A simple and efficient phosphorescent probe for iodide-specific detection based on crystallization-induced phosphorescence of organic ionic crystals†
Guilin
Chen‡
a,
Jin
Zhou‡
 b,
Hui
Feng
b,
Hui
Feng
 a,
Feifei
Feng
a,
Pengfei
Xu
a,
Saifei
Pan
a,
Jing
Xu
a and
Zhaosheng
Qian
a,
Feifei
Feng
a,
Pengfei
Xu
a,
Saifei
Pan
a,
Jing
Xu
a and
Zhaosheng
Qian
 *a
*a
aKey Laboratory of the Ministry for Advanced Catalysis Materials, College of Chemistry and Life Sciences, Zhejiang Normal University, Jinhua 321004, People's Republic of China. E-mail: qianzhaosheng@zjnu.cn
bCollege of Pharmacy, Weifang Medical University, Weifang 261053, People's Republic of China
First published on 24th November 2018
Abstract
It is very challenging to develop luminescent detection methods for iodide ions due to their significant fluorescence quenching of general fluorophores induced by the heavy-atom effect. Herein, we reveal a unique crystallization-induced bright phosphorescence of tetraphenylphosphonium iodide (TPP I) and develop a novel time-gated detection method for iodide ions to minimize the autofluorescence from complex biological samples. Both the involvement of heavy iodide ion and accessible ionic interaction-induced organic crystals contribute to the accomplishment of a very high phosphorescence quantum yield (0.42) for TPP I. This specific iodide-triggered bright organic phosphorescence enables the development of a novel time-gated detection method for iodide ions in the luminescence turn-on manner and further offers an opportunity to establish a facile test strip for the visual detection of iodide ions based on solid-state phosphorescence on a solid substrate. Excellent performance of this probe in imaging iodide in live cells and intriguing use in double encryption illustrate the versatile and broad application prospects of highly efficient organic ionic crystals in various areas.
Utilization of long-lived emissive probes for sensing and imaging via the time-gated photoluminescence technique is particularly valuable in minimizing interference from autofluorescence and scattering light.1 A variety of long-lived luminescent probes have been developed to attain high signal-noise ratio spectra and high-quality images with minimized autofluorescence interference, and these include lanthanides,2 transition-metal complexes,3 inorganic nanomaterials4 and persistent phosphors.5 However, these long-lived luminescent materials usually show certain toxicity to cells due to the inclusion of heavy metal elements in them; thereby, such a situation has forced scientists to design long-lived organic materials with room-temperature phosphorescence. Recent advances demonstrate that crystallization-induced phosphorescence (CIP) acts as a general design strategy to achieve efficient room-temperature phosphorescence,6 and a preliminary application in bioimaging has been achieved using red CIP nanocrystals.7 To maintain bright room-temperature phosphorescence (RTP), a strict crystalline state is required for these organic phosphorescent materials during sensing and imaging processes, and the relatively low brightness of long-lived emission is not advantageous for sensitive sensing and high-quality imaging. As a result, the design and development of phosphorescent organic probes with facile crystallization-induced highly bright phosphorescence are urgently required for sensing and bioimaging to minimize interference from autofluorescence.
Iodine as one key micronutrient is involved in neurological development and normal function of thyroid gland; consequently, an abnormal change in the amount of iodine can give rise to thyroid diseases.8 Several luminescent assays for iodide ions have been proposed, and most of them rely on the luminescence quenching effect of iodide ion to metal nanoclusters9 and fluorescent dyes10 due to its significant heavy-atom effect. In contrast to these luminescence quenching-based assays, luminescence turn-on detection methods are more desirable because they can minimize the interference from the surroundings to a great extent. Two replacement strategies based on Au nanoparticles and graphene oxide were proposed to achieve fluorescence turn-on detection of iodide.11 In comparison to nanoprobes, molecular probes in a signal turn-on manner possess appreciable merits in reproducibility and sensitivity. However, to the best of our knowledge, only one study has reported a fluorescence turn-on detection method for iodide based on an elimination reaction, and this detection strategy relies on the iodide ion-triggered elimination of two adjacent bromine atoms in the fluorophore.12 This assay performs well in imaging the iodide ion in live cells, but other species that lead to the elimination reaction might interfere with the detection of the iodide ion. Consequently, it is very challenging to establish a highly specific iodide ion detection method in a luminescence turn-on manner.
Quaternary phosphonium salts are a fundamentally significant class of organophosphorus compounds and are generally used as valuable arylating reagents13 and powerful chiral phase-transfer catalysts.14 Among them, the triphenylphosphonium moiety has been recognized as an effective targeting functional group for mitochondria in the design of mitochondria-selective indicators.15 However, its unique solid-state photoluminescence has not been explored. In this study, we demonstrate unique and efficient crystallization-induced room-temperature phosphorescence of an organic ionic crystal with the involvement of iodide ion; the specific iodide-triggered crystallization-induced phosphorescence (CIP) provides a controllable platform through external stimuli. As shown in Scheme 1, the tetraphenylphosphonium (TPP) cation can strongly bind with iodide ion to form water-insoluble TPP I microcrystals, whereas other ordinary inorganic anions cannot precipitate the TPP cation in water. The generated TPP I microcrystals exhibit bright blue room-temperature phosphorescence due to the significant heavy-atom effect of iodide. The iodide-triggered bright phosphorescence of the organic ionic crystal enables the development of a highly specific assay for iodide ion in a time-gated manner to avoid the autofluorescence of complex matrices. Its applications in sensing iodide ion in live cells and in double encryption were further evaluated.
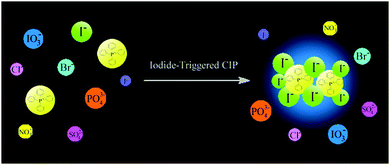 | ||
| Scheme 1 Schematic illustration of iodide-triggered crystallization-induced phosphorescence based on tetraphenylphosphonium cation. | ||
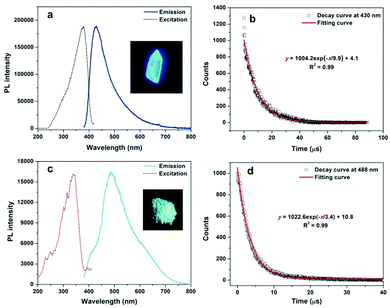
1H NMR and 13C NMR spectra of TPP halides in CD3CN (Fig. S1 and S2, ESI†) demonstrate that all of them have satisfactory purities. All TPP halides can dissolve well in acetonitrile, but no detectable fluorescence for such solutions can be observed by the naked eye or with a fluorospectrophotometer, implying that the dissolved TPP halides do not fluoresce. All the TPP halides consist of a positive TPP cation and a negative halide and are thus ionic compounds. Their solid forms as crystals or microcrystals were further confirmed by the X-ray powder diffraction technique. The sharp peaks and ordered patterns in Fig. S3 (ESI†) clearly indicate that solid TPP halides exist in the crystalline state. It is found that the crystals of four tetraphenylphosphonium halides can exhibit different luminescences under UV light irradiation. As shown in Fig. S4 (ESI†), the major emissions of TPP F, TPP Cl and TPP Br crystals are located around 500 nm, but only weak green emissions can be observed for them because their quantum yields are relatively low (less than 0.05). The time-resolved PL decay curves in Fig. S5 (ESI†) show that TPP F, TPP Cl and TPP Br exhibit ultralong emission in millisecond magnitude; this indicates that significant electronic coupling occurs inside the crystals because it has been shown that strong intermolecular electronic coupling is responsible for the occurrence of ultralong afterglow in organic crystals.16 In comparison with the results for others, the emission maximum of TPP I is appreciably blue-shifted to 430 nm (Fig. 1a). The most impressive property of TPP I is its extremely bright blue emission, and its quantum yield is determined to be 0.42 using the absolute method. The lifetime of TPP I crystal is determined to be 9.9 μs, according to its time-resolved luminescence decay curve (Fig. 1b), and such a microsecond lifetime clearly indicates that the bright blue luminescence is due to phosphorescence caused by the heavy-atom effect of iodide. Such a high phosphorescence emission efficiency for TPP I is very rare, and only two examples including 1,2,3,4,5,6-hexakis(arylthio)benzenes17 and 1,4-dibenzoyl-2,5-bis(siloxy)-benzenes18 with higher quantum yields have been reported till now. A similar bright room-temperature phosphorescence is also found for methyltriphenylphosphonium iodide (MTPP I). In contrast to the observation for TPP I, its triplet emission is red-shifted to 488 nm (Fig. 1c), and its quantum yield decreases to 0.26. The microsecond lifetime of the MTPP I crystal from Fig. 1d illustrates the triplet nature of the emission, which is consistent with that for TPP I. It is found that TPP Cl and MTPP Cl are soluble in aqueous solutions, but TPP I and MTPP I are almost insoluble in water, which enables TPP Cl and MTPP Cl to quantify iodide in the phosphorescence turn-on manner. TPP Cl was chosen as the phosphorescent probe to quantitate iodide ions in the following section because of the higher quantum yield of the precipitation product TPP I with the iodide ion.
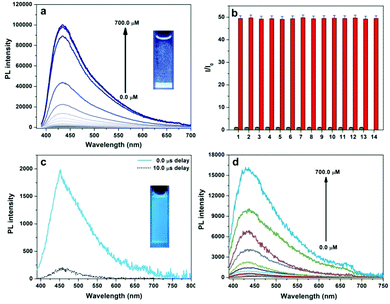
Ultrabright phosphorescence and poor solubility of tetraphenylphosphonium iodide (TPP I) in water inspire us to develop a novel phosphorescence turn-on assay for iodide ions. Water-soluble TPP Cl was employed as the probe, and the introduction of iodide ions into the probe solution resulted in many microcrystals of TPP I with a bright blue emission. Fig. 2a demonstrates a phosphorescence enhancement trend at 430 nm as the iodide concentration increases from 0.0 to 700.0 μM, and more than 500-fold enhancement at maximum is achieved. The calibration curve in Fig. S6 (ESI†) shows a linear relationship between the natural logarithm of the PL intensity (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I) and iodide concentration in the range of 366.7–633.3 μM. The selectivity test in Fig. 2b illustrates that significant PL enhancement is only caused by the presence of iodide ions (500.0 μM), whereas an equivalent amount of the other anions including F−, Cl− and Br− cannot interfere with the detection of iodide, indicating the excellent specificity of the luminescent assay for iodide ions. The long-lived microsecond emission of TPP I enables us to perform time-gated detection of iodide to avoid significant autofluorescence from biological samples such as serum. As shown in Fig. 2c, human serum has intense blue autofluorescence with an average lifetime of 3.4 ns (Fig. S7, ESI†); however, most emission signals from the serum can be suppressed when a delay time of 10.0 μs is used. This effective reduction of autofluorescence provides an opportunity to establish a time-gated assay for iodide with a proper delay time. Fig. 2d displays the time-gated PL spectra of TPP versus the concentration of iodide ions in the range of 0.0–700.0 μM with a delay time of 10.0 μs; a similar relationship between the natural logarithm of the PL intensity (ln
I) and iodide concentration in the range of 366.7–633.3 μM. The selectivity test in Fig. 2b illustrates that significant PL enhancement is only caused by the presence of iodide ions (500.0 μM), whereas an equivalent amount of the other anions including F−, Cl− and Br− cannot interfere with the detection of iodide, indicating the excellent specificity of the luminescent assay for iodide ions. The long-lived microsecond emission of TPP I enables us to perform time-gated detection of iodide to avoid significant autofluorescence from biological samples such as serum. As shown in Fig. 2c, human serum has intense blue autofluorescence with an average lifetime of 3.4 ns (Fig. S7, ESI†); however, most emission signals from the serum can be suppressed when a delay time of 10.0 μs is used. This effective reduction of autofluorescence provides an opportunity to establish a time-gated assay for iodide with a proper delay time. Fig. 2d displays the time-gated PL spectra of TPP versus the concentration of iodide ions in the range of 0.0–700.0 μM with a delay time of 10.0 μs; a similar relationship between the natural logarithm of the PL intensity (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I) and the iodide amount in a closer concentration range of 400.0–700.0 μM to the assay without time-gating is obtained (Fig. S8, ESI†).
I) and the iodide amount in a closer concentration range of 400.0–700.0 μM to the assay without time-gating is obtained (Fig. S8, ESI†).
To further evaluate the tracking ability for iodide ions in complex matrices, imaging of iodide ions in live human HepG2 cells was conducted using TPP Cl as the probe. Cytotoxicity test of TPP Cl was first assessed using a standard MTT assay, and the results in Fig. S9 (ESI†) indicate that more than 80% viability of HepG2 cells was retained, and no apparent change in cell morphology was observed even when a large concentration of TPP Cl (200.0 μM) was used to treat the cells. Thus, a lower amount of TPP Cl (100.0 μM) was used to image the iodide ions in live HepG2 cells. It is noted that no emission can be recorded for HepG2 cells when they are only treated with TPP Cl (Fig. 3a); however, a clear blue color is observed after a fixed amount of iodide ions is introduced into the HepG2 cells. In Fig. 3b, we see that the relatively bright green pseudocolor labels the entire cytoplasm part of a cell; the intensity of the blue phosphorescence emitted from HepG2 cells does not change even after 15 min of irradiation at 405 nm. The above observations apparently suggest that TPP Cl as an ionic probe can penetrate live cells and respond to iodide ions in such a complex matrix. In contrast to soluble fluorescent probes,12 the location of the targets inside the live cells can be fixed at their original positions due to the deposition of the generated phosphorescent crystals. Moreover, no appreciable photobleaching takes place during the imaging process due to the stability of these microcrystals.
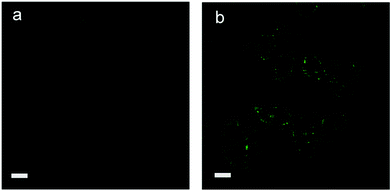 | ||
| Fig. 3 Laser scanning confocal microscopy images of human HepG2 cells with tetraphenylphosphonium chloride (TPP Cl): (a) TPP Cl (100.0 μM) (b) TPP Cl and I− (100.0 μM). Scale bar: 20 μm. | ||
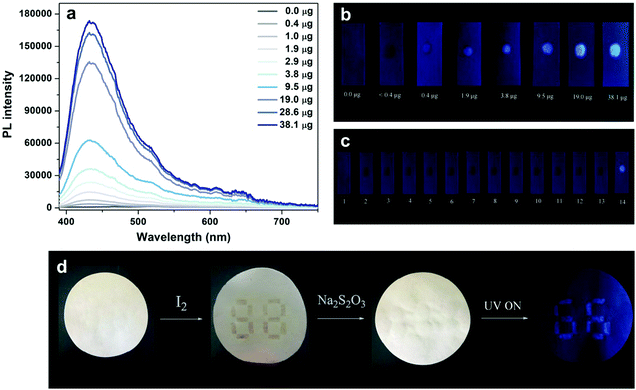
The facile detection of iodine depends on an intense blue or purple-blue starch-iodine complex with a unique charge-transfer nature;19 hence, a conversion reaction from the iodide ion to iodine is required prior to the detection of iodide-containing samples. The bright solid-state phosphorescence of TPP I enables the construction of a novel phosphorescent test strip for iodide ions with high sensitivity and selectivity based on an RTP solid substrate. Fig. 4a shows PL spectra of TPP Cl-containing test strips in the presence of different amounts of iodide ions from 0.0 to 38.1 μg, and almost 350-fold increase in phosphorescence at 430 nm can be attained relative to the background emission from the test strips when a small amount of iodide ions (38.1 μg) is added. The amounts of added iodide ions on the test strips can be quantified with simple visual detection by the naked eye with the aid of a handheld UV lamp, as shown in Fig. 4b. According to the calibration curve in Fig. S10 (ESI†), the amount of iodide ions is directly proportional to the observed phosphorescence intensity, and the lower detection limit is determined to be 0.2 μg. The iodide test strips also possess highly selective response to iodide ions without interference from other common anions (Fig. 4c), and this high specificity toward iodide ions guarantees the practical use in real samples. Furthermore, the ionic interaction-induced intense blue phosphorescence of TPP I can be used in the coloration of invisible information on the paper for double encryption by combining the traditional chromogenic reaction between iodine and starch. As displayed in Fig. 4d, the number “88” is first written on a white paper with a starch solution, and a pattern “66” is then covered on the number “88” with a concentrated TPP Cl solution. A purple “88” number appears upon the addition of iodine on the paper, but no patterns can be observed in the dark under a UV lamp. However, continuous introduction of a solution of a proper reductant such as Na2S2O3 leads to disappearance of the purple “88” number and simultaneous emergence of a blue “66” pattern when excited by UV light due to the conversion reaction from iodine to iodide ions. Such a double encryption function is useful in designing smart materials and anti-counterfeit labels.
In summary, unique room-temperature phosphorescence of tetraphenylphosphonium iodide crystal is revealed, and the iodide-triggered bright phosphorescence enables the development of a time-gated phosphorescent detection method for iodide ions. The specific heavy-atom effect of iodide and low solubility of tetraphenylphosphonium iodide in aqueous solutions guarantee high specificity for iodide detection. The bright solid-state phosphorescence of tetraphenylphosphonium iodide on the solid substrates provides an opportunity for developing a facile test strip for visual detection of iodide ions. In contrast to previous reports, our detection method not only quantifies iodide ions in an emission turn-on manner, but also offers a novel time-gated assay for iodide using an organic probe to avoid autofluorescence of complex matrices. The excellent performance in imaging iodide in live cells and double encryption demonstrates the versatile and broad application prospects of phosphorescent organic ionic crystals.
We gratefully acknowledge financial support from the National Natural Science Foundation of China (Grant No. 21675143, 21775139 and 21705120), Natural Science Foundation of Zhejiang Province (Grant No. LR18B050001 and LY17B050003) and Shandong Province (Grant No. ZR2017LB016).
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Y. Zhang, Q. Yu, H. Wei, S. Liu, Q. Zhao and W. Huang, Chem. Rev., 2018, 118, 1770–1839 CrossRef CAS PubMed.
- (a) K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed; (b) J.-C. G. Bunzli, Acc. Chem. Res., 2006, 39, 53–61 CrossRef; (c) E. G. Moore, A. P. S. Samuel and K. N. Raymond, Acc. Chem. Res., 2009, 42, 542–552 CrossRef CAS PubMed.
- (a) Q. Zhao, F. Li and C. Huang, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC; (b) D.-L. Ma, V. P.-Y. Ma, D. S.-H. Chan, K.-H. Leung, H.-Z. He and C.-H. Leung, Coord. Chem. Rev., 2012, 256, 3087–3113 CrossRef CAS.
- (a) J. Yao, M. Yang and Y. Duan, Chem. Rev., 2014, 114, 6130–6178 CrossRef CAS; (b) L. Shang, S. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401–418 CrossRef CAS.
- (a) Y. Li, M. Gecevicius and J. Qiu, Chem. Soc. Rev., 2016, 45, 2090–2136 RSC; (b) S.-K. Sun, H.-F. Wang and X.-P. Yan, Acc. Chem. Res., 2018, 51, 1131–1143 CrossRef CAS.
- (a) S. Mukherjee and P. Thilagar, Chem. Commun., 2015, 51, 10988–11003 RSC; (b) M. Baroncini, G. Bergamini and P. Ceroni, Chem. Commun., 2017, 53, 2081–2093 RSC; (c) S. Hirata, Adv. Opt. Mater., 2017, 1700116 CrossRef.
- S. M. A. Fateminia, Z. Mao, S. Xu, Z. Yang, Z. Chi and B. Liu, Angew. Chem., Int. Ed., 2017, 56, 12160–12164 CrossRef CAS PubMed.
- (a) G. Dai, O. Levy and N. Carrasco, Nature, 1996, 379, 458–460 CrossRef CAS PubMed; (b) A. M. Leung and L. E. Braverman, Nat. Rev. Endocrinol., 2014, 10, 136–142 CrossRef CAS PubMed.
- (a) L. Feng, Z. Sun, H. Liu, M. Liu, Y. Jiang, C. Fan, Y. Cai, S. Zhang, J. Xu and H. Wang, Chem. Commun., 2017, 53, 9466–9469 RSC; (b) Z. Chen, Y. Niu, G. Cheng, L. Tong, G. Zhang, F. Cai, T. Chen, B. Liu and B. Tang, Analyst, 2017, 142, 2781–2785 RSC; (c) Y. Wang, H. Zhu, X. Yang, Y. Dou and Z. Liu, Analyst, 2013, 138, 2085–2089 RSC; (d) S.-C. Wei, P.-H. Hsu, Y.-F. Lee, Y.-W. Lin and C.-C. Huang, ACS Appl. Mater. Interfaces, 2012, 4, 2653–2658 Search PubMed; (e) W. Hou, Y. Chen, Q. Lu, M. Liu, Y. Zhang and S. Yao, Talanta, 2018, 180, 144–149 CrossRef CAS PubMed.
- (a) D. Y. Lee, N. Singh, M. J. Kim and D. O. Jang, Org. Lett., 2011, 13, 3024–3027 CrossRef CAS PubMed; (b) N. Ahmed, B. Shirinfar, S. Youn, M. Yousuf and K. S. Kim, Org. Biomol. Chem., 2013, 11, 6407–6413 RSC; (c) R. X. Zhang, P. F. Li, W. J. Zhang, N. Li and N. Zhao, J. Mater. Chem. C, 2016, 4, 10479–10485 RSC; (d) C. Kar, A. Basu and G. Das, Tetrahedron Lett., 2012, 53, 4754–4757 CrossRef CAS; (e) V. Suresh, N. Ahmed, I. S. Youn and K. S. Kim, Chem. – Asian J., 2012, 7, 658–663 CrossRef CAS PubMed.
- (a) Y.-M. Chen, T.-L. Cheng and W.-L. Tseng, Analyst, 2009, 134, 2106–2112 RSC; (b) D. Dinda, B. K. Shaw and S. K. Saha, ACS Appl. Mater. Interfaces, 2015, 7, 14734–14749 CrossRef.
- F. Kong, X. Meng, R. Chu, K. Xu and B. Tang, Chem. Commun., 2015, 51, 6925–6927 RSC.
- (a) L. K. Hwang, Y. Na, J. Lee, Y. Do and S. Chang, Angew. Chem., Int. Ed., 2005, 44, 6166–6169 CrossRef CAS PubMed; (b) X. Zhang and A. McNally, Angew. Chem., Int. Ed., 2017, 56, 9833–9836 CrossRef CAS PubMed.
- (a) R. He, X. Wang, T. Hashimoto and K. Maruoka, Angew. Chem., Int. Ed., 2008, 47, 9466–9468 CrossRef CAS PubMed; (b) C. L. Zhu, F. G. Zhang, W. Meng, J. Nie, D. Cahard and J. A. Ma, Angew. Chem., Int. Ed., 2011, 50, 5869–5872 CrossRef CAS PubMed; (c) S. Liu, Y. Kumatabara and S. Shirakawa, Green Chem., 2016, 18, 331–341 RSC.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- S. Pan, Z. Chen, X. Zheng, D. Wu, G. Chen, J. Xu, H. Feng and Z. Qian, J. Phys. Chem. Lett., 2018, 9, 3939–3945 CrossRef CAS PubMed.
- G. Bergamini, A. Fermi, C. Botta, U. Giovanella, S. D. Motta, F. Negri, R. Presutti, M. Gingras and P. Ceroni, J. Mater. Chem. C, 2013, 1, 2717–2724 RSC.
- M. Shimizu, R. Shigitani, M. Nakatani, K. Kuwabara, Y. Miyake, K. Tajima, H. Sakai and T. Hasobe, J. Phys. Chem. C, 2016, 120, 11631–11639 CrossRef CAS.
- S. Madhu, H. A. Evans, V. V. T. Doan-Nguyen, J. G. Labram, G. Wu, M. L. Chabinyc, R. Seshadri and F. Wudl, Angew. Chem., Int. Ed., 2016, 55, 8032–8035 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, NMR and luminescence spectra, and calibration curves. See DOI: 10.1039/c8tc04781b |
| ‡ These authors contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2019 |