of the mixtures. Therefore, the sample was removed from the PCA. PC1 explained 51.1% of the total variance and separated bio-crudes of microalgae and residue from mixtures and macroalgae, which were further separated from bio-crudes of lignocellulosics. PC2 explained 13.8% of the total variance and separated bio-crudes with increasing scores in the order wood, microalgae, macroalgae, mixtures, grass, and DDGS. Small organic acids (C2–C4) in the form of acetic acid, propionic acid, butyric acid, glycolic acid, and lactic acid were characteristic for bio-crudes of grass while formic acid and C5 acids were characteristic of DDGS and >C6 acids were found in bio-crudes of microalgae. Alcohols were less well-grouped but smaller alcohols of ethylene glycol, propylene glycol, and butane-2,3-diol were found in higher concentrations in lignocellulosics, while monoalcohols were most prevalent for microalgae, and glycerol was found in highest concentrations in DDGS bio-crude.
Cyclic oxygenates were highly characteristic of lignocellulosics, which has previously been attributed to their formation from degradation of carbohydrates.32 Especially ketones of C5 ring structures are abundant, as previously reported.33 In this work cyclopentanones had positive values and cyclopent-2-enones had negative values on PC2 indicating that higher concentrations of cyclopentanones are obtained from grass feedstocks compared to wood feedstocks and vice versa for cyclopent-2-enones.
Cyclic nitrogenates were characteristic of microalgae and DDGS. The main cyclic nitrogenates of DDGS were hydroxypyridines and pyrrolidine, while microalgae showed higher concentrations of piperidines and indoles.
Phenolics and benzenediols are mainly produced from degradation of lignin and to some extent from carbohydrates.32 Generally it was observed that alkylated phenols and catechols had positive loading on PC1-3 separating M. x giganteus from clover and wood. Alkylated phenols were also characteristic of DDGS.
Microalgae were generally characterized by higher concentrations of C16–17 fatty acids and hydrocarbons from degradation of pigments, while DDGS were characterized by higher concentrations of C18 fatty acids. Despite a significantly lower lipid content in macroalgae and lignocellulosics these bio-crudes were characterized by higher concentrations of monoglycerides which could originate from partial breakdown of triglycerides. Bio-crudes from grass were further characterized by higher concentrations of 2-monopalmitoyl glycerol and 2-monostearin glycerol while macroalgae and wood bio-crudes had higher concentrations of 1-monopalmitoyl glycerol and 1-monostearin glycerol. Furthermore, it is interesting to note that neither monopalmitoleoyl glycerol nor monooleyl glycerol were detected.
Selected abundant compounds from each compound class were quantitated in order to evaluate the quantitative effects of the different feedstocks and their mixtures (Table 2). Results of bio-crude from household waste have been included for comparison, despite the apparent outlier nature of this sample.
| M. x giganteus mg g−1 | Willow mg g−1 | Poplar mg g−1 | Clover mg g−1 | L. digitata mg g−1 | L. hyperborea mg g−1 | C. vulgaris mg g−1 | Spirulina mg g−1 | N. gaditana mg g−1 | DDGS mg g−1 | HHW mg g−1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-Methylcyclopentanone | 0.46 ± 0.02 | 0.35 ± 0.01 | 0.04 ± 0.02 | 0.36 ± 0.05 | 0.14 ± 0.01 | 0.052 ± 0.02 | 0.005 ± 0.002 | ND | 0.007 ± 0.001 | 0.023 ± 0.003 | 0.18 ± 0.06 |
| 2,3-Dimethylcyclopent-2-enone | 3.71 ± 0.18 | 3.23 ± 0.09 | 2.93 ± 0.27 | 2.90 ± 0.15 | 1.42 ± 0.07 | 1.06 ± 0.12 | 0.47 ± 0.02 | 0.15 ± 0.02 | 0.27 ± 0.02 | 0.67 ± 0.19 | 1.70 ± 0.18 |
| 3-Hydroxypyridine | 0.094 ± 0.022 | 0.054 ± 0.005 | 0.059 ± 0.07 | 0.34 ± 0.01 | 0.18 ± 0.02 | 0.16 ± 0.00 | 0.21 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.40 ± 0.06 | 0.34 ± 0.08 |
| Palmitic acid | 6.54 ± 0.59 | 5.02 ± 0.37 | 8.33 ± 0.05 | 10.52 ± 0.07 | 14.31 ± 1.12 | 20.15 ± 2.75 | 27.85 ± 1.97 | 41.52 ± 0.14 | 31.36 ± 0.07 | 28.91 ± 4.99 | 17.61 ± 1.12 |
| Phenol | 5.81 ± 0.22 | 1.36 ± 0.02 | 5.17 ± 0.15 | 3.97 ± 0.28 | 0.53 ± 0.01 | 0.81 ± 0.05 | 1.85 ± 0.00 | 2.83 ± 0.12 | 1.36 ± 0.02 | 1.95 ± 0.20 | 1.15 ± 0.10 |
| 2-Methoxyphenol | 2.73 ± 0.51 | 2.28 ± 0.05 | 2.54 ± 0.08 | 1.03 ± 0.04 | ND | ND | ND | ND | ND | 0.022 ± 0.004 | 0.24 ± 0.01 |
| 4-Ethylphenol | 5.71 ± 0.66 | 0.21 ± 0.02 | 0.45 ± 0.01 | 2.01 ± 0.07 | 0.045 ± 0.000 | 0.54 ± 0.07 | 0.65 ± 0.03 | 1.71 ± 0.31 | 0.62 ± 0.04 | 1.02 ± 0.05 | 0.41 ± 0.05 |
| Ethyleneglycol | 0.50 ± 0.09 | 0.37 ± 0.02 | 0.32 ± 0.02 | 0.40 ± 0.28 | 0.32 ± 0.02 | 0.08 ± 0.01 | 0.035 ± 0.003 | 0.014 ± 0.003 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.48 ± 0.05 |
| Propyleneglycol | 0.42 ± 0.04 | 0.31 ± 0.01 | 0.28 ± 0.01 | 0.34 ± 0.15 | 0.27 ± 0.03 | 0.17 ± 0.01 | 0.14 ± 0.03 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.17 ± 0.02 | 0.39 ± 0.04 |
| Glycerol | 0.22 ± 0.03 | 0.18 ± 0.02 | 0.19 ± 0.01 | 0.26 ± 0.10 | 0.27 ± 0.01 | 0.24 ± 0.01 | 0.36 ± 0.11 | 0.43 ± 0.13 | 0.56 ± 0.15 | 0.93 ± 0.27 | 1.67 ± 0.35 |
| Formic acid | 0.37 ± 0.04 | 0.20 ± 0.01 | 0.13 ± 0.01 | 0.39 ± 0.05 | 0.32 ± 0.04 | 0.44 ± 0.04 | 0.32 ± 0.06 | 0.36 ± 0.03 | 0.28 ± 0.01 | 1.00 ± 0.01 | 0.42 ± 0.00 |
| Acetic acid | 2.43 ± 1.43 | 1.94 ± 0.21 | 0.31 ± 0.03 | 2.40 ± 1.01 | 1.54 ± 0.25 | 1.04 ± 0.09 | 0.79 ± 0.57 | 0.88 ± 0.04 | 0.61 ± 0.26 | 1.55 ± 0.01 | 4.10 ± 0.43 |
| Glycolic acid | 0.81 ± 0.47 | 0.21 ± 0.02 | 0.14 ± 0.01 | 0.92 ± 0.48 | 0.41 ± 0.12 | 0.25 ± 0.02 | 0.030 ± 0.014 | 0.11 ± 0.02 | 0.086 ± 0.024 | 0.29 ± 0.09 | 0.67 ± 0.06 |
| Lactic acid | 2.84 ± 1.55 | 0.85 ± 0.01 | 0.61 ± 0.01 | 4.28 ± 2.14 | 5.13 ± 0.98 | 2.33 ± 0.03 | 0.45 ± 0.23 | 0.98 ± 0.17 | 1.04 ± 0.43 | 2.22 ± 0.62 | 6.76 ± 0.69 |
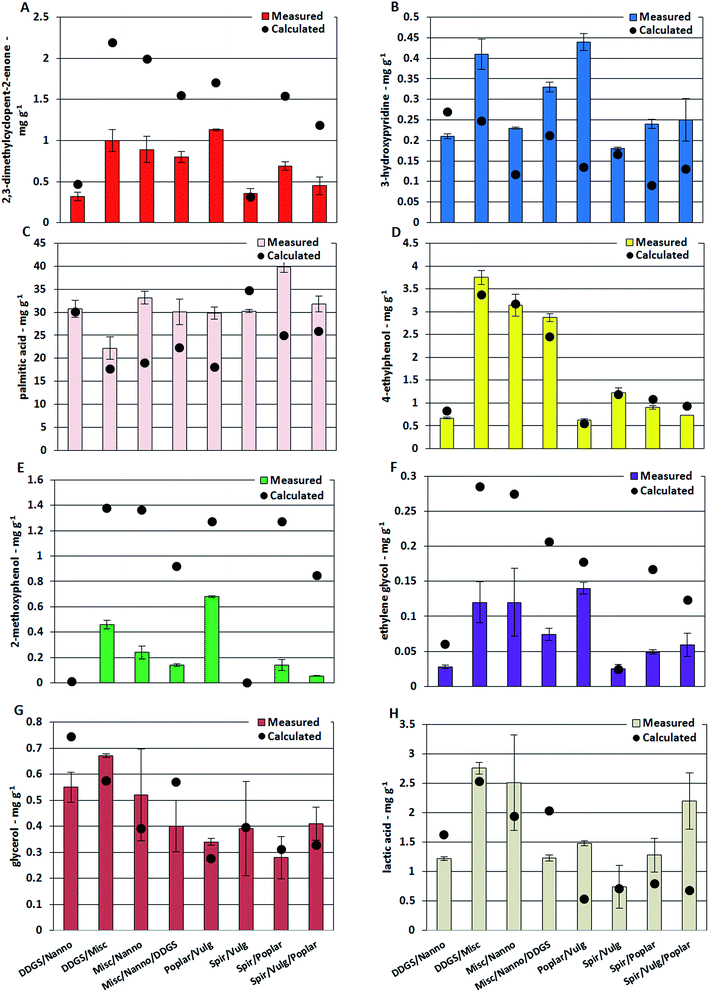
The highest concentrations of 3-methylcyclopentanone and 2,3-dimethylcyclopent-2-enone were found in bio-crude from M. x giganteus with 0.46 mg g−1 and 3.71 mg g−1, respectively. These compounds are primarily produced from carbohydrates and the different carbohydrate structures of macroalgae are likely the reason for the lower concentrations of these cyclic oxygenates in bio-crudes from L. digitate and L. hyperborea.34 The concentration of cyclic oxygenates was significantly lower for bio-crudes of microalgae and DDGS while the concentration of 3-hydroxypyridine was highest for bio-crude of DDGS (0.40 mg g−1). Previous studies have suggested that 3-hydroxypyridines are formed from reaction between ammonia and furfurals and 2-acetylfurans which are formed from degradation of especially cellulose.35,36 Several studies have identified the formation of Schiff bases, in what is often referred to as Maillard type reactions, as a key reaction for formation of nitrogen containing compounds during HTL. In this work, the formation of Schiff bases from degradation products of carbohydrates and protein is proposed to account for the decreased oxygen content and increased nitrogen content observed for the bio-crudes of mixtures of biomasses. Fig. 3 shows the concentrations of single compounds in bio-crudes from mixtures of biomass. Bio-crudes from mixtures of microalgae and DDGS had concentrations of 2,3-dimethylcyclopent-2-enone and 3-hydroxypyridine, which could be calculated from the averages detected in bio-crudes from the respective feedstocks (Fig. 4A and B). In contrast, the measured concentrations were approximately 0.5 times for 2,3-dimethylcyclopent-2-enone and 2 times for 3-hydroxypyridine compared to the calculated concentrations when mixing biomasses of lignocellulosics and microalgae. Hence, the degradation products which lead to the formation of cyclic oxygenates are converted into nitrogen-containing ring structures which include hydroxypyridines, pyrazines (further discussed in Section 3.2.2), and quinolines (submitted for publication). Pyrazines were not present in the bio-crude due to the work up method employed but are known to be highly prevalent from DDGS.20
Palmitic acid was the most abundant compound in most bio-crudes with up to 41.5 mg g−1 from Spirulina while the lowest concentration was detected from willow with 5.0 mg g−1. The measured concentration of palmitic acid in bio-crudes from mixtures of microalgae and DDGS was in agreement with the calculated concentration, while measured concentrations in mixtures of lignocellulosics (high carbohydrate) and microalgae (high protein) were higher than the calculated concentrations (Fig. 4C). This could have two reasons; (1) carbohydrate and protein degrades significantly faster than triglycerides during the HTL process leading to reaction of ammonia with products from carbohydrates; (2) the ammonia reacts significantly faster with products from carbohydrates (aldehydes and ketones) than with free fatty acids. Consequently, more fatty acids and less fatty amides are formed. The combined effect of increased fatty acid and Schiff base formation with less formation of small alcohols and organic acids could partly explain the increased carbon content observed for bio-crudes from mixtures of lignocellulosics and microalgae, which was not present for microalgae and DDGS mixtures. Further palmitic acid and stearic acid was found to be present in the form of monoglycerides, which had an estimated concentration of 1–10 mg g−1. Assuming a linear dependency, the concentration of 1-palmitoyl glycerol and 1-stearoyl glycerol were approximately two times higher in bio-crude from M. x giganteus and wood compared to algae and DDGS, while 2-palmitoyl glycerol and 2-stearoyl glycerol were approximately three times higher in macroalgae compared to microalgae and DDGS with lignocellulosics having concentrations in between (ESI II†). Furthermore, it was observed that roughly 10 times more 1-palmitoyl glycerol and 1-stearoyl glycerol were found compared to 2-palmitoyl glycerol and 2-stearoyl glycerol.
Oxygenated aromatics are highly abundant in bio-crude from lignocellulosics due to degradation of lignin in particular and to some extent from carbohydrates and protein. Protein and lignin are the main contributors to 4-ethylphenol, and its highest concentrations were found in bio-crude from M. x giganteus, Clover, and Spirulina with 5.71 mg g−1, 2.01 mg g−1, and 1.71 mg g−1, respectively, while 10–20 times lower concentrations were observed in bio-crudes from wood. The measured and calculated concentrations of 4-ethylphenol in bio-crudes from mixtures of biomass were in accordance showing that it is formed from degradation of biochemical constituents for which intermediates are not involved in other pathways (Fig. 4D). Similar observations could be made for phenol, which can be formed from degradation of cellulose, indicating that the formation of phenol and cyclopent-2-enones constitute two separate reaction pathways. A phenolic compound produced solely from lignin is 2-methoxyphenol which was abundant in bio-crudes of lignocellulosics such as M. x giganteus (2.73 mg g−1) while being absent in algae bio-crudes. The measured concentration of 2-methoxyphenol in bio-crudes from mixtures was between 1.6 and 15 times lower than the calculated concentrations (Fig. 4E). This indicates that the formation of 2-methoxyphenol from lignin occurs through reactive intermediates that are most likely aldehydes or ketones involved in Schiff base formation.
Another interesting observation is that the measured concentration of ethylene glycol (and butane-2,3-diol) in bio-crudes from mixtures of biomass shows the exact same trend compared to the calculated concentrations as observed for 2,3-dimethylcyclopent-2-enone and 2-methoxyphenol (Fig. 4F). Previous studies have proposed that monosaccharides are degraded through either a retro-aldol reaction or dehydration. The retro-aldol reaction produces small oxygenated compounds which can further deoxygenate to diols, while dehydration produces amongst others furans, furfurals, and γ-keto acids which can ultimately produce hydroxypyridines and pyrrolidin-2-ones in the presence of ammonia. Hence, it is proposed that the retro-aldol and dehydration reaction occur in equilibrium and the addition of nitrogen pulls the equilibrium towards dehydration as furans, furfurals, and γ-keto acids are converted.
Glycerol is generally the most abundant alcohol observed in bio-crudes and up to 0.93 mg g−1 and 1.67 mg g−1 was found from DDGS and household waste, respectively. Bio-crudes from microalgae, macroalgae, and lignocellulosics contained approximately 0.40 mg g−1, 0.25 mg g−1, and 0.20 mg g−1 of glycerol, respectively. Experiments with model compounds have shown that only minor amounts of glycerol are obtained in bio-crudes from cellulose, hemicellulose, and protein meaning that glycerol is almost exclusively obtained from triglycerides (data not shown). DDGS and household waste had the highest lipid content along with the highest glycerol contents of the bio-crude. The significant amount of glycerol found in bio-crudes of macroalgae and lignocellulosics is likely due to the presence of other compounds creating micro-emulsions similar to what is observed for fatty acids of the AqP. The solubility of glycerol is likely linked to the presence of small organic acids of which acetic acid and lactic acid were particularly abundant. Bio-crudes of household waste, L. digitata, and Clover contained some of the highest amounts of acetic acid (4.10 mg g−1, 1.54 mg g−1, and 2.4 mg g−1, respectively) and lactic acid (6.76 mg g−1, 5.13 mg g−1, and 4.28 mg g−1, respectively) while concentrations were significantly lower for bio-crudes of microalgae. The concentration of glycerol and lactic acid in bio-crudes from mixtures showed similar tendencies where the measured concentration of these compounds was lower than the calculated concentration when mixing biomasses of high lipid content together (DDGS and N. gaditana). Meanwhile, the measured concentration was higher or similar to the calculated concentration for the remaining mixtures (Fig. 4G and H).
3.2 Analysis of aqueous phase
The AqP has been recognized as an important fraction for making the HTL process economically feasible due to the distribution of significant amounts of carbon. In this section we report TOC, TN, pH, and quantitative GC-MS analysis of compound classes similar to the bio-crude.| TOC – mg L−1 | TN – mg L−1 | pH | |
|---|---|---|---|
| M. x giganteus | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845 ± 148 845 ± 148 |
231 ± 11 | 6.52 ± 0.40 |
| Willow | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 42 800 ± 42 |
83 ± 1 | 5.71 ± 0.08 |
| Poplar | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095 ± 573 095 ± 573 |
87 ± 4 | 5.76 ± 0.06 |
| Clover | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 ± 424 010 ± 424 |
600 ± 29 | 7.16 ± 0.03 |
| L. digitata | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 ± 445 365 ± 445 |
542 ± 78 | 7.35 ± 0.35 |
| L. hyperborea | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 ± 396 550 ± 396 |
1355 ± 35 | 7.70 ± 0.08 |
| C. vulgaris |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 785 ± 693 785 ± 693 |
6850 ± 375 | 9.09 ± 0.14 |
| Spirulina |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 715 ± 7 715 ± 7 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 150 ± 71 150 ± 71 |
9.49 ± 0.15 |
| N. gaditana |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 660 ± 516 660 ± 516 |
7135 ± 127 | 9.00 ± 0.08 |
| DDGS |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 310 ± 283 310 ± 283 |
5010 ± 693 | 8.14 ± 0.00 |
| HHW | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705 ± 346 705 ± 346 |
2210 ± 863 | 7.15 ± 0.12 |
The TOC values were lowest for AqP from feedstock of lignocellulosics and macroalgae, which were approximately 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1. However, the TOC value increased substantially when feedstocks with >30% protein were used (microalgae and residues), which also lead to pH > 8.00. Furthermore, the TOC value increases steadily with increasing protein content and thereby increasing pH values. It is proposed that the increase in TOC value could be due to enhanced deprotonation of phenolics (phenol pKa 10) and protonation of nitrogenated aromatics (pyridine pKb 8.8) leading to exponential increase in their ionized form and thus higher solubility in water.
000 mg L−1. However, the TOC value increased substantially when feedstocks with >30% protein were used (microalgae and residues), which also lead to pH > 8.00. Furthermore, the TOC value increases steadily with increasing protein content and thereby increasing pH values. It is proposed that the increase in TOC value could be due to enhanced deprotonation of phenolics (phenol pKa 10) and protonation of nitrogenated aromatics (pyridine pKb 8.8) leading to exponential increase in their ionized form and thus higher solubility in water.
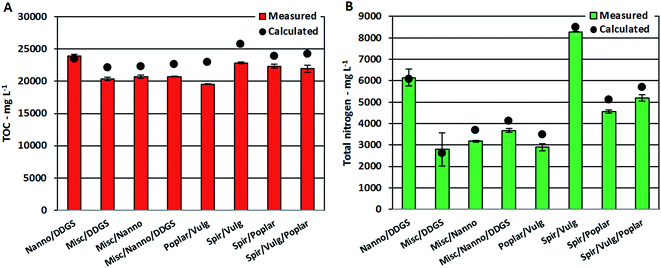
Despite the apparent linear dependency of TOC and TN on protein content, measured TOC and TN values for AqPs from mixtures were approximately 10% lower than calculated values in most cases (Fig. 5A and B). It has previously been shown that increasing concentrations of nitrogenated compounds in the AqP leads to further displacement into the bio-crude.26 Hence, the increasing formation of nitrogenated compounds observed in the bio-crude and AqP (Section 3.2.2) caused by reaction of ammonium with degradation products leads to less ammonium in the AqP and more nitrogen ending up in the bio-crude. Furthermore, the change in equilibria towards dehydration of monosaccharides means that less small polar compounds (<C5) soluble in AqP are formed, which lowers the TOC value.
| Small organic acids mg L−1 | Fatty acids mg L−1 | Oxygenated aromatics mg L−1 | Cyclic oxygenates mg L−1 | Nitrogenates mg L−1 | |
|---|---|---|---|---|---|
| M. x giganteus | 7552 | 40.5 | 392.7 | 1683 | 155.4 |
| Willow | 8063 | 126.5 | 97.8 | 1703 | 73.9 |
| Poplar | 7831 | 109.0 | 345.3 | 1580 | 78.4 |
| Clover | 6200 | 21.3 | 167.5 | 1926 | 464.9 |
| L. digitata | 4446 | 12.1 | 42.0 | 1878 | 186.4 |
| L. hyperborea | 5568 | 13.3 | 66.4 | 1274 | 268.9 |
| C. vulgaris | 7703 | 975.0 | 182.8 | 630.9 | 1966 |
| Spirulina | 6720 | 256.6 | 238.7 | 340.6 | 796.0 |
| N. gaditana | 6651 | 2401 | 194.9 | 497.5 | 899.0 |
| DDGS | 6804 | 237.0 | 119.0 | 1178 | 1927 |
| Household waste | 7388 | 23.3 | 112.7 | 1933 | 1253 |
| Nanno/DDGS | 6484 | 2101 | 164.8 | 870.7 | 1366 |
| DDGS/Misc | 7010 | 37.2 | 266.2 | 1552 | 1233 |
| Misc/Nanno | 7417 | 105.6 | 300.6 | 1144 | 960.5 |
| Misc/Nanno/DDGS | 8024 | 37.4 | 255.0 | 1198 | 1336 |
| Pop/Chlo | 7407 | 17.5 | 256.7 | 1311 | 1704 |
| Spir/Chlo | 7009 | 208.4 | 193.9 | 483.1 | 1301 |
| Spir/Pop | 7874 | 134.6 | 298.4 | 971.5 | 1404 |
| Spir/Pop/Chlo | 8035 | 267.7 | 254.9 | 939.6 | 1633 |
Small organic acids are a major part of the AqP and have a potential for production of value-added chemicals.38 They are also the most diverse group of compounds detected in this work. Total concentrations of small organic acids ranged from a minimum of 4446 mg L−1 in AqP from L. digitata to a maximum of 8063 mg L−1 for willow. However, most AqPs maintained concentrations of approximately 6200–8000 mg L−1.
Acetic acid was the most abundant compound detected in the AqP, with the analytical method used in this work. Acetic acid is especially prevalent from lignocellulosics with concentrations of up to 5226 mg L−1 for willow, which is comparable to previously published results.18 A previous study of model compounds showed the largest formation of acetic acid in the AqP from protein feedstock.37 However, the model compounds had been purified with removal of protective groups, probably leading to reduced formation of acetic acid. DDGS is a residue from bio-ethanol production and has also been purified and showed low concentrations of acetic acid while macroalgae had the lowest concentration despite the feedstock having carbohydrate concentrations similar to lignocellulosics. Microalgae led to AqP with intermediate acetic acid concentrations. Hence, acetic acid is predominantly formed from hydrolysis of acetyl groups especially prevalent in lignocellulosics with contributions from degradation of carbohydrates and proteins. This observation was further supported by the observation that measured concentrations of small organic acids (predominantly acetic acid) showed only minor difference from calculated concentrations (ESI IV†).
Addition of potassium carbonate as catalyst entails some degree of saponification, which will affect the fatty acid concentrations. Fatty acids are insoluble in water, and it has been suggested that fatty acids are present as micro-emulsions.20 The concentration of fatty acids in AqP from HTL of grass species and macroalgae were <41 mg L−1 while wood species gave 109–127 mg L−1 indicating that triglycerides from wood is more easily hydrolyzed in HTL than triglycerides from grass and macroalgae. It is noteworthy that fatty acid concentration of AqP from N. gaditana was approximately 2.3 times higher than C. vulgaris, which is similar to the difference in lipid content, while C. vulgaris had fatty acid concentrations 4 times higher than DDGS despite having similar biochemical composition, with C. vulgaris even having lower lipid content. Comparison of the bio-crude from these samples shows similar fatty acid profiles; hence the difference is unlikely to be explained by the lipid profile of the feedstock. Furthermore, the pH value for AqP of C. vulgaris (pH 9.0) was higher than for DDGS (pH 8.1) which could indicate that saponification is significantly enhanced across this range. The saponification effect is apparent for AqP of mixtures, as measured fatty acid concentrations are significantly lower than calculated concentrations when mixing microalgae and lignocellulosics, while it increases for the mixture of DDGS and N. gaditana (ESI IV†). However, saponification seems to be only part of the explanation as mixtures with lignocellulosics in many cases lead to values that were significantly lower than for lignocellulosic feedstock alone which had acidic AqP. Furthermore, a significantly lower concentration of free fatty acids from Spirulina with C. vulgaris is observed which is due to the higher protein content leading to greater formation of fatty acid amides.39
The most commonly reported oxygenated aromatics were quantitated (phenols, cresols, hydroquinones). Concentrations amongst lignocellulosics varied greatly with M. x giganteus and poplar having the greatest concentrations of 393 and 345 mg L−1 in their AqPs, respectively, while clover and willow had only 168 and 98 mg L−1, respectively. The lowest concentrations were obtained in AqP from macroalgae with less than 70 mg L−1, while AqP from microalgae contained approximately 200 mg L−1 supporting that lignin is the main contributor of oxygenated aromatics but also emphasizing that significant amounts are produced from protein. The substantial concentrations found in AqP from microalgae may be attributed to the alkaline pH of the AqP which is nearing the pKa value of phenol (pKa ∼10) leading to a greater degree of deprotonation and thereby solubility in water leading to increasing TOC values. The formation of oxygenated aromatics seems to be independent of mixing of biomasses as accurate calculation of concentrations in AqP from mixtures could be made (ESI IV†). Thus, these compounds look to be formed mainly from simple degradation of lignin and amino acids while smaller contributions occur from degradation and intramolecular reaction of carbohydrates which do not compete with other reaction pathways.
In previous work with model compounds a linear dependency on carbohydrate content was observed for formation of cyclic oxygenates in AqP.37 The concentration of cyclic oxygenates indeed decreases in the following order lignocellulosics/macroalgae > DDGS > microalgae. The highest concentration was observed for 3-methylcyclopent-2-enone with 1060 mg L−1 in AqP from clover. It is interesting to note that the measured concentration of cyclic oxygenates from mixtures could be calculated (ESI IV†) and did not correlate with observations in the bio-crude. However, it has previously been shown that the concentration of cyclic oxygenates in bio-crude does not necessarily correlate with AqP concentrations.26
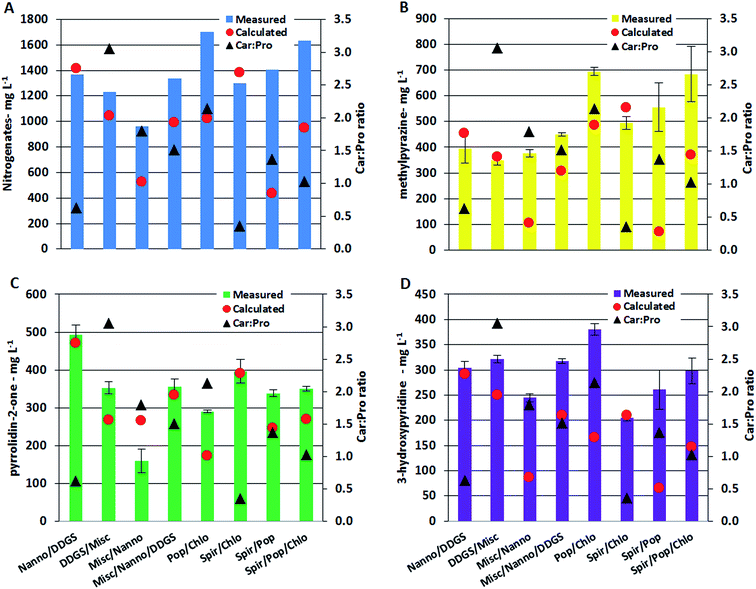
Nitrogenated compounds reported from HTL AqP are most often amides, pyrroles, pyrazines, pyrrolidinones, and hydroxypyridines with pyrazines constituting the most varied compound class. The analytical method used in this work allowed quantification of pyrazines, pyrrolidin-2-one, and 3-hydroxypyridine. Generally the total concentration varied more than one order of magnitude (with up to two orders of magnitude for some pyrazines) between AqP from HTL of different feedstocks. Particularly high concentrations were found in the AqP from HTL of DDGS and C. vulgaris. Pyrazines are formed via several pathways which include (i) self-condensation of amino acids, (ii) Amadori rearrangement (AR) of carbohydrate products and ammonium/amines, and (iii) Strecker type degradation (SD) of carbohydrate products and amino acids.40 Previous work on model compounds showed that the formation of pyrazines highly depends on the ratio of carbohydrate to protein of the feedstock.37 In that work, it was found that a linear fit was required when protein to carbohydrate was <1, a 2nd order polynomial was required when the ratio was >1, and an exponential fit was required when the ratio was ∼1. Similar observations were made in this work which is especially noticed for AqP of mixtures supporting the observations made for TN and TOC values in AqP and elemental distribution in bio-crude. The measured and calculated concentration in AqP of mixtures for nitrogenates, methylpyrazine, pyrrolidin-2-one, and 3-hydroxypyridine are presented along with carbohydrate to protein ratios in the mixtures in Fig. 6A–D. Mixing microalgae together or with DDGS leads to excess protein and the measured and calculated values of the compounds are consistent as expected from a linear dependency. Mixing lignocellulosics with microalgae leads to carbohydrate to protein ratios between 1 and 3, and increasing discrepancy between measured and calculated values are observed as ratios approach 1 which is expected from 2nd order polynomial and exponential functions. The largest differences are generally observed with the mixture of Spirulina and poplar, where a 10 fold higher concentration of methylpyrazine is observed. The relative difference between measured and calculated concentrations is highest for pyrazines, which could mean that if the bio-crude is not solvent extracted a more significant amount of nitrogen will be found in the bio-crude of feedstocks with carbohydrate to protein ratios close to 1.
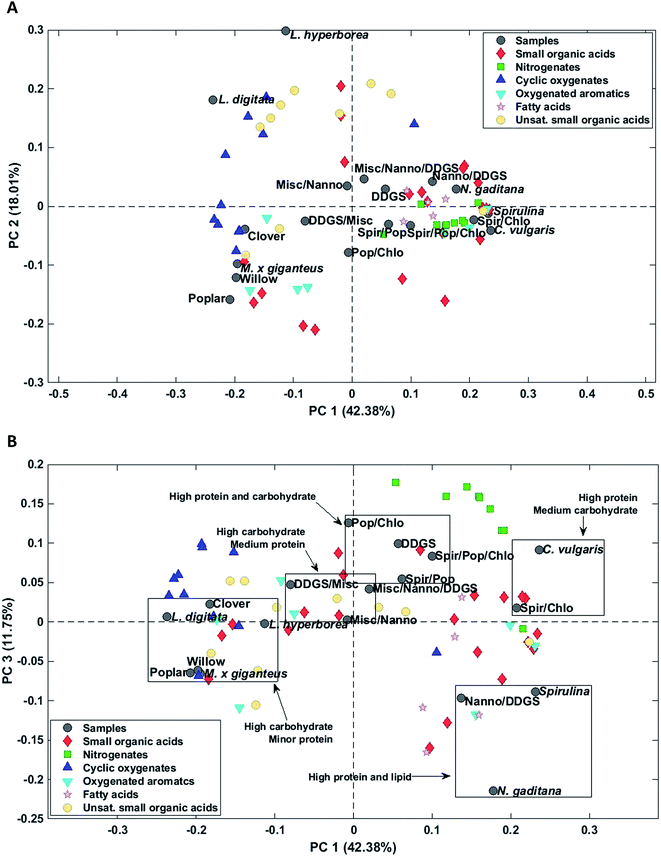
Again AqP from HTL of household waste displayed a highly different composition than the remaining samples and was removed as an outlier. Three principal components explained 69.5% of the total variance. PC1 explained 42.4% of the total variance and separated AqP samples obtained from high carbohydrate feedstocks from samples obtained from high protein and lipid feedstocks (Fig. 7A). Microalgae and most biomass mixtures were clustered together and characterized by higher concentrations of fatty acids, nitrogenates, and numerous small organic acids as well as oxygenated aromatics formed from degradation of amino acids. The major compounds of these compound classes from microalgae were palmitoleic acid (952 mg L−1, N. gaditana), methylpyrazine (969 mg L−1, C. vulgaris), succinic acid (1568 mg L−1, C. vulgaris), and hydrocinnamic acid (88.9 mg L−1, Spirulina/C. vulgaris).
PC2 explained 18.0% of the total variance and separated samples of macroalgae from the remaining samples. AqPs from macroalgae were distinctly different from the other samples and were characterized by higher concentrations of especially unsaturated small organic acids and several cyclic oxygenates and significantly lower concentration of acetic acid (1171 mg L−1, L. hyperborea). The higher concentration of unsaturated small organic acids, mainly C3–5, in AqP from macroalgae is due to the presence of acidic polysaccharides, such as alginate, which decompose and dehydrate. However, the highest concentration was found for methacrylic acid, which was only 124 mg L−1 (L. hyperborea).
AqP of lignocellulosics showed negative scores on PC2 and were characterized by higher concentrations of several small organic acids of which acetic acid is especially prevalent (5226 mg L−1, willow). Thus, PC2 displays either the difference in carbohydrate composition of macroalgae and lignocellulosics, such as the degree of acetylation, or the difference in their processing due to the higher ash content of macroalgae.
The change of reaction pathways for nitrogenate formation is well described by PC1 and 3 which explains 54.1% of the total variance. Fig. 7B supports our previous findings that when carbohydrate to protein ratios move closer to 1 in the feedstock, the formation of nitrogenates significantly increases and mixtures of samples are grouped close to samples from DDGS and C. vulgaris.37 This is especially notable for the formation of methylpyrazine in poplar (4.3 mg L−1), Spirulina (139 mg L−1), C. vulgaris (969 mg L−1), and the mixture of Poplar/Spirulina/C. vulgaris (684 mg L−1). Due to the increasing displacement of nitrogenates from AqP to bio-crude, the increasing pyrazine formation in mixtures explains the higher nitrogen contents of the bio-crudes observed in Section 3.1.1, making mixtures of high protein biomasses and lignocellulosics less desirable.
Additional PCs, encompassing up to 90% of the total variance, were investigated in order to determine potential matrix effects or additional interactions from mixing of biomasses. This did not reveal any hidden structures in the dataset. However, mixtures containing macroalgae were not prepared and it is likely that mixtures with high protein biomass could yield different nitrogenates, considering the differences in formation of cyclic oxygenates from lignocellulosics and macroalgae.
3.3 Reaction pathways
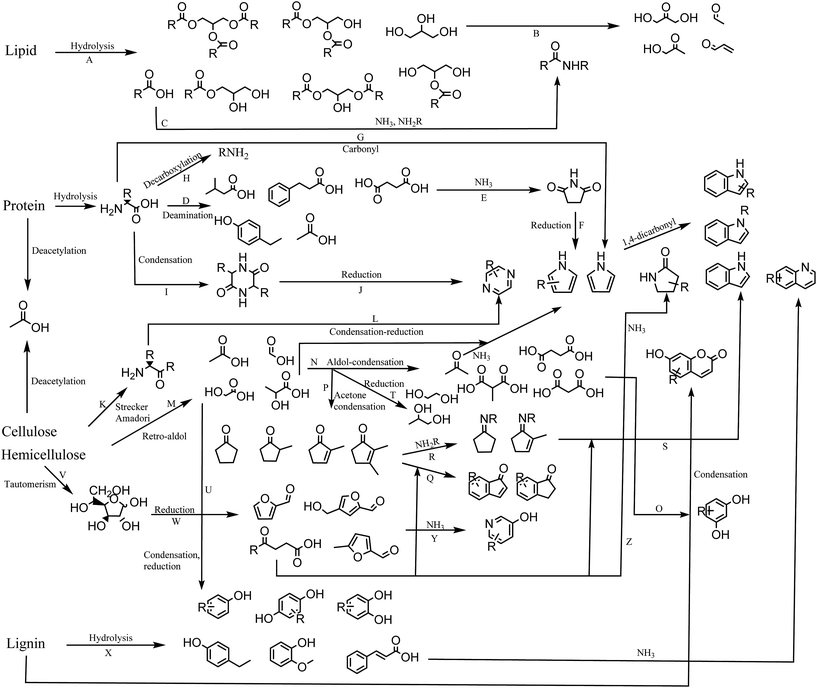
In this section we propose a reaction network for formation of products from HTL of biomass (Fig. 8). Each reaction is assigned a capital letter, which is presented in parenthesis in the description below.High lipid containing biomass has generally been considered the most applicable to HTL due to the ease of hydrolysis (A) and the high resulting bio-crude yields. The current work shows that only partial hydrolysis occurs, which has also been observed in other studies from model compounds.36 Glycerol released from hydrolysis has been proposed to degrade to a number of aldehydes and ketones,41 which are highly reactive in the presence of especially ammonia and amines (B). Fatty acids are often the most abundant compounds of the biomass and are thermally stable. In the presence of protein they can be converted to a range of fatty amides (C).39 Recent work shows that the conversion to fatty amides is dependent on the ratios of carbohydrate, protein, and lipids and their abundance is often overlooked due to the larger presence of fatty acids.42 The ammonia is obtained from deamination of amino acids hydrolyzed from protein, resulting in a large number of small organic acids mainly dispersed to the AqP (D).43 Especially succinic acid is abundant and can react with the released ammonia or amines to form succinimides (E),44 which in a reductive environment can produce pyrroles (F). Pyrroles have previously been shown to be abundant when bio-crudes are recovered without solvent extraction.20 Amino acids can also condensate with carbonyls in a Knorr pyrrole reaction leading to substituted pyrroles (G). Decarboxylation of amino acids leads to formation of amines, which are highly reactive and capable of the same reactions mentioned for ammonia (H). Amino acids can self-condensate into diketopiperazines (I),45 which at elevated temperatures may reduce to pyrazines (J). Pyrazines can also be formed via products from Strecker degradation and Amadori rearrangement (K),46 which condensate and reduce to pyrazines (L).
It has previously been established that two main pathways for degradation of cellulose and hemicellulose occur in HTL, namely dehydration and retro-aldol reaction.33 Retro-aldol leads to formation of small organic acids such as formic acid, acetic acid, glycolic acid, and lactic acid found to be highly abundant in the AqP (M).47 The formation of these compounds is furthermore found to be highly dependent on the pH of the medium.48 These can again perform aldol reactions to form acetone and small dicarboxylic acids (N) of which malonic acids are highly reactive and could be reacting with resorcinols resulting in umbelliferone derivatives (O). Resorcinols are abundant in plant material but are typically not reported in bio-crudes from HTL making the proposed reaction doubtful. The acetone produced from aldol condensation could be part of condensation reaction with for example glycolic acid and lactic acid to form cyclopentanones and cyclopent-2-enones (P),33 potentially reacting further with ketoacids to form indanones and indenones (Q). Acetone and small organic acids may also degrade further to gaseous products.49
In the presence of protein, cyclic ketones may form Schiff bases (R) potentially reacting with ketoacids to form indoles (S). Often indoles are reported as N-alkylated suggesting that indoles are formed from the reaction of the amine moiety from amino acids, which could suggest the pathway through cyclic ketones. The highly abundant glycolic acid and lactic acid may also reduce to glycols (T) reported in AqP from HTL of wood or they may condensate to produce oxygenated aromatics (U) such as phenol and catechol observed from HTL of cellulose and hemicellulose.
Monosaccharides exist as tautomers (V), which can be reduced to different furans, furfurals, and levulinic acid derivatives (W). Furans and furfurals have been suggested to react with ammonia to produce 3-hydroxypyridines (Y),36 while levulinic acid derivatives may react with ammonia to form pyrrolidin-2-ones (Z) which are often reported in the literature.19 Furfurals may also be an additional source of indanones and indenones.50
When carbohydrate is mixed with protein we observe decreasing concentrations of lactic acid, glycols and cyclic ketones along with increasing concentrations of pyrazines and 3-hydroxypyridines, which suggests that equilibria shift towards formation of furfurals, Strecker products, and Amadori products.
Degradation products of lignin consist predominantly of oxygenated aromatics (X). The decomposition involves the formation of aldehydes and ketones of which vanillin is most often reported. These compounds are likely involved in either Schiff base formation or nucleophilic attack on the aromatic ring by ammonia. More work is required to elucidate these effects. Lignin is also known to be the source of a number of aromatic oligomers, alkylated benzenes, and small aldehyde, ketones, carboxylic acids, and alcohols, which are not displayed in this scheme.42,51
The reaction network presented in this work is by no means comprehensive due to the limitation of GC to detect volatile and semi-volatile compounds. Furthermore, compounds formed from pigments, triterpenoids, and waxes were not included for simplicity. Additional characterization is required on the non-volatile fraction with analytical techniques such as NMR, FT-ICR MS, pyrolysis GC-MS, and LC-MS.
4. Conclusion
Feedstocks of lignocellulosics, macroalgae, microalgae, and residues were subjected to HTL and quantitative analysis of bio-crudes and AqPs was conducted. The oxygen content in bio-crudes decreased in the order lignocellulosics > algae > residues. Nitrogen and sulfur contents increased compared to the biomass when the initial value was <3% nitrogen while it decreased for initial values of >3% nitrogen. Mixtures of lignocellulosics and microalgae led to decreasing oxygen contents and increasing carbon, nitrogen, and sulfur contents in bio-crudes when compared to calculated values. These effects were linked to the formation of Schiff bases by ammonia/amines with furans and furfurals in the bio-crude leading to less formation of smaller oxygenated compounds such as diols. Therefore, mixing of microalgae and lignocellulosics leads to a bio-crude of lower than expected quality.Decreasing concentrations of TOC and TN were observed for AqPs of mixtures of lignocellulosics and microalgae when compared to calculated values. This was due to the formation of pyrazines which was significantly increased when carbohydrate to protein ratios were close to 1. The substantially increased pyrazine formation could lead to a lower quality bio-crude without solvent extraction.
This work shows that detailed chemical analyses provide important information for elucidating reaction pathways during HTL and for optimizing feedstock composition/blend to obtain a desired bio-crude composition.
Acknowledgements
The authors would like to acknowledge funding of this work by Innovation Fund Denmark Grant No. 1305-00030B, the Danish National Research Foundation (DNRF93), and the Danish Centre for Food and Agriculture.References
- D. C. Elliott, P. Biller, A. B. Ross, A. J. Schmidt and S. B. Jones, Bioresour. Technol., 2015, 178, 147–156 CrossRef CAS PubMed.
- P. Biller, B. K. Sharma, B. Kunwar and A. B. Ross, Fuel, 2015, 159, 197–205 CrossRef CAS.
- F. Behrendt, Y. Neubauer, M. Oevermann, B. Wilmes and N. Zobel, Chem. Eng. Technol., 2008, 31, 667–677 CrossRef CAS.
- D. R. Vardon, B. K. Sharma, J. Scott, G. Yu, Z. Wang, L. Schideman, Y. Zhang and T. J. Strathmann, Bioresour. Technol., 2011, 102, 8295–8303 CrossRef CAS PubMed.
- S. Karagöz, T. Bhaskar, A. Muto and Y. Sakata, Fuel, 2005, 84, 875–884 CrossRef.
- P. Biller and A. B. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- K. Anastasakis and A. B. Ross, Bioresour. Technol., 2011, 102, 4876–4883 CrossRef CAS PubMed.
- A. B. Ross, P. Biller, M. L. Kubacki, H. Li, A. Lea-Langton and J. M. Jones, Fuel, 2010, 89, 2234–2243 CrossRef CAS.
- A. J. Mørup, J. Becker, P. R. Christensen, K. Houlberg, E. Lappa, M. Klemmer, R. B. Madsen, M. Glasius and B. B. Iversen, Ind. Eng. Chem. Res., 2015, 54, 5935–5947 CrossRef.
- R. B. Madsen, P. R. Christensen, K. Houlberg, E. Lappa, A. J. Mørup, M. Klemmer, E. M. Olsen, M. M. Jensen, J. Becker, B. B. Iversen and M. Glasius, Bioresour. Technol., 2015, 192, 826–830 CrossRef CAS PubMed.
- Y. Zhu, M. J. Biddy, S. B. Jones, D. C. Elliott and A. J. Schmidt, Appl. Energy, 2014, 129, 384–394 CrossRef CAS.
- M. N. Nabi, M. M. Rahman, M. A. Islam, F. M. Hossain, P. Brooks, W. N. Rowlands, J. Tulloch, Z. D. Ristovski and R. J. Brown, Energy Convers. Manage., 2015, 96, 588–598 CrossRef CAS.
- M. Klemmer, R. B. Madsen, K. Houlberg, A. J. Mørup, P. S. Christensen, J. Becker, M. Glasius and B. B. Iversen, Ind. Eng. Chem. Res., 2016, 55, 12317–12325 CrossRef CAS.
- C. Xu and P. E. Savage, Algal Res., 2014, 6, 1–7 CrossRef.
- P. Duan and P. E. Savage, Bioresour. Technol., 2011, 102, 1899–1906 CrossRef CAS PubMed.
- D. L. Barreiro, B. R. Gómez, F. Ronsse, U. Horning, A. Kruse and W. Prins, Fuel Process. Technol., 2016, 148, 117–127 CrossRef.
- P. Biller, A. B. Ross, S. C. Skill, A. Lea-Langton, B. Balasundaram, C. Hall, R. Riley and C. A. Llewellyn, Algal Res., 2012, 1, 70–76 CrossRef CAS.
- E. Panisko, T. Wietsma, T. Lemmon, K. Albrecht and D. Howe, Biomass Bioenergy, 2015, 74, 162–171 CrossRef CAS.
- B. Maddi, E. Panisko, T. Wietsma, T. Lemmon, M. Swita, K. Albrecht and D. Howe, Biomass Bioenergy, 2016, 93, 122–130 CrossRef CAS.
- S. R. Villadsen, L. Dithmer, R. Forsberg, J. Becker, A. Rudolf, S. B. Iversen, B. B. Iversen and M. Glasius, Energy Fuels, 2012, 26, 6988–6998 CAS.
- Y. Li, S. Leow, A. C. Fedders, B. K. Sharma, J. S. Guest and T. J. Strathmann, Green Chem., 2017, 19, 1163–1174 RSC.
- D. W. F. Brilman, N. Drabik and M. Wadrzyk, Biomass Convers. Biorefin., 2017 DOI:10.1007/s13399-017-0241-2.
- L. Yang, Q. He, P. Havard, K. Corscadden, C. Xu and X. Wang, Bioresour. Technol., 2017 DOI:10.1016/j.biortech.2017.02.087..
- K. P. R. Dandamudi, T. Muppaneni, N. Sudasinghe, T. Schaub, F. O. Holguin, P. J. Lammers and S. Deng, Bioresour. Technol., 2017, 236, 129–137, DOI:10.1016/j.biortech.2017.03.165..
- R. B. Madsen, M. M. Jensen, A. J. Mørup, K. Houlberg, P. R. Christensen, M. Klemmer, J. Becker, B. B. Iversen and M. Glasius, Anal. Bioanal. Chem., 2016, 408, 2171–2183 CrossRef CAS PubMed.
- P. Biller, R. B. Madsen, M. Klemmer, J. Becker, B. B. Iversen and M. Glasius, Bioresour. Technol., 2016, 220, 190–199 CrossRef CAS PubMed.
- S. M. Gerchakov and P. G. Hatcher, Limnol. Oceanogr., 1972, 17, 938–943 CrossRef CAS.
- F. C. Moreira-Vilar, R. C. Siqueira-Soares, A. Finger-Teixeira, D. M. Oliveira, A. P. Ferro, G. J. Rocha, M. L. L. Ferrarese, W. D. Santos and O. Ferrarese-Filho, PLoS One, 2014, 9 DOI:10.1371/journal.pone.0110000.
- R. Bro and A. K. Smilde, Anal. Methods, 2014, 6, 2812–2831 RSC.
- G. C. Laredo, C. R. López, R. E. Álvarez and J. L. Cano, Fuel, 2004, 83, 1689–1695 CrossRef CAS.
- N. Sudasinghe, B. Dungan, P. Lammers, K. Albrecht, D. Elliott, R. Hallen and T. Schaub, Fuel, 2014, 119, 47–56 CrossRef CAS.
- M. Carrier, A. Loppinet-Serani, C. Absalon, C. Aymonier and M. Mench, Biomass Bioenergy, 2012, 43, 65–71 CrossRef CAS.
- T. H. Pedersen and L. Rosendahl, Biomass Bioenergy, 2015, 83, 206–215 CrossRef CAS.
- K. A. Jung, S. Lim, Y. Kim and J. M. Park, Bioresour. Technol., 2013, 135, 182–190 CrossRef CAS PubMed.
- E. Jakab, K. Liu and H. L. C. Meuzelaar, Ind. Eng. Chem. Res., 1997, 36, 2087–2095 CrossRef CAS.
- A. Croce, E. Battistel, S. Chiaberge, S. Spera, F. De Angelis and S. Reale, ChemSusChem, 2016, 9, 1–12 CrossRef.
- R. B. Madsen, P. Biller, M. M. Jensen, J. Becker, B. B. Iversen and M. Glasius, Energy Fuels, 2016, 30, 10470–10483 CrossRef CAS.
- K. Wu, M. Yang, Y. Chen, W. Pu, H. Hu and Y. Wu, AIChE J., 2017 DOI:10.1002/aic.15687.
- S. Chiaberge, I. Leonardis, T. Fiorani, G. Bianchi, P. Cesti, A. Bosetti, M. Crucianelli, S. Reale and F. De Angelis, Energy Fuels, 2013, 27, 5287–5297 CrossRef CAS.
- V. A. Yaylayan, Food Sci. Technol. Res., 2003, 9, 1–6 CrossRef CAS.
- T. H. Pedersen, L. Jasiunas, L. Casamassima, S. Singh, T. Jensen and L. Rosendahl, Energy Convers. Manage., 2015, 106, 886–891 CrossRef CAS.
- R. B. Madsen, H. Zhang, P. Biller, A. H. Goldstein and M. Glasius, Energy Fuels, 2017 DOI:10.1021/acs.energyfuels.7b00160.
- R. B. Madsen, P. Biller, M. M. Jensen, J. Becker, B. B. Iversen and M. Glasius, Energy Fuels, 2016, 30, 10470–10483 CrossRef CAS.
- B. Maddi, E. Panisko, T. Wietsma, T. Lemmon, M. Swita, K. Albrecht and D. Howe, ACS Sustainable Chem. Eng., 2017, 5, 2205–2214 CrossRef CAS.
- D. Klingler, J. Berg and H. Vogel, J. Supercrit. Fluids, 2007, 43, 112–119 CrossRef CAS.
- A. Kruse, P. Maniam and F. Spieler, Ind. Eng. Chem. Res., 2007, 46, 87–96 CrossRef CAS.
- S. Yin and Z. Tan, Appl. Energy, 2012, 92, 234–239 CrossRef CAS.
- S. Yin, A. K. Mehrotra and Z. Tan, Bioresour. Technol., 2011, 102, 6605–6610 CrossRef CAS PubMed.
- A. Kruse and A. Gawlik, Ind. Eng. Chem. Res., 2003, 42, 267–279 CrossRef CAS.
- M. Deniel, G. Haarlemmer, A. Roubaud, E. Weiss-Hortala and J. Fages, Sustainable Energy Fuels, 2017 10.1039/c6se00065g.
- J. Barbier, N. Charon, N. Dupassieux, A. Loppinet-Serani, L. Mahé, J. Ponthus, M. Courtiade, A. Ducrozet, A. Quoineaud and F. Cansell, Biomass Bioenergy, 2012, 46, 479–491 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00104e |
| This journal is © The Royal Society of Chemistry 2017 |