Enabling the metal fuel economy: green recycling of metal fuels
Philippe
Julien
and
Jeffrey M.
Bergthorson
*
Alternative Fuels Laboratory, Department of Mechanical Engineering, McGill University, 817 Sherbrooke Street West, Montreal, QC, Canada. E-mail: jeff.bergthorson@mcgill.ca
First published on 9th March 2017
Abstract
Energy carriers are required to store and transport clean, renewable energy. Currently, most scientific efforts are invested in batteries and hydrogen despite several drawbacks, such as low energy densities and, for hydrogen, a serious risk of accidental or intentional explosions. Metal fuels, which present an inherently low safety risk and have high energy densities, have been proposed as advanced storage and transport systems for large quantities of clean energy but have received far less attention. Metal powders or sprays can be burned with air to produce heat, or can be reacted with water to produce heat and hydrogen. The hydrogen produced can then fuel either an internal combustion engine or a fuel cell. Metal fuel utilization produces metallic oxides, which can be captured and cyclically reduced back into metal fuels ad infinitum. The recycling process to produce metal fuels must use renewable energy to ensure an environmentally friendly cycle. A set of 5 constraints guiding the choice for potential metal fuels, along with the available commercial and research-stage green recycling techniques, lead to a selection of 7 different metals that could be used as recyclable fuels in the near future.
1. Introduction
1.1 Background
The scientific community has reached a strong consensus1 that human activity is responsible for climate change and global warming and, yet, the world still relies heavily on fossil fuels for energy production.2 Combustion of coal and hydrocarbon fuels is the major contributor of greenhouse gases by releasing carbon dioxide, methane and other gases, on top of numerous other pollutants that affect human health and natural ecosystems.3 Limited fossil-fuel supplies and production rates also limit the economic growth potential of the global economy.4In an attempt to mitigate climate change, the share of electricity produced from renewable sources, such as wind or solar, is increasing. Unfortunately, renewable power faces several challenges, with one of the most serious being the intermittent nature of its production. The sun only shines during the day and can be blocked with clouds, while wind strength varies over the day and seasons, and the period of high-energy demand does not necessarily coincide with periods of large production. This problem can be partially addressed by using temporary methods of storing energy,5 such as thermal storage,6 compressed air7,8 or flywheels,9 but these methods are limited in terms of low energy density, short storage duration, and lack of mobility. Furthermore, the optimal location for producing electricity in an economically feasible fashion, i.e. where winds are strong and constant enough or where solar insolation is sufficient, might be situated at very large distances from the main sites of power consumption. An example is the production of solar energy in the Sun Belt while much of the population of the developed world lives at northern latitudes.
Using non-polluting sources of energy for transportation is even more challenging. Transportation systems are limited by mass, such that the gravimetric specific energy of the fuel used is paramount. Fossil fuels are, thus, difficult to displace and no adequate replacements have yet been found. Batteries, despite the increasing electrification of automobiles, still limit cars to short ranges and long charging times. Larger vehicles, such as trucks, trains, ships and heavy machinery, are unlikely to ever run on batteries. Hydrogen, easily produced from water using electrolysis, can be efficiently used in fuel cells to produce electricity. Cryogenic hydrogen10 and metal hydrides11 are two solutions currently put forward to carry hydrogen, but greatly reduce the specific energy of the fuel by adding structural materials to the overall system. A better storage system, that is both energy dense and presents an inherently low safety risk, is required.
It is necessary to develop an energy carrier that can be replenished with renewable energy, that can be stored for long time periods, and that can be shipped over long distances to the final consumer, where it can be used for heating, electricity production and transportation. The ideal energy carrier should be energy dense, safe, and easy to carry. It should also have a long shelf-life and be available in large quantities. More importantly, the ideal energy carrier should be green, non-polluting, and recyclable in an environmentally friendly way.
1.2 Metal fuels
Metal powders are the only energy carrier that can be used in large quantities to store green, renewable energy that meets all the criteria for a future low-carbon fuel.12,13 Aluminum has been used for decades to increase the energy density of propellants and condensed explosives.14,15 NASA has used aluminum powder in combination with other compounds in the Space Shuttle's reusable solid-propellant booster rockets.16 More recently, a rocket prototype fueled exclusively by aluminum and water was developed.17,18 In these applications, the metal fuel is only used once and discarded after it has released its energy. In order to be green, the spent metal oxides, the only combustion products of metal fuels, must be collected and recycled.Metal fuels have long been used to produce hydrogen on demand, through their reaction with water or basic solutions.19–21 If the metal–water reaction is operated at high temperatures, it can produce hot hydrogen, potentially along with steam, that can be used in a fuel cell or burned directly in internal combustion engines.22
More recently, the direct combustion of metals with air has been proposed as another metal-fuel utilization technology.12,13,23,24 Many metals can be burned, in powder form, with air to produce heat that can be used for process heating, residential or commercial heating, or drive an external-combustion engine that can produce motive or electrical power.12
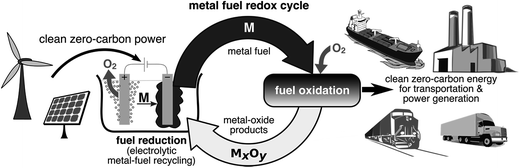
In both approaches to metal–fuel utilization, the only products of the reaction are metallic oxides, essentially an ultra-pure version of the original ore. These oxides would then be collected and reduced back to metallic form using a green energy source, such as wind or solar. Efficient metal-oxide collection following metal–fuel utilization would enable the metal to be reduced and oxidized over an unlimited number of recycle loops. Metal fuels are thus a perfect example of the concept of a circular economy, required for sustainable development.25Fig. 1 illustrates the concept of the metal fuel cycle, through which clean primary energy is stored, transported, and delivered to end users while minimizing wastes to the environment. These metal fuels could act as renewable energy commodities for global clean-energy trade and could be stored as strategic renewable energy reserves to protect against energy-supply disruptions.
Ensuring that the entire cycle is green is essential to the concept of metal fuels. From ancient times to the present day, most of the planet's metal has been chemically reduced using carbon. This releases at least a stoichiometric amount of CO2, even if the smelter uses a green energy source like hydro-electric power. Furthermore, many metallurgical processes still use toxic chemicals that are harmful to the environment and to human health.26,27
The present paper further investigates the idea of using metal powders as recyclable zero-carbon fuels that are safe and environmentally friendly. A set of 5 constraints is proposed for the selection of promising metal–fuel candidates. Existing reduction techniques of metal oxides, along with novel green technologies, are also presented and discussed. Finally, a selection of metal fuels that could be used today, or in the near future, is identified.
2. Identifying the best chemical energy carrier: the periodic table of fuels
The energy released during the combustion of any fuel is equal to the difference in the enthalpy of formation of the fuel, and its oxidizer, and that of the combustion products. Such enthalpy differences are the same as those that enable these fuels to power batteries. In fact, both batteries and recyclable fuels, such as hydrogen or metal fuels, operate on reduction–oxidation, or redox, cycles (see Fig. 1). During reduction, an oxidized form of an element is reduced to the pure element, releasing the bound oxygen and storing chemical energy for later release. During oxidation, the element is chemically combined with oxygen, or another oxidizer, releasing the stored chemical energy. This redox cycle can be repeated indefinitely if the reaction products are retained.Fossil fuels contain carbon, which produces carbon dioxide when burned with the oxygen present in air. The fundamental problem that limits carbon-based fuels from being efficiently recycled is the fact that carbon dioxide is a gas, under normal conditions, that is emitted into the atmosphere. Although convenient, and leading to high energy densities, carbon dioxide emissions are the main contributor to climate change. Chemical reactions between different elements of the periodic table need not be limited between carbon and oxygen for energy production. Keeping this in mind, it is worthwhile exploring the rest of the periodic table to determine what other elements could eventually replace carbon as humanity's fuels.
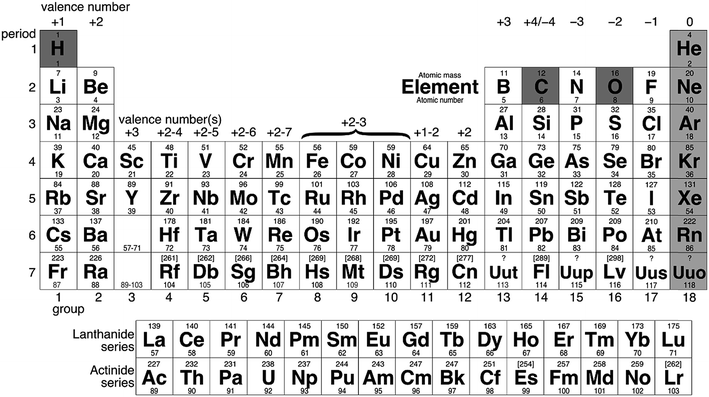
As can be seen from Fig. 2, even by excluding hydrogen, carbon, oxygen, and the noble gases, over 100 elements are left as potential fuels. Not all elements can be used as fuels and the candidates must be filtered by applying appropriate constraints. Note that the majority of elements that are of interest as potential fuels are metals or metalloids, as discussed below.
3. Constraints for identifying metals that could be used as a fuel
3.1 Constraint #1: element must react with oxygen
A fuel is a reduced form of any electropositive element that can donate electrons to an oxidizer, releasing chemical energy. In theory, a fuel can react with any type of oxidizer to release energy. Fossil fuels have the advantage of burning with oxygen, a free oxidizer that is taken from the air. Only one of the two reactants must then be transported, which partially accounts for the higher energy densities of fossil fuels over batteries. In batteries, the fuel and the oxidizer are embedded inside the battery and must be transported together. Carrying both reactants, in addition to the structural material of the battery, creates an energy device that has a very low energy density (see lithium battery in Fig. 3 and compare to pure lithium metal). | ||
| Fig. 3 Energy density vs. specific energy for various fuels reacting with air compared to a Li-ion battery. The DOE target represents the goal set by the Department of Energy of the United States for the overall energy density and specific energy of hydrogen-storage systems.41 | ||
Metals can react with many different oxidizers, whether they are elemental, such as oxygen28,29 and sulfur,30 or molecules, like water and carbon dioxide.12,31–37 Most fuel-oxidation reactions are exothermic and could be harnessed, in theory, to release the chemical energy contained in metal fuels. For practical and economic considerations, however, metal fuels should react with oxygen drawn from ambient air, similarly to fossil fuels. This justifies the first constraint, which states that, in order to be considered a viable fuel, the element must be able to react with oxygen in an exothermic reaction.
The Gibbs free energy, ΔG, shown in eqn (1), is defined as the difference in enthalpy between the products and the reactants, ΔH, minus the product of the temperature, T, and the difference in entropy between the products and the reactants, ΔS.
| ΔG = ΔH − TΔS | (1) |
For the reaction of a metal with oxygen to be thermodynamically favored, the Gibb's free energy has to be negative. This is the case, for example, of iron,† as shown in eqn (2), which spontaneously rusts in the presence of oxygen,38 and also burns with air,39,40 when iron is made into small particles.
| 2Fe(s) + O2(g) → 2FeO(s), ΔG° = −488.7 kJ mol−1 | (2) |
Metals like iron can be classified as reactive metals, in opposition to metals that do not spontaneously react with oxygen, often called noble metals. Gold is an example of a noble metal, which are found in nature in their elemental states. Generally, only elements from groups 1–14, and specifically 1–8 and 12–14, can react with oxygen with sufficient energy release to be of interest as fuels.
3.2 Constraint #2: competitive fuel energy density
To be competitive with, and even be superior to, other proposed energy carriers, the ideal metal fuel should be sufficiently energy dense on a mass and volume basis. A high volumetric energy density but low specific energy means large masses of metal need to be carried around for little energy. Conversely, a low volumetric energy density but high gravimetric specific energy requires an oversized fuel tank that would be difficult to fit in a transportation system—this is one of the serious problems for hydrogen as a fuel.The specific energy (by mass) is a function of the number of valence electrons versus the number of protons and neutrons contained in the nuclei of the fuel. Fossil fuels are essentially composed of hydrogen and carbon, two light reactive elements that gives them very high gravimetric specific energy. Metals with large nuclei, such as those in periods 5 and higher, have a small ratio of chemical energy (valence electrons) to atomic mass, and would not likely be high-performing metal fuels. The region of light reactive fuels includes, besides hydrogen and carbon, only metal and metalloid elements.
Fig. 3 shows the comparison of different fuels and energy carriers in terms of energy density and specific energy. The values for fuels are calculated using oxygen as the oxidizer. The energy density (by volume) depends on the phase of the fuel. Liquid hydrocarbons have higher energy densities than gaseous hydrocarbons or hydrogen. Metal fuels, which are solid at room temperature, generally have higher energy densities than hydrocarbons. As can be seen, some of the metals are as good as hydrocarbons on a per mass basis, and beat hydrocarbons on a per volume basis. Furthermore, metal fuels perform better than all other low-carbon solutions currently proposed (batteries, hydrogen, etc.).
3.3 Constraint #3: no toxic, radioactive or dangerous elements
The third set of constraints eliminates all elements that can be harmful for the environment, human health, and overall safety. The fuels chosen should respect the proposed guidelines for Green Chemistry,42 and their production should minimize all forms of pollution. This includes eliminating toxic compounds used for smelting metals that can affect the environment or human health, minimizing waste production, and favoring safer processes, such as low-temperature and low-pressure processes. Elements that are toxic, or that form toxic oxides after combustion, should also be eliminated.The third constraint eliminates many elements, because they are radioactive (uranium, polonium, radium), toxic (lead, mercury, selenium, manganese, cadmium), or form toxic oxides, such as beryllium.43 Furthermore, all alkali metals, such as sodium and lithium, react violently with water and are, thus, not desirable metal fuels from a safety standpoint. They could, nonetheless, be used in closed cycles.37
3.4 Constraint #4: oxide reduction must be done without CO2 emissions
To be considered as green energy carriers, metal fuels must be recyclable, which means that the metal oxide, the product of metal fuel combustion or oxidation, must be reducible in an environmentally friendly fashion, without CO2 emissions.For reactive metals, the ΔG of the reduction reaction, going from metal oxide to pure metal and pure oxygen, is necessarily positive and is, thus, not thermodynamically favored. Energy, or a reducing agent, must be provided to reduce the oxides back to metals, a process referred to as smelting. Some current industrial reduction techniques are discussed below.
3.4.1.1 Chemico-thermal reduction techniques.
3.4.1.1.1 Carbothermic reduction. In ancient times, humanity overcame the positive ΔG by using charcoal to smelt metals like copper, in the bronze age, followed by steel, in the iron age.26 This charcoal was produced through the incomplete combustion of wood, which was later replaced by coal and metallurgical coke, a purer and carbon-dense fuel produced by the destructive distillation of coal in an oxygen-starved high-temperature furnace.
Typically, coke is both used to increase the temperature of the furnace to favour reduction and acts as a reducing agent.44 Modern furnaces can also use other fuels, such as natural gas,45 to heat the environment in an effort to reduce exploitation costs, while coke is still employed as the reducing agent. This process is generally called carbothermic reduction, referring to the high temperatures and use of carbon to reduce metal oxides. More than 90% of the world's iron and steel is produced with this process, and the rest is reduced with natural gas as direct-reduced iron (DRI).46 Vanadium, chromium, silicon and manganese are other examples of metals that are produced by carbothermic reduction in a smelting furnace, with release of CO2.27,47 For nickel, as shown in eqn (3) and (4), the reaction first produces a stoichiometric amount of CO, which later reacts with air to make CO2:27
| NiO(s) + C(s) → Ni(s) + CO(g) | (3) |
| CO(g) + ½O2(g) → CO2(g) | (4) |
The Ellingham diagram, which plots the Gibb's free energy on the y-axis versus the temperature of the reaction on the x-axis, is a useful tool for determining which elements can reduce which metal oxides. Fig. 4 shows an example of an Ellingham diagram that is relevant for metal fuels.
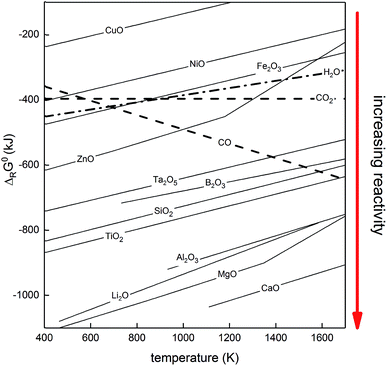 | ||
| Fig. 4 Ellingham diagram (ΔG°versus temperature) for some oxides.48 Each line corresponds to the reaction of type 2x/yMe + O2(g) = 2/yMexOy. All values are thus expressed per mole of O2, in order to allow comparison between metals of different numbers of valence electrons. Note the negative slope of the carbon monoxide line, which explains the use of high-temperature carbothermic processes for many metals, such as iron or silicon. | ||
The sign of the slope of the line on the Ellingham diagram depends on the number of gaseous moles on both sides of the chemical equation. For example, magnesium oxidizes according to eqn (5), with 1 mole of gas on the reactant side and zero moles of gas on the product side. Since gases are consumed, the final products are more ordered, which results in a decrease in entropy, making the “−TΔS” term positive, resulting in a positive slope on the Ellingham diagram.
| 2Mg(s) + O2(g) → 2MgO(g), less moles of gas afterwards | (5) |
Unlike metal oxidation, the generation of CO from carbon produces more moles of gas in the products, as can be seen in eqn (6), resulting in a positive entropy change and, thus, a negative slope on the Ellingham diagram (see Fig. 4). When producing carbon dioxide, as shown in eqn (7), the number of moles of gases on both sides of the reaction is identical, resulting in essentially no change in entropy and, thus, a flat line on the diagram.
| 2C(s) + O2(g)→ 2CO(g), more moles of gas afterwards | (6) |
| C(s) + O2(g)→ CO2(g), same moles of gas afterwards | (7) |
In order for one element (element 1) to act as a reducing agent for another element (element 2), the change in Gibbs energy during the oxidation of element 1 must be more negative than that of element 2. The different signs of the slopes for carbon oxidation (eqn (6)), compared to the other metals, create intersection points on the Ellingham diagram, which explains why most carbothermic reduction processes happen at high temperature. Silicon oxide, for example, cannot be reduced at room temperature with carbon given the fact that the carbon monoxide line is higher on the diagram than the silicon oxide line. However, the two curves cross at around 1600 K, which means that silicon oxide can be reduced with carbon above that temperature.
3.4.1.1.2 Hydrogen reduction. Hydrogen, similarly to carbon, can also be used to reduce some metal oxides.46,49 The oxidation line of hydrogen is higher than most metals on the Ellingham diagram and is, therefore, able to reduce only a minority of metal oxides. Furthermore, unlike carbon monoxide, oxidation of hydrogen (eqn (8)) reduces the number of moles of gaseous species, which results in a positive slope for the line on the diagram.
| 2H2(g) + O2(g)→ 2H2O(g), fewer moles of gas afterwards | (8) |
Increasing the reaction temperature may only marginally help to thermodynamically favour the reduction reaction. Hydrogen can, nonetheless, reduce some of the more noble metals, including commercially important ones like copper,50 nickel,51 and cobalt.52 Hydrogen reduction of nickel oxide is shown in eqn (9).
| NiO(s) + H2(g) → Ni(s) + H2O(g) | (9) |
Hydrogen can also reduce the world's most used metal, iron.49,53–55 Iron has multiple oxides, and there are a number of intermediate reactions through which hydrogen reduces Fe(III) into Fe(II), before achieving full metallization to Fe(0). The net reactions are shown in eqn (10)–(12).
| FeO(s) + H2(g) → Fe(s) + H2O(g) | (10) |
| Fe2O3(s) + 3H2(g) → 2Fe(s) + 3H2O(g) | (11) |
| Fe3O4(s) + 4H2(g) → 3Fe(s) + 4H2O(g) | (12) |
3.4.1.1.3 Metallothermic reduction. Thermo-chemical reduction of a metal oxide can also be performed with a less noble, or more reactive, metal as the reducing agent, with these reactive metals acting in place of carbon. The most stable metal oxides, or most reactive metals, have a more negative ΔG of oxidation and lie lower on the Ellingham diagram. The pure metal associated with those oxides can then be used to reduce a more noble metal (less stable metal oxide). For example, calcium could, in theory, reduce any of the metal oxides shown on the diagram since CaO has the most negative Gibb's free energy of all oxides shown. This process is generally referred to as metallothermic reduction.
Carbothermic processes have the advantage of using a fuel and reactant that is readily available in nature. Metallothermic processes, on the other hand, require the first pure metal to be produced before it can be used to reduce the second metal oxide. In order to be green, the first metal must then be produced without the emission of greenhouse gases. The entire process also runs the risk of being less efficient, given that it requires additional thermodynamic steps compared to the direct reduction of a single metal.
For example, magnesium from dolomites ore is reduced with the Pidgeon process, whose steps include silicothermic reduction of magnesium oxide to produce pure magnesium.56 The silicon oxide line on the Ellingham diagram is above the line of magnesium oxide, which suggests that the reaction is not normally thermodynamically favoured. This process is made possible by using a vacuum, which removes magnesium vapours as they are produced, forcing the Le Châtelier equilibrium in the desired direction. It is important to note that production of silicon is generally done using a carbothermic process,57 such that the Pidgeon process cannot be used to produce low-net-carbon magnesium fuel.
3.4.1.2 Electrodeposition of metals. Fortunately, nature provides an alternative route for reducing metal oxides—electrolysis. This technique does not involve the reaction of a metal oxide with another compound at high temperatures. Instead, the chemical reaction is driven forward by an electric current in an electrolysis cell, with a cathode (−) and an anode (+) connected to an external DC power source. The working principle is similar to charging a battery, where reduction and oxidation reactions are performed during charging and discharging, respectively. It is also equivalent to the electrolysis of water, the oxide of hydrogen, where the water is split into hydrogen fuel and oxygen by applying a voltage, and current, to an electrolysis cell.
3.4.1.2.1 Electrolysis cell voltage. Applying a voltage between the cathode and the anode can overcome the positive ΔG of the desired reaction that is, otherwise, not thermodynamically favourable. The theoretical voltage required, denoted E°, is directly proportional to ΔG, according to eqn (13), where n is the number of electrons per metal atom and F is the Faraday constant:58
| ΔG = −nFE° | (13) |
Eqn (13) gives the theoretical reversible voltage, which puts the electrolysis cell exactly in equilibrium but produces no metal. To drive the reaction to produce metal, it is necessary to apply an overvoltage, which must also counter internal resistances of the electrical circuit, the resistance of the electrolyte, and various effects at the electrodes.59 A greater voltage will favor a greater production rate, but results in an inevitable loss of efficiency. The operational voltage will be an economic compromise between minimizing losses while maintaining high production rates of the plant—both time and energy have value.
3.4.1.2.2 Electrolytic solutions. Before undergoing electrolysis, the metal oxide must first be dissolved in an electrolytic solution. Some metal oxides can easily be dissolved in mineral acids like hydrochloric or sulphuric acids, which are liquids at room temperature.60 This technique can be conducted at low temperatures and is easy to operate; however, these acids are water based and, if the voltage is high enough, performing electrolysis of metals will also electrolyze the water within the cell into hydrogen.
This is not an issue for metals like copper and nickel, which lie higher on the Ellingham diagram than hydrogen. It is, nevertheless, possible to use this technique for metals whose oxidation line is slightly below that of hydrogen on the diagram. If the ratio of chemical reaction rates is favorable for metal production, which means that metal oxide reduction rates are greater than hydrogen production rates, then this technique will mostly produce pure metal and evolve only a minimal quantity of pure hydrogen. Zinc is a metal commonly produced through electrolysis within an acidic solvent that is not thermodynamically favored but is possible due to the overvoltage of hydrogen on zinc that suppresses hydrogen evolution.61,62
Metals with very negative ΔG of oxidation cannot be reduced in an aqueous electrolyte, due to preferential hydrogen evolution, and require different mixtures. Aluminium, for example, is dissolved in molten fluoride salt (Na3AlF6) at a temperature of around 1000 °C.59 The anode is made of carbon and gets consumed during the process, resulting in a stoichiometric release of CO2. Even though it is an electrolysis process, the carbon-anode consumption contributes some of the smelting power, effectively lowering the voltage of the cell and, therefore, reducing the electricity consumption.
Magnesium electrolysis uses a chloride salt (anhydrous MgCl2) at around 650 °C.63,64 Magnesium compounds are extremely hygroscopic and eliminating water upstream of the electrolysis cell is a very difficult and complex process. Only a handful of companies worldwide have proven magnesium production technologies with electrolysis, and these are proprietary.65 Lithium is another example of a metal reduced by molten salt electrolysis and requires complete water removal before electrolysis.66 Typically, its production includes the intermediate compound lithium carbonate (Li2CO3), which releases CO2 in the process.
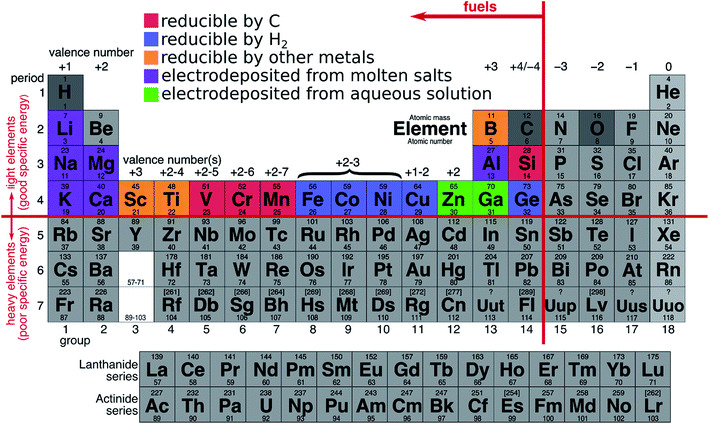
3.4.1.3 Summary of reduction techniques. Fig. 5 illustrates the different reduction techniques possible for some selected metals. Production methods that produce carbon dioxide cannot be used for producing green metal fuels. Most metals can be reduced by more than one technique.
The constraint of no greenhouse-gas emissions could, in theory, be satisfied through carbon capture and sequestration methods.67 This process would not be sustainable, however, since fossil fuels would still be consumed, and the efficacy of carbon capture technologies has yet to be proven.68
3.4.2.1 Industrial-scale green metallurgy. Some electrolysis techniques for producing metals are carbon-free, provided also that the electricity comes from a green source. Zinc, for example, is electrolyzed in an acidic solution without evolution of CO2,61 and, if wastes are kept minimal, could readily be used as a metal fuel. Magnesium can also be obtained from electrolysis without evolving carbon emissions.63,64
Iron can be directly reduced with hydrogen,46 and, if this hydrogen comes from a water electrolyser unit fed by green electricity,69 or by artificial leafs,70 then the entire reduction process can be accomplished without CO2 emissions. It is important to note that the majority of the hydrogen consumed today comes from fossil fuels.71
Zinc, magnesium and iron are, thus, potential green metal fuels that can be produced commercially, today, without carbon-dioxide emissions using renewable energy.
3.4.2.2 Laboratory-scale green metallurgy. Many green reduction techniques have been proven at the laboratory scale, but have yet to be commercialized. The majority of these techniques are based on the electrolysis of metal oxides and could be performed with green electricity in the near future. The main challenges for green electrolysis include the development of the appropriate electrolytic solution (acids, molten salts), and appropriate inert electrodes, both of which needs to be tailored to each metal. Electrolysis techniques can also include novel features, preventing undesired reactions inside the cells, such as solid oxide membranes.72–79 Many metals could then, in theory, be produced with green electrolysis. Molten salts80,81 can be used to dissolve many metal oxides, such as aluminum,82,83 titanium,84 iron,85–87 magnesium,88 and silicon.89
Even though iron can be produced with green hydrogen,46,53,55 electrolysis of iron oxide would prove to be a more efficient technique and help decrease the cost of the fuel. Some research groups are investigating low-temperature alkaline-based electrolysis for iron production.86,87,90 Iron, along with other metals, can also be produced using molten-oxide electrolysis using inert anodes under high-temperature conditions (above 1800 K).87,91–95 These techniques are being developed to make the steel industry greener and might prove to be economically advantageous in the near future.
Titanium is currently made using the Kroll process,96 an expensive, long and energy intensive process that requires magnesium as a precursor. The FFC Cambridge process, recently developed by Fray, Farthing and Chen, uses molten oxides to dissolve directly titania, before undergoing electrolysis.84 This new methodology promises to reduce the costs of titanium as a structural material and can be applied for the production of metal fuel.
Green, carbon-free aluminium production processes are being actively investigated to reduce the carbon footprint and costs of aluminium production. The present high-temperature Hall–Héroult process is expensive and evolves a significant amount of carbon dioxide from the consumable carbon anode.97,98 Inert electrodes, which are essential for the production of carbon-free aluminum, are currently being developed by different companies and laboratories.82,99 Aluminum is one of the most promising candidate for metal fuels given its light weight, abundance, and high energy density. Rusal,100 a large aluminum producer, is planning on introducing inert anodes in its production plants powered by hydro-electricity.99 Once inert anodes are commercialized, aluminum will be able to be used as a low-carbon metal fuel.
3.4.2.3 Using fuels to produce other fuels. It is possible to use a metal fuel that has a very negative ΔG of oxidation to reduce other metal oxides. With additional reaction steps, however, the conversion efficiency between the initial source of energy, whether it is solar, hydro or wind, and the metal fuel goes down. It may, therefore, be more advantageous to burn directly the fuel that has a very negative ΔG instead of using it for reducing another metal. Furthermore, the metal fuels that could be used to produce other types of metal typically have greater energy densities, so downgrading their energy into less-reactive metals appears counterproductive.
Special circumstances could, nonetheless, justify utilization of such a process. For example, in certain applications, an inexpensive type of metal could be used for a single utilization without recycling. One could imagine a scenario where silicon is produced from sand, a cheap and abundant source of the metal, using a rarer, and more expensive, metal as the reducing agent. Spent silicon fuel could be easily and safely discarded, while the expensive metal remains in a loop without ever leaving the production plant. An example cycle for production of silicon without carbon emissions is based on using magnesium electrolysis followed by the magnesiothermic reduction of silicon.67,101 Silicon is, therefore, a potential metal fuel. Boron can also be produced from magnesium, calcium and aluminum,102 and, given its high energy density, could be used in niche applications for remote locations and large distances.
3.5 Constraint #5: technology must be scalable
The literature contains many other new and exotic pathways for reducing metals, such as heating magnesium oxide to thousands of degrees Kelvin using lasers.103 Another proposition is to concentrate solar radiation to increase the temperature of a reaction cell to reduce metal oxide, effectively saving electricity for this process.104 Whether such techniques could prove advantageous in terms of the overall energy-cycle efficiency or cost of the system operation and, thereby, lead to commercialization remains to be determined.Basing the novel technique on an already industrially proven technique could help diminish the obstacles and speed up the commercialization process. A rule of thumb in primary metallurgy is that it takes at least 10 years to bring a new technology to full industrial scale.105,106 This would favor electrolysis techniques with an alternative electrolyte, such as ionic liquids, or low-temperature molten salts that are composed entirely of ions and are liquid at room temperature.83,95,107,108 These low-temperature electrolysis techniques would have less losses than high-temperature processes and, thus, a higher conversion efficiency from electricity into the metal fuel.
Furthermore, millions of tons of metals would be required for a metal fuel economy, such that the chosen technique must be scalable. Electrolysis techniques are already employed to produce large quantities of metals and are, therefore, promising for the production of metal fuels on a global scale.
4. Selected metals
The 5 constraints discussed above produces a short list of metals that could be used as fuels in the foreseeable future. Of course, as research-stage technology keeps improving, the list can be updated to include other metals whose low-carbon production becomes feasible on a commercial scale. Fig. 6 shows the metals that are identified by the authors of this paper as potential candidates for metal fuels.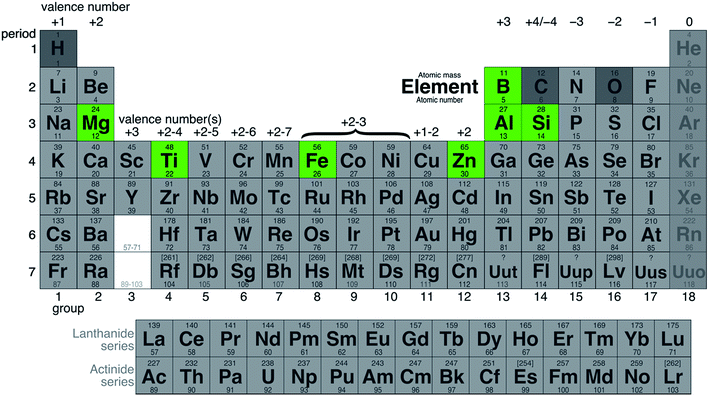 | ||
| Fig. 6 Periodic table illustrating final selection of metal fuels: boron (B), magnesium (Mg), aluminium (Al), silicon (Si), titanium (Ti), iron (Fe), and zinc (Zn). | ||
The figure includes three of the most abundant metals on earth: aluminum, iron and silicon. These metals are produced in large quantities and are relatively inexpensive, making them interesting candidates. Four more elements, boron, magnesium, titanium, and zinc, could also be used as metal fuels, potentially in niche applications given their higher prices.
5. Conclusions
The world still lacks a viable solution for storing and transporting large quantities of green energy as part of a low-carbon economy. The concept of recyclable metal fuels appears to be one of the most promising options and is consistent with the vision of a circular economy.Many different metals could potentially be used as fuels; however, different constraints must be applied to select the best candidates. Here, we propose that the chosen metal fuels must react with oxygen, must have a sufficiently large energy density, must be non-toxic, non-radioactive, and safe. Furthermore, the corresponding metal oxides must be reducible in a green process without CO2 emissions, and the technology used for reducing the metal oxides must be scalable to large industrial scales. The present paper has discussed the feasibility of producing and recycling metal fuels without carbon dioxide emissions, demonstrating the viability of the metal–fuel concept.
The proposed set of 5 constraints yields a selection of 7 metals that can be envisaged as fuels in the foreseeable future: boron, magnesium, aluminum, silicon, titanium, iron, and zinc. As reduction technologies evolve, more metals could potentially be considered for use as fuels. Having a wide variety of metal fuels could prove beneficial from both a technological and economical point of view, making the metal fuel economy robust through diversity of supplies. Further studies on the overall energy-cycle efficiency and other related life-cycle analyses are needed for these potential metal fuels to provide guidance regarding the optimal recyclable fuel for a specific application.
Acknowledgements
The authors wish to thank Robert Harrison for his guidance on this work and George Demopoulos, Kristian Waters, Sylvain Coulombe, Douglas Robinson, Andre Noel, and Roger Urquhart for their advice and suggestions. The authors also want to thank colleagues Samuel Goroshin, David Frost, Andrew Higgins, Michael Soo, Jan Palecka, James Vickery, Keena Trowell, and Michelle Mcrae for collaborating on the research in combustion of metal powders and promoting the idea of metal fuels.References
- N. Oreskes, Science, 2004, 306, 1686 CrossRef CAS PubMed.
- International Energy Agency, www.iea.org, accessed November 2016.
- J. M. Bergthorson and M. J. Thomson, Renewable Sustainable Energy Rev., 2015, 42, 1393–1417 CrossRef CAS.
- D. J. Murphy and C. A. S. Hall, Ann. N. Y. Acad. Sci., 2011, 1219, 52–72 CrossRef PubMed.
- J. P. Barton and D. G. Infield, IEEE Transactions on Energy Conversion, 2004, 19, 441–448 CrossRef.
- B. Zalba, J. M. Marín, L. F. Cabeza and H. Mehling, Appl. Therm. Eng., 2003, 23, 251–283 CrossRef CAS.
- H. Lund and G. Salgi, Energy Convers. Manage., 2009, 50, 1172–1179 CrossRef.
- P. Denholm and R. Sioshansi, Energy Policy, 2009, 37, 3149–3158 CrossRef.
- I. Hadjipaschalis, A. Poullikkas and V. Efthimiou, Renewable Sustainable Energy Rev., 2009, 13, 1513–1522 CrossRef.
- S. M. Aceves, F. Espinosa-Loza, E. Ledesma-Orozco, T. O. Ross, A. H. Weisberg, T. C. Brunner and O. Kircher, Int. J. Hydrogen Energy, 2010, 35, 1219–1226 CrossRef CAS.
- B. Sakintuna, F. Lamari-Darkrim and M. Hirscher, Int. J. Hydrogen Energy, 2007, 32, 1121–1140 CrossRef CAS.
- J. M. Bergthorson, S. Goroshin, M. J. Soo, P. Julien, J. Palecka, D. L. Frost and D. J. Jarvis, Appl. Energy, 2015, 160, 368–382 CrossRef CAS.
- D. B. Beach, A. J. Rondinone, B. G. Sumpter, S. D. Labinov and R. K. Richards, J. Energy Resour. Technol., 2007, 129, 29 CrossRef CAS.
- J. L. Sabourin, R. A. Yetter, B. W. Asay, J. M. Lloyd, V. E. Sanders, G. A. Risha and S. F. Son, Propellants, Explos., Pyrotech., 2009, 34, 385–393 CrossRef CAS.
- L. Galfetti, L. T. DeLuca, F. Severini, G. Colombo, L. Meda and G. Marra, Aerosp. Sci. Technol., 2007, 11, 26–32 CrossRef CAS.
- Space Shuttle, http://www.nasa.gov/returntoflight/system/system_SRB.html, accessed November 2016.
- T. D. Wood, M. A. Pfeil, T. L. Pourpoint and S. F. Son, in 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, 2009, p. 2009-4877 Search PubMed.
- D. S. Sundaram, V. Yang, T. L. Connell, G. A. Risha and R. A. Yetter, Proc. Combust. Inst., 2013, 34, 2221–2228 CrossRef CAS.
- E. R. Weaver, J. Ind. Eng. Chem., 1920, 12, 232–240 CrossRef CAS.
- E. I. Shkolnikov, A. Z. Zhuk and M. S. Vlaskin, Renewable Sustainable Energy Rev., 2011, 15, 4611–4623 CrossRef CAS.
- Y. Yavor, S. Goroshin, J. M. Bergthorson and D. L. Frost, Int. J. Hydrogen Energy, 2015, 40, 1026–1036 CrossRef CAS.
- J. M. Bergthorson, Y. Yavor, J. Palecka, W. Georges, M. Soo, J. Vickery, S. Goroshin, D. L. Frost and A. J. Higgins, Appl. Energy, 2017, 186, 13–27 CrossRef CAS.
- D. Wen, Energy Environ. Sci., 2010, 3, 591–600 CAS.
- C. Mandilas, G. Karagiannakis, A. G. Konstandopoulos, C. Beatrice, M. Lazzaro, G. Di Blasio, S. Molina, J. V Pastor and A. Gil, Energy Fuels, 2014, 28, 3430–3441 CrossRef CAS.
- K. E. Boulding, in Environmental Quality in a Growing Economy, ed. H. Jarrett, Johns Hopkins University Press, Baltimore, 1966, pp. 3–14 Search PubMed.
- F. Habashi, A History of Metallurgy, Métallurgie Extractive Québec, Québec, 1994 Search PubMed.
- F. Habashi, Handbook of extractive metallurgy, Wiley-VCH, New York, 1997 Search PubMed.
- P. Julien, J. Vickery, S. Goroshin, D. L. Frost and J. M. Bergthorson, Combust. Flame, 2015, 162, 4241–4253 CrossRef CAS.
- P. Julien, S. Whiteley, M. Soo, S. Goroshin, D. Frost and J. Bergthorson, Proc. Combust. Inst., 2017, 36, 2291–2298 CrossRef CAS.
- S. Goroshin, A. Miera, D. L. Frost and J. H. S. Lee, Proc. Combust. Inst., 1996, 26, 1883–1889 CrossRef.
- M. Soo, P. Julien, S. Goroshin, J. M. Bergthorson and D. L. Frost, Proc. Combust. Inst., 2013, 34, 2213–2220 CrossRef CAS.
- P. Julien, M. Soo, S. Goroshin, D. L. Frost, J. M. Bergthorson, N. Glumac and F. Zhang, J. Propul. Power, 2014, 30, 1047–1054 CrossRef CAS.
- A. Ishihara and M. Q. Brewster, Combust. Sci. Technol., 1993, 87, 275–290 CrossRef CAS.
- L. Meda, G. Marra, L. Galfetti, F. Severini and L. De Luca, Mater. Sci. Eng., C, 2007, 27, 1393–1396 CrossRef CAS.
- S. Yuasa and A. Fukuchi, Proc. Combust. Inst., 1994, 25, 1587–1594 CrossRef.
- E. Y. Shafirovich and U. I. Goldshleger, Combust. Sci. Technol., 1992, 84, 33–43 CrossRef CAS.
- M. Schiemann, J. Bergthorson, P. Fischer, V. Scherer, D. Taroata and G. Schmid, Appl. Energy, 2016, 162, 948–965 CrossRef CAS.
- D. R. Lide and T. J. Bruno, CRC handbook of chemistry and physics, CRC Press, New York, NY, 2012 Search PubMed.
- P. Julien, S. Whiteley, S. Goroshin, M. J. Soo, D. L. Frost and J. M. Bergthorson, Proc. Combust. Inst., 2015, 35, 2431–2438 CrossRef CAS.
- F.-D. Tang, S. Goroshin and A. J. Higgins, Proc. Combust. Inst., 2011, 33, 1975–1982 CrossRef CAS.
- D. J. Durbin and C. Malardier-Jugroot, Int. J. Hydrogen Energy, 2013, 38, 14595–14617 CrossRef CAS.
- D. Allen and D. Shonnard, Green Engineering: Environmentally Conscious Design of Chemical Processes, Prentice Hall, Upper Saddle River, 2002 Search PubMed.
- M. J. Brisson and A. A. Ekechukwu, Beryllium: environmental analysis and monitoring, Royal Society of Chemistry, Cambridge, 2009 Search PubMed.
- J. G. Peacey and W. G. Davenport, in The Iron Blast Furnace, ed. D. Hopkins, Elsevier, 1979, pp. 1–15 Search PubMed.
- A. Babich, Y. Yaroshevskii, A. Formoso, A. Cores, L. Garcia and V. Nozdrachev, ISIJ Int., 1999, 39, 229–238 CrossRef CAS.
- H. Y. Sohn, Suspension Hydrogen Reduction of Iron Oxide Concentrate, 2007 Search PubMed.
- C. K. Gupta and N. Krishnamurthy, Extractive metallurgy of vanadium, Elsevier, Amsterdam, 1992 Search PubMed.
- Ellingham Diagrams, http://www.doitpoms.ac.uk/tlplib/ellingham_diagrams/index.php, accessed November 2016.
- D. Wagner and O. Devisme, in Sohn International Symposium, San Diego, pp. 111–120 Search PubMed.
- R. N. Pease and H. S. Taylor, J. Am. Chem. Soc., 1921, 43, 2179–2188 CrossRef CAS.
- G. Parravano, J. Am. Chem. Soc., 1952, 74, 1194–1198 CrossRef CAS.
- N. G. Gallegos and J. M. P. Lopez, Mater. Chem. Phys., 1988, 19, 431–446 CrossRef CAS.
- J. Bessieres, A. Bessieres and J. J. Heizmann, Int. J. Hydrogen Energy, 1980, 5, 585–595 CrossRef CAS.
- W. K. Jozwiak, E. Kaczmarek, T. P. Maniecki, W. Ignaczak and W. Maniukiewicz, Appl. Catal., A, 2007, 326, 17–27 CrossRef CAS.
- J. W. Halley and J. E. Mcconnel, Reduction of iron ore with hydrogen, US3140168 A, 1964.
- M. Halmann, A. Frei and A. Steinfeld, Ind. Eng. Chem. Res., 2008, 47, 2146–2154 CrossRef CAS.
- W. T. Fairchild, JOM, 1970, 22, 55–58 CrossRef CAS.
- R. H. Perry and D. W. Green, Perry's chemical engineers' handbook, McGraw-Hill Professional, 1999 Search PubMed.
- H. Kvande and W. Haupin, JOM, 2000, 52, 31–37 CrossRef CAS.
- K. I. Popov, S. S. Djokic and B. N. Grgur, Fundamental aspects of electrometallurgy, Kluwer Academic/Plenum Publishers, New York, 2002 Search PubMed.
- B. C. Tripathy, S. C. Das, G. T. Hefter and P. Singh, J. Appl. Electrochem., 1997, 27, 673–678 CrossRef CAS.
- F. C. Porter, Zinc handbook: properties, processing, and use in design, CRC Press, New York, 1991 Search PubMed.
- H. E. Friedrich and B. L. Mordike, Magnesium Technology: metallurgy, design data, applications, Springer, 2006 Search PubMed.
- E. Aghion and G. Golub, in Magnesium Technology: Metallurgy, Design Data, Applications, ed. H. E. Mordike and B. L. Friedrich, Springer, 2006 Search PubMed.
- International Magnesium Association, http://www.intlmag.org/, accessed November 2016.
- B. de Closset, in International Symposium on Production and Electrolysis of Light Metals, Pergamon Press, Halifax, p. 278 Search PubMed.
- W. E. Bardsley, in International Engineering Conference on Hot Arid Regions, Al-Ahsa, 2010 Search PubMed.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- L. Bertuccioli, A. Chan, D. Hart, F. Lehner, B. Madden and E. Standen, Development of Water Electrolysis in the European Union, Switzerland: Fuel Cells and Hydrogen Joint Undertaking, Lausanne, 2014 Search PubMed.
- H. Zhou, X. Li, T. Fan, F. E. Osterloh, J. Ding, E. M. Sabio, D. Zhang and Q. Guo, Adv. Mater., 2010, 22, 951–956 CrossRef CAS PubMed.
- R. Kothari, D. Buddhi and R. L. Sawhney, Renewable Sustainable Energy Rev., 2008, 12, 553–563 CrossRef CAS.
- U. B. Pal and A. C. Powell, JOM, 2007, 59, 44–49 CrossRef CAS.
- U. B. Pal, D. E. Woolley and G. B. Kenney, JOM, 2001, 53, 32–35 CrossRef CAS.
- U. B. Pal, JOM, 2008, 60, 43–47 CrossRef CAS.
- X. Guan, U. B. Pal and A. C. Powell, Metall. Mater. Trans. E, 2014, 1, 132–144 CAS.
- X. Guan, P. A. Zink, U. B. Pal and A. C. Powell, Metall. Mater. Trans. B, 2013, 44, 261–271 CrossRef CAS.
- X. Guan, S. Su, U. B. Pal and A. C. Powell, Metall. Mater. Trans. B, 2014, 45, 2138–2144 CrossRef CAS.
- A. Martin, D. Lambertin, J.-C. Poignet, M. Allibert, G. Bourges, L. Pescayre and J. Fouletier, JOM, 2003, 55, 52–54 CrossRef CAS.
- X. Lu, X. Zou, C. Li, Q. Zhong, W. Ding and Z. Zhou, Metall. Mater. Trans. B, 2012, 43, 503–512 CrossRef CAS.
- X. Y. Yan and D. J. Fray, Mineral Processing and Extractive Metallurgy, 2007, 116, 17–24 CrossRef CAS.
- Y. Ito and T. Nohira, Electrochim. Acta, 2000, 45, 2611–2622 CrossRef CAS.
- D. R. Sadoway, J. Mater. Res., 1995, 10, 487–492 CrossRef CAS.
- M. Zhang, V. Kamavarum and R. G. Reddy, JOM, 2003, 55, 54–57 CrossRef CAS.
- G. Chen, D. Fray and T. Farthing, Nature, 2000, 407, 361–364 CrossRef CAS PubMed.
- A. Cox and D. J. Fray, Ironmaking Steelmaking, 2008, 38, 1401–1407 CAS.
- H. Kim, J. Paramore, A. Allanore and D. R. Sadoway, J. Electrochem. Soc., 2011, 158, 101–105 CrossRef.
- S. Wang, J. Ge, Y. Hu, H. Zhu and S. Jiao, Electrochim. Acta, 2013, 87, 148–152 CrossRef CAS.
- E. S. Gratz, X. Guan, J. D. Milshtein, U. B. Pal and A. C. Powell, Metall. Mater. Trans. B, 2014, 45, 1325–1336 CrossRef CAS.
- K. Yasuda, T. Nohira, K. Amezawa, Y. H. Ogata and Y. Ito, J. Electrochem. Soc., 2005, 152, D69–D74 CrossRef CAS.
- A. Allanore and H. L. J. P. Birat, J. Appl. Electrochem., 2010, 1957–1966 CrossRef CAS.
- H. Kim, J. Paramore, A. Allanore and D. R. Sadoway, J. Electrochem. Soc., 2011, 158, E101–E105 CrossRef CAS.
- A. Allanore, L. Yin and D. R. Sadoway, Nature, 2013, 497, 353–356 CrossRef CAS PubMed.
- D. Wang, A. J. Gmitter and D. R. Sadoway, J. Electrochem. Soc., 2011, 158, E51–E54 CrossRef CAS.
- A. Allanore, J. Electrochem. Soc., 2015, 162, E13–E22 CrossRef CAS.
- D. Fray, Faraday Discuss., 2016, 190, 11–34 RSC.
- M. Ma, D. Wang, W. Wang, X. Hu, X. Jin and G. Z. Chen, J. Alloys Compd., 2006, 420, 37–45 CrossRef CAS.
- J. Evans, H. Kvande and V. Mann, JOM, 2008, 60, 25–31 CrossRef.
- D. R. Sadoway, JOM, 2001, 53, 34–35 CrossRef CAS.
- Inert Anode Technology, http://www.rusal.ru/en/development/innovations/inert_anode/, accessed November 2016.
- Rusal, http://www.rusal.ru/en/, accessed November 2016.
- N. Auner and S. Holl, Energy, 2006, 31, 1395–1402 CrossRef CAS.
- A. F. Zhigach and D. C. Stasinevich, in Boron and Refractory Borides, ed. V. I. Matkovich, Springer Berlin Heidelberg, New York, 1977, pp. 214–226 Search PubMed.
- T. Yabe, S. Uchida, K. Ikuta, K. Yoshida, C. Baasandash, M. S. Mohamed, Y. Sakurai, Y. Ogata, M. Tuji, Y. Mori, Y. Satoh, T. Ohkubo, M. Murahara, A. Ikesue, M. Nakatsuka, T. Saiki, S. Motokoshi and C. Yamanaka, Appl. Phys. Lett., 2006, 89, 20–23 CrossRef.
- A. Steinfeld, P. Kuhn, A. Reller, R. Palumbo, J. Murray and Y. Tamaura, Int. J. Hydrogen Energy, 1998, 23, 767–774 CrossRef CAS.
- T. McNulty, in Conference Of Metallurgist, Canadian Institute of Mining, Metallurgy and Petroleum, 2014 Search PubMed.
- R. P. Harrisson, K. Waters and M. C. Patoine, in 50th conference of metallurgists, 2011 Search PubMed.
- M. Galinski, A. Lewandowski and I. Stepniak, Electrochim. Acta, 2006, 51, 5567–5580 CrossRef CAS.
- V. Kamavaram, D. Mantha and R. G. Reddy, J. Min. Metall., 2003, 39, 43–58 CrossRef CAS.
Footnote |
| † In this paper the word iron is used to denote the element, Fe, and not the industrial product. Commercial Fe is called either “iron” or “steel” depending on its carbon content. Confusingly, “steel” usually contains a higher concentration of Fe than does “iron”. |
| This journal is © The Royal Society of Chemistry 2017 |