DOI:
10.1039/C8RA06500D
(Paper)
RSC Adv., 2018,
8, 34525-34535
A comparison of TiF3 and NbF5 catalytic effects on hydrogen absorption and desorption kinetics of a ball-milled Mg85Zn5Ni10 alloy
Received
2nd August 2018
, Accepted 22nd September 2018
First published on 8th October 2018
Abstract
In this investigation, the as-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) composites were successfully produced via ball milling. The different influences between the catalysts TiF3 and NbF5 on the hydrogen storage behavior and microstructure of the composites were investigated by XRD, SEM, TEM and hydrogen absorption/desorption tests. The as-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys contain the major phase Mg, the secondary phase Mg2Ni, a small amount of MgZn2, TiF3 and NbF5. After hydrogenation, MgH2 and Mg2NiH4 are formed, which convert back into Mg and Mg2Ni after dehydrogenation indicating that MgZn2 and the catalysts TiF3 and NbF5 do not react with hydrogen. Compared with NbF5 catalyzed alloy, the TiF3 catalyzed alloy has a faster hydrogen absorption/desorption kinetics. On the basis of Arrhenius equation, the dehydrogenation activation energy values of the as-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys are 75.514 and 82.367 kJ mol−1 H2, respectively, while the value of ball-milled Mg85Zn5Ni10 alloy is 109.830 kJ mol−1 H2. As a result, both TiF3 and NbF5 can significantly ameliorate the hydrogen storage thermodynamics. TiF3 shows better catalytic influence on hydrogen storage property of Mg85Zn5Ni10 than NbF5.
Introduction
For nearly half a century, rapid economic development and energy crisis have threatened our existence because of excessive exploitation of non-renewable energy sources. Meanwhile, the consequent environmental problems arising from the excessive use of fossil fuels are becoming increasingly prominent. There is a growing demand for a new kind of renewable energy. Therefore, many scholars pay attention to hydrogen owing to its high efficiency, environment friendly nature, and renewable characteristics.1–13 However, the problem that is severely restricting the application of hydrogen is availability of a safe and credible storage mode.14–16 Because of the merits of high gravimetric storage density and safety, many researchers have great expectations from metal hydrides as a hydrogen storage material.15–20 Most researchers believe that among the different metals which can form hydrides, Mg is the most potential candidate to be widely used as a hydrogen storage material because of its low price, excellent reversibility, abundance, and high gravimetric hydrogen storage densities (7.6 wt%).21,22 However at the same time, the inherent shortcomings of Mg, such as the high decomposition temperature of its hydride and poor storage kinetics seriously impede its commercial development. Therefore, it is essential to ameliorate the kinetics and thermodynamics of the hydrogen storage alloys.
Some researches have confirmed that alloying Mg with transition metals such as Ni,23–25 Zn,26,27 Ti, Cr, Nb, Cu and Fe28–31 can enhance the hydrogenation/dehydrogenation rates. Also, some rare earth elements,32–34 intermetallic compounds,35 metallic oxides,36,37 and fluorides38–40 are proven to have a good catalytic effect on accelerating the storage and release of hydrogen. Yavari et al.41 added FeF3 into MgH2 to manufacture nanostructured MgH2 composites and found that hydrogenation/dehydrogenation rates were clearly enhanced. Lee et al.42 synthesized Mg–5NbF5 and showed that Mg–5NbF5 had faster initial hydrogen absorption and desorption and a larger effective storage capacity. Ma et al.39 mechanically prepared a MgH2 + TiF3 system and proved that mechanical milling with 4 mol% TiF3 could enhance the sorption kinetics markedly and reduce operation temperatures in particular. The hydrogenation could be accomplished in about 25 s even at 313–373 K. Recham et al.43 showed that NbF5 could reduce the dehydriding temperature while enhancing the kinetics of ball-milled MgH2 with 2 mol% of NbF5 as the optimum concentration. It is believed that the transition metal fluorides play a key role in enhancing the hydrogenation kinetics of Mg.42
In this study, we designed a new kind of Mg based Mg85Zn5Ni10 ternary alloy, and ball milled it with TiF3 and NbF5 as catalysts to ameliorate the hydrogen storage properties. We also thoroughly investigated the influence of doping TiF3 and NbF5 on the microstructure and hydrogen storage performances of experimental materials. The storing and releasing characteristics and activation energies were also analysed.
Experimental
Mg85Zn5Ni10 alloy was prepared using a vacuum induction furnace under 0.04 MPa helium atmosphere. Extra 10 wt% Mg was added to reduce the volatilization of magnesium during melting. The as-cast alloy ingots were mechanically crushed into powders (particle size < 75 μm). Then the as-cast alloy powder was mixed with 4 wt% TiF3 and NbF5 and transferred to a mechanical ball mill. A planetary-type mill was used under an Ar atmosphere for milling. The weight ratio of the alloy powder and Cr–Ni stainless steel balls was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The milling speed was 350 rpm. During ball milling, in order to prevent excess heating, the mill was set to rest for 1 h after every 1 h of working. The samples were milled for a total of 5 h. The as-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) in this research are named as Mg85Zn5Ni10–4C (C = TiF3, NbF5). Phase structures, morphologies and crystalline states of the ball-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) alloys were investigated and observed by X-ray diffraction (XRD) (D/max/2400), SEM and high resolution transmission electron microscopy (HRTEM) (JEM-2100F).
40. The milling speed was 350 rpm. During ball milling, in order to prevent excess heating, the mill was set to rest for 1 h after every 1 h of working. The samples were milled for a total of 5 h. The as-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) in this research are named as Mg85Zn5Ni10–4C (C = TiF3, NbF5). Phase structures, morphologies and crystalline states of the ball-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) alloys were investigated and observed by X-ray diffraction (XRD) (D/max/2400), SEM and high resolution transmission electron microscopy (HRTEM) (JEM-2100F).
A Sieverts-type apparatus was used to measure the pressure–composition isotherms (P–C-I) and hydrogen absorption/desorption kinetics. First, 6 hydrogen absorption and desorption cycles were performed at 360 °C to completely activate the samples. PCI curves were tested at 360, 340 and 320 °C. The hydrogen absorption was conducted at a hydrogen pressure of 3 MPa, at 360, 340, 320, 300, 280, 260, 240, 220, 200 and 150 °C respectively. The hydrogen desorption was tested at a pressure of 1 × 10−4 MPa at 360, 340, 320, 300 and 280 °C.
The dehydrogenation process of the ball-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) alloys was studied by differential scanning calorimetry (DSC) on a NETZSCH, STA 449F3 instrument. The flow rate of argon was 50 mL min−1. The hydrogen saturated-samples were gradually heated from room temperature to 500 °C with an increase of 5 °C min−1.
Results and discussion
Phase and microstructural characteristics
X-ray diffraction (XRD) has been utilized in studying the phase compositions and structures of ball-milled Mg85Zn5Ni10 and Mg85Zn5Ni10–4C (C = TiF3, NbF5) before and after hydrogen absorption/desorption, as described in Fig. 1. We can see that after 5 h ball milling, the broadened diffraction peaks reveal that mechanical ball milling causes the reduction of grain size and amorphization. According to Fig. 1, the ball-milled Mg85Zn5Ni10 contains three phases: Mg as the major phase, the secondary phase Mg2Ni, and a small MgZn2 phase. Meanwhile, after adding TiF3 and NbF5, no new phase appeared in the XRD curves, indicating no reaction occurred between the catalysts TiF3 and NbF5 and Mg85Zn5Ni10 during ball milling. Mg and Mg2Ni are still the major and the secondary phase in Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys, respectively. After hydrogen absorption, it is clear that the Mg phase is converted into MgH2. Simultaneously, Mg2Ni converts to Mg2NiH4. It is worth noting that among all three samples, only MgZn2 existed after hydrogen absorption, showing that it does not react with hydrogen. After hydrogen desorption, MgH2 and Mg2NiH4 change into Mg and Mg2Ni, respectively. MgZn2 is also found in the dehydrogenated alloys. Based on the XRD patterns, there are two reversible reactions for hydrogenation and dehydrogenation of the alloys. The reaction path ways can be inferred as follows:
 |
| | Fig. 1 XRD patterns of the ball-milled Mg85Zn5Ni10 and Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys before and after 30 hydrogen absorption and desorption cycles: (a) ball-milled, (b) C = TiF3, (c) C = NbF5. | |
The Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloy samples after hydrogen absorption and desorption were detected by HRTEM and ED, as described in Fig. 2. After ball milling with the catalysts and hydrogen absorption and desorption, partial nanocrystalline and amorphous phases form in the Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys. Evidently, after hydrogen absorption, Mg85Zn5Ni10–4TiF3 contains MgH2, Mg2NiH4, MgZn2 and TiF3 phases, and Mg85Zn5Ni10–4NbF5 possesses MgH2, Mg2NiH4, MgZn2 and NbF5 phases, which are sustained through ED patterns and also consistent with the XRD test. According to the structural analysis and index of ED rings, MgH2 and Mg2NiH4 convert into Mg and Mg2Ni respectively. MgZn2, TiF3 and NbF5 are found after hydrogen desorption, suggesting absence of reaction among MgZn2, TiF3, NbF5 and hydrogen. The catalysts TiF3 and NbF5 exist stably in the Mg85Zn5Ni10–4C (C = TiF3, NbF5) composites after ball milling, even after 30 hydrogenation and dehydrogenation cycles. The results of HRTEM and ED coincide with the XRD results.
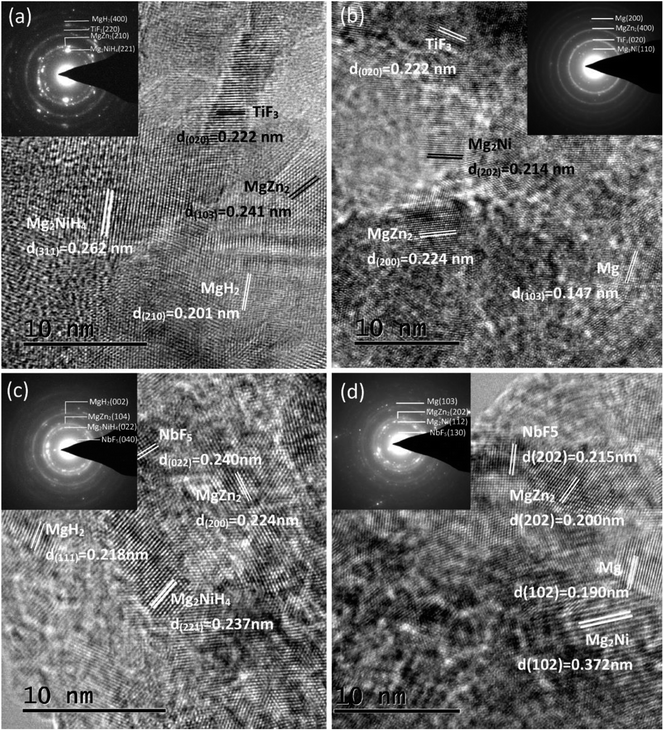 |
| | Fig. 2 HRTEM micrographs and SAED patterns of the Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys after hydrogen absorption and desorption: (a) hydrogenated C = TiF3, (b) dehydrogenated C = TiF3, (c) hydrogenated C = NbF5, (d) dehydrogenated C = NbF5. | |
Fig. 3 and 4 present the bright field FETEM images and EDS mapping of the Mg85Zn5Ni10–4TiF3 and Mg85Zn5Ni10–4NbF5 alloys after dehydrogenation cycles, respectively. The different colours express the distributions of different elements. As can be seen from Fig. 3(a) and (b), Mg is the major component covering the main matrix of a single Mg85Zn5Ni10–4TiF3 alloy particle. In Fig. 3(c) and (d), the Ni and Zn elements are mainly distributed in the bright regions of the alloy particle illustrating that Mg–Ni and Mg–Zn metal compounds are distributed on the surface of the alloy particle. The Ti and F elements are relatively well-distributed around the alloy particle indicating uniform inlaying of TiF3 on Mg matrix which is corroborated by XRD and TEM results. Similarly, according to Fig. 4, Nb and F elements are also well-distributed on the Mg85Zn5Ni10–4NbF5 alloy particles. Based on XRD, TEM and EDS mapping, the catalysts TiF3 and NbF5 are both present after hydrogenation and dehydrogenation cycles suggesting that they do not react with hydrogen or decompose after hydriding and dehydriding cycles.
 |
| | Fig. 3 FETEM micrographs and EDS mapping of the Mg85Zn5Ni10–4TiF3 alloy after hydrogen desorption cycles. | |
 |
| | Fig. 4 FETEM micrographs and EDS mapping of the Mg85Zn5Ni10–4NbF5 alloy after hydrogen desorption cycles. | |
Hydrogenation and dehydrogenation kinetics
With the aim to study the influence of different catalysts on hydrogenation kinetics of Mg85Zn5Ni10, the hydrogen absorptions of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys were measured at a pressure of 3 MPa and at 360, 340, 320, 300, 280, 260, 240, 220, 200 and 150 °C, as represented in Fig. 5. We can observe that at the beginning of the hydrogenation process, the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys have a very fast hydrogenation rate and the alloys need relatively long time to get saturated. This is because the hydride layer that is formed swiftly covers the particle surfaces and blocks the hydrogen diffusion into the alloys. For the convenience of comparison, the hydrogen absorption capacity at 600 s has been taken as the reference. According to Fig. 5, the C = TiF3 alloy can absorb 4.404, 4.33, 4.238, 4.153, 4.117 wt% at 360, 340, 320, 300, 280 °C, respectively in 600 s. Simultaneously, the C = NbF5 alloy can absorb 4.313, 4.244, 4.235, 4.197, 4.06 wt% at the same condition. Distinctly, the hydrogenation absorption rate within 600 s of C = TiF3 alloy is a little faster than the C = NbF5 alloy, also at relatively low temperatures. From Fig. 5(c) and (d), even at 150 °C, the C = TiF3 alloy shows a faster absorption rate comparing with C = NbF5 alloy. This explains that TiF3 has more effective catalytic effect than NbF5. The ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys show very fast hydrogenation rate. This is probably due to the TiF3 and NbF5 catalysts promoting H2 molecules to dissociate into H atoms and distribute on the surfaces of the Mg particles. Also, after ball milling with TiF3 and NbF5, the uniform mixing powders generated defects and cracks during the hydriding/dehydriding process, as illustrated in Fig. 6. These defects and cracks provide hydrogen diffusion channels and nucleation positions to form magnesium hydrides. As a comparison, the cracks formed on the C = TiF3 alloy particles are evidently more than those on the C = NbF5 alloy. This is the reason why the C = TiF3 alloy has faster hydrogen absorption kinetics, even at relatively low temperatures.
 |
| | Fig. 5 Hydrogen absorption kinetic curves of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at different temperatures: (a) and (c) C = TiF3, (b) and (d) C = NbF5. | |
 |
| | Fig. 6 SEM images of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys: (a) C = TiF3 alloy after ball milling, (b) C = TiF3 alloy after hydrogenation, (c) C = TiF3 alloy after dehydrogenation, (d) C = NbF5 alloy after ball milling, (e) C = NbF5 alloy after hydrogenation, (f) C = NbF5 alloy after dehydrogenation. | |
The hydrogen desorptions of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys were tested to investigate the different catalytic effects between TiF3 and NbF5 on the hydrogen desorption kinetics, as depicted in Fig. 7. The hydrogen desorption from the alloys was tested at 360, 340, 320, 300 and 280 °C. Evidently, the temperature significantly affects the hydrogen desorption kinetics. The ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys have very fast desorption kinetics over 320 °C. The ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys could release all the hydrogen in less than 300 s at 360 °C, but took more than 30 minutes at 280 °C. According to Fig. 7, the time taken for releasing 4 wt% H2 at 360, 340, 320, 300 and 280 °C is 102, 156, 318, 726 and 1836 s, respectively for the C = TiF3 alloy, and 150, 222, 348, 756 and 1860 s, respectively for the C = NbF5 alloy. This indicates the C = TiF3 alloy has better desorption kinetics than C = NbF5 alloy owing to the defects and cracks (Fig. 6(c) and (f)) formed on the particles which promote the hydrogen diffusion. Certainly, both catalysts have significant effects on the hydrogenation kinetics compared with the milled MgH2, which hardly decomposes at 300 °C.44
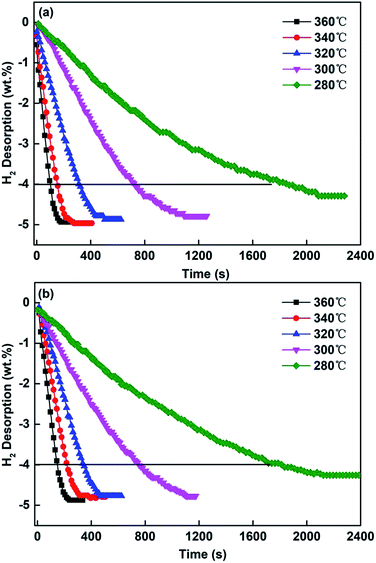 |
| | Fig. 7 Hydrogen desorption kinetic curves of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at different temperatures: (a) C = TiF3, (b) C = NbF5. | |
Hydrogen desorption property
To further study the different catalytic effects between TiF3 and NbF5 on hydrogen desorption property of Mg85Zn5Ni10, the composites were characterized by DSC measurement as shown in Fig. 8. There is a big difference between the DSC curves of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys, as seen in Fig. 8. There are two endothermic peaks in the curve of C = NbF5 alloy at 228.84 °C and 349.93 °C. On the basis of XRD and TEM results, there are two reversible reactions in every hydrogenation and dehydrogenation reactions. The dehydrogenation reactions are MgH2 → Mg + H2 and Mg2NiH4 → Mg2Ni + H2. Therefore, the peak at 228.84 °C belongs to the endothermic peak of Mg2NiH4 and the peak at 349.93 °C corresponds to MgH2.43,45–47 However, for the C = TiF3 alloy, there seems to be only one endothermic peak at 256.88 °C in the DSC curve. This indicates that the catalyst TiF3 strongly decreases the endothermic peak temperature of MgH2, and even causes the two peaks of Mg2NiH4 and MgH2 to coincide. It illustrates that the reaction for MgH2 decomposition becomes more easy by adding TiF3. Conversely, there is no such significant catalytic effect by NbF5. This also proves that TiF3 improves the hydrogen desorption property of Mg85Zn5Ni10 more effectively than NbF5. This result is in line with previous discussions of hydrogenation and dehydrogenation kinetics. Moreover, the onset temperatures of dehydrogenations are 218.63 and 210.08 °C of C = TiF3 and NbF5 alloys, respectively.
 |
| | Fig. 8 DSC curves of the desorption process of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys. | |
Hydrogenation and dehydrogenation cyclic stability
Fig. 9 and 10 depict the hydrogen absorbing and desorbing cyclic stability curves of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at 360 °C. According to Fig. 9 and 10, basically there is no change of hydrogen absorption and desorption capacities for both C = TiF3 alloy and C = NbF5 alloy after 15 cycles. It is remarkable that the hydrogenation rate of first cycle for the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys is quite slow owing to a long process of complete activation of the alloys. However, after the second cycle, the hydrogenation and dehydrogenation rates are significantly accelerated. Subsequently, with the increase of cycle times, the hydrogenation and dehydrogenation rates gradually improve and show good kinetics. This is because defects and cracks are generated during the hydrogenation and dehydrogenation cycles. These defects and cracks can provide more hydrogen diffusion channels and nucleation positions for Mg hydrides which was discussed previously. Therefore, the hydrogen absorption and desorption cyclic curves are almost identical. Also, there is no capacity loss even after 30 hydrogenation/dehydrogenation cycles. On the basis of Fig. 9 and 10, the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys reveal good hydrogenation and dehydrogenation cyclic stability. Furthermore, the capacity without any loss indicates that there is no other stable hydride formation during the hydrogenation/dehydrogenation process. Subsequently, the decomposition of TiF3/NbF5 and formation of TiH2/NbH2 is always accompanied by the loss of capacity owing to the formation of TiH2. TiH2/NbH2 cannot release hydrogen during dehydrogenation of MgH2.48–50 Therefore, this also proves that both TiF3 and NbF5 exist stably in the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys.
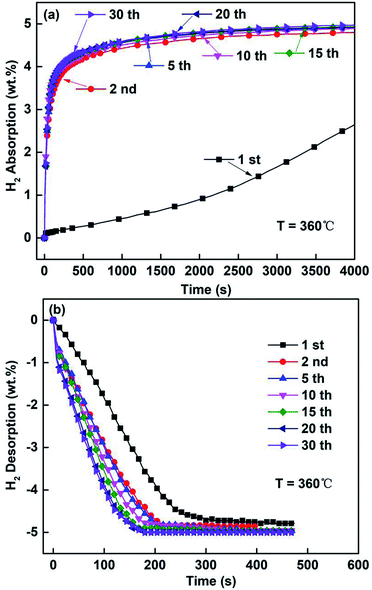 |
| | Fig. 9 Hydrogenation/dehydrogenation cycling curves of Mg85Zn5Ni10–4TiF3 alloy at 360 °C: (a) hydrogenation cycles, (b) dehydrogenation cycles. | |
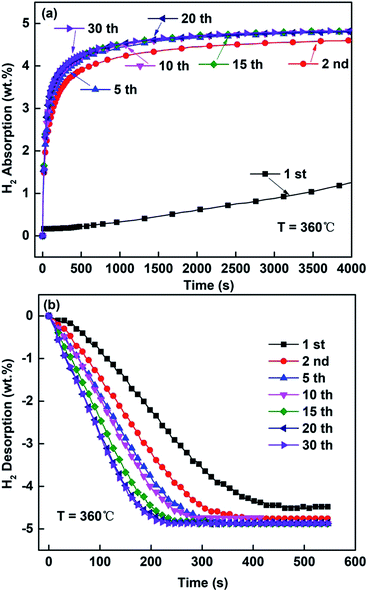 |
| | Fig. 10 Hydrogenation/dehydrogenation cycling curves of Mg85Zn5Ni10–4NbF5 alloy at 360 °C: (a) hydrogenation cycles, (b) dehydrogenation cycles. | |
Hydrogen desorption activation energy
In order to further study the catalytic mechanism of the differences between TiF3 and NbF5, the dehydriding activation energy of the Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys is estimated by Arrhenius method. As a general rule, in a gas–solid reaction, the total energy barrier which is to be crossed determines the activation energy. The energy barrier of H2 released from MgH2 is the leading cause which may explain the dehydrogenation rate.34 During hydrogenation, the activation energy is considered to be relative to the total energy barrier. As we know, the dehydrogenation reaction is accomplished by nucleation and growth processes. In addition, the Johnson–Mehl–Avrami (JMA) model always simulates the nucleation and growth processes during hydrogen desorption, which is represented by the following equation:51| |
ln[−ln(1 − α)] = η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + η k + η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t
| (1) |
where α, k and η represent the reaction fraction at time t, an effective kinetic parameter, and the Avrami exponent reaction order, respectively. Fig. 11 expresses the linear plots of ln[−ln(1 − α)] vs. the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t. Evidently, the JMA plots described in Fig. 11 are nearly linear, illustrating that dehydriding reactions of the samples follow instantaneous nucleation followed by interface controlled-three-dimensional growth process.52 According to the slope and intercept of the linear fitting, η and η
t. Evidently, the JMA plots described in Fig. 11 are nearly linear, illustrating that dehydriding reactions of the samples follow instantaneous nucleation followed by interface controlled-three-dimensional growth process.52 According to the slope and intercept of the linear fitting, η and η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values at each temperature can be calculated. On the basis of η and η
k values at each temperature can be calculated. On the basis of η and η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values, the rate constant k is calculated, then the activation energy (Ea) for dehydrogenation can be computed from the following Arrhenius equation:53,54
k values, the rate constant k is calculated, then the activation energy (Ea) for dehydrogenation can be computed from the following Arrhenius equation:53,54| |
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT) exp(−Ea/RT)
| (2) |
where, A, R and T represent a temperature independent coefficient, universal gas constant and the absolute temperature, respectively. The Arrhenius plots for the dehydrogenation kinetics are described in Fig. 12. Accordingly, on the basis of the slopes of these plots, the activation energy, Ea (de), can be calculated. The Ea (de) values of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys are 75.514 and 82.367 kJ mol−1 H2, respectively, and are listed in Table 1. Clearly, the Ea (de) of C = TiF3 alloy is much lower than that of the C = NbF5 alloy. In this study, the Ea (de) value of ball-milled Mg85Zn5Ni10 is calculated to be 109.83 kJ mol−1 H2, suggesting that both catalysts remarkably decreased the dehydriding activation energy when compared with the Ea (de) value of pure milled MgH2 (158.5 kJ mol−1 H2).55
 |
| | Fig. 11 JMA graphs of the as-milled of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at different temperatures: (a) C = TiF3, (b) C =NbF5. | |
 |
| | Fig. 12 P–C–I curves of the as-milled of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at different temperatures: (a) C = TiF3, (b) C = NbF5. | |
Table 1 Hydrogen desorption activation energy (Ea (de)), enthalpy change (ΔH) and entropy change (ΔS) of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5, none) alloy
| Mg85Zn5Ni10–4C alloys |
Ea (de) (kJ mol−1) |
ΔHab (kJ mol−1) |
ΔSab (J mol−1 K−1) |
ΔHde (kJ mol−1) |
ΔSde (J mol−1 K−1) |
| C = TiF3 |
75.514 |
−70.100 |
−122.473 |
80.054 |
137.781 |
| C = NbF5 |
82.367 |
−75.869 |
−128.945 |
79.005 |
132.683 |
| C = none |
109.830 |
−82.446 |
−140.628 |
86.187 |
146.114 |
Hydrogen storage thermodynamics
The pressure–composition Isotherms (PCI) tests were measured at 360, 340 and 320 °C for the sake of studying the influence of different catalysts on the hydrogen storage thermodynamics of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloy. The PCI curves are represented in Fig. 13. Evidently, in each PCI curves there are two distinct plateaus. The lower pressure plateau belongs to MgH2, and the higher one is the pressure plateau of Mg2NiH4 according to the reported results for Mg–10Ni–xMm alloy.56 All the curves exhibit flat plateaus. On the basis of the plateau pressures in PCI curves, both enthalpy change ΔH and entropy change ΔS can be derived from the Van't Hoff equation:57| | |
ln[P(H2)/P0] = ΔH/(RT) − ΔS/R
| (3) |
where, P(H2), P0, T and R represent the equilibrium hydrogen gas pressure, the standard atmospheric pressure, the sample temperature and the gas constant, respectively. Fig. 14 shows the Van't Hoff plots and the enthalpy value (ΔH) and entropy value (ΔS) of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloy. Therefore, the thermodynamic parameters can be easily calculated by intercepts and slopes of the Van't Hoff plots which are listed in Table 1. The calculations reveal that the hydrogen absorption/desorption ΔH and ΔS of the C = TiF3 alloy are smaller than of the C = NbF5 alloy indicating that TiF3 is more effective than NbF5 as a catalyst to improve the hydrogen storage thermodynamics of Mg85Zn5Ni10. Meanwhile, the hydrogen absorption/desorption ΔH and ΔS of ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys are smaller than those of ball-milled Mg85Zn5Ni10 alloy, suggesting both TiF3 and NbF5 are helpful to ameliorate the thermodynamics of Mg85Zn5Ni10.
 |
| | Fig. 13 P–C–I curves of the as-milled of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at different temperatures: (a) C = TiF3, (b) C = NbF5. | |
 |
| | Fig. 14 Van't Hoff plots of the as-milled of the ball-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys at different temperatures: (a) C = TiF3, (b) C = NbF5. | |
Conclusions
In this investigation, as-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) specimens have been prepared successfully via ball milling. The different influences between the catalysts TiF3 and NbF5 on the microstructure and hydrogen storage properties were studied. The main conclusions drawn are as follows:
(1) After 5 h ball milling, and 30 hydrogenation/dehydrogenation cycles, the catalysts TiF3 and NbF5 always exist in the Mg85Zn5Ni10–4C (C = TiF3, NbF5) composites, indicating that both TiF3 and NbF5 do not decompose and have a good stability.
(2) The C = TiF3 alloy possesses a faster hydrogen absorption/desorption rate than the C = NbF5 alloy. The dehydrogenation activation energy values calculated by Arrhenius equation of the as-milled Mg85Zn5Ni10–4C (C = TiF3, NbF5) alloys are 75.514 and 82.367 kJ mol−1 H2, respectively, while the value of ball-milled Mg85Zn5Ni10 alloy is 109.830 kJ mol−1 H2, indicating that the catalysts TiF3 and NbF5 visibly reduce the dehydrogenation activation energy and effectively improve the hydrogen absorption/desorption kinetics.
(3) Compared with the ball-milled Mg85Zn5Ni10 alloy, the composites catalyzed by TiF3 and NbF5 possess lower thermodynamic parameters (ΔH and ΔS), suggesting that TiF3 and NbF5 can significantly ameliorate the hydrogen storage thermodynamics.
(4) TiF3 has a better effect to generate more defects and cracks during the hydriding/dehydriding process than NbF5, which is responsible for the faster hydrogen absorption/desorption rate. During hydrogenation and dehydrogenation, defects and cracks existing on alloy particles play an effective and important role in increasing the hydrogen absorption and desorption rates. TiF3 has a better catalytic effect on the hydrogen storage property of Mg85Zn5Ni10 than NbF5.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This study is financially supported by the National Natural Science Foundations of China (51761032, 51471054 and 51871125).
References
- W. J. Song, J. S. Li, T. B. Zhang, X. J. Hou and H. C. Kou, RSC Adv., 2015, 5, 54258–54265 RSC.
- N. Juahir, N. S. Mustafa, A. M. Sinin and M. Ismail, RSC Adv., 2015, 5, 60983–60989 RSC.
- R. F. Bill, D. Reed, D. Book and P. A. Anderson, J. Alloys Compd., 2015, 645, S96–S99 CrossRef CAS.
- L. Z. Ouyang, Z. J. Cao, H. Wang, J. W. Liu, D. L. Sun, Q. A. Zhang and M. Zhu, J. Alloys Compd., 2014, 586(6), 113–117 CrossRef CAS.
- Y. J. Yang, Y. F. Liu, Y. Zhang, Y. Li, M. X. Gao and H. G. Pan, J. Alloys Compd., 2014, 585, 674–680 CrossRef CAS.
- L. Z. Ouyang, J. Tang, Y. Zhao, H. Wang, X. Yao, J. Liu, J. Zou and M. Zhu, Sci. Rep., 2015, 5, 10776 CrossRef CAS PubMed.
- Z. M. Yuan, W. Zhang, P. L. Zhang, Y. H. Zhang, W. G. Bu, S. H. Guo and D. L. Zhao, RSC Adv., 2017, 7, 56365–56374 RSC.
- Y. H. Zhang, M. Ji, Z. M. Yuan, W. G. Bu, Y. Qi and S. H. Guo, RSC Adv., 2017, 7, 37689–37698 RSC.
- Y. F. Liu, H. G. Pan, M. X. Gao, R. Li and Y. Q. Lei, J. Alloys Compd., 2004, 376, 296–303 CrossRef CAS.
- Y. H. Zhang, P. P. Wang, W. G. Bu, Z. M. Yuan, Y. Qi and S. H. Guo, RSC Adv., 2018, 8, 23353–23363 RSC.
- Y. F. Zhu, H. G. Pan, M. X. Gao, J. X. Ma, S. Q. Li and Q. D. Wang, Int. J. Hydrogen Energy, 2002, 27, 287–293 CrossRef CAS.
- M. Chourashiya, D. C. Yang, C. N. Park and C. J. Park, Int. J. Hydrogen Energy, 2012, 37, 4238–4245 CrossRef CAS.
- Y. Bai, C. Wu, F. Wu, J. H. Yang, L. L. Zhao, F. Long and B. L. Yi, Int. J. Hydrogen Energy, 2012, 37, 12973–12979 CrossRef CAS.
- H. Wang, A. K. Prasad and S. G. Advani, Int. J. Hydrogen Energy, 2012, 37, 290–298 CrossRef CAS.
- Y. F. Liu, H. F. Du, X. Zhang, Y. X. Yang, M. X. Gao and H. G. Pan, Chem. Commun., 2015, 52, 705–708 RSC.
- H. J. Lin, J. Matsuda, H. W. Li, M. Zhu and E. Akiba, J. Alloys Compd., 2015, 645, S392–S396 CrossRef CAS.
- D. F. Wu, L. Z. Ouyang, C. Wu, H. Wang, J. W. Liu, L. X. Sun and M. Zhu, J. Alloys Compd., 2015, 642, 180–184 CrossRef CAS.
- X. L. Luo, D. M. Grant and G. S. Walker, J. Alloys Compd., 2015, 645, S23–S26 CrossRef CAS.
- H. Wang, H. J. Lin, W. T. Cai, L. Z. Ouyang and M. Zhu, J. Alloys Compd., 2016, 658, 280–300 CrossRef CAS.
- D. Milcius, J. Grbovic-Novakovic, R. Zostautiene, M. Lelis, D. Girdzevicius and M. Urbonavicius, J. Alloys Compd., 2015, 647, 790–796 CrossRef CAS.
- I. E. Malka, T. Czujko and J. Bystrzycki, Int. J. Hydrogen Energy, 2010, 35, 1706–1712 CrossRef CAS.
- F. Y. Cheng, Z. L. Tao, J. Liang and J. Chen, Chem. Commun., 2012, 48, 7334–7343 RSC.
- J. J. Reilly Jr and R. H. Wiswall Jr, J. Inorg. Chem., 1968, 7, 2254–2256 CrossRef.
- E. Akiba, K. Nomura, S. Ono and S. Suda, Int. J. Hydrogen Energy, 1982, 7, 787–791 CrossRef CAS.
- T. Fujimoto, S. Ogawa, T. Kanai, N. Uchiyama, T. Yoshida and S. Yagi, Int. J. Hydrogen Energy, 2015, 40, 11890–11894 CrossRef CAS.
- T. Liu, T. W. Zhang, X. Z. Zhang and X. G. Li, Int. J. Hydrogen Energy, 2011, 36, 3515–3520 CrossRef CAS.
- J. Y. Wang, C. Y. Wu, J. K. Nieh, H. C. Lin, K. M. Lin and H. Y. Bor, Int. J. Hydrogen Energy, 2010, 35, 1250–1256 CrossRef CAS.
- J. J. Reilly Jr and R. H. Wiswall Jr, J. Inorg. Chem., 1967, 6, 2220–2223 CrossRef.
- K. I. Kim and T. W. Hong, Korean J. Met Mater., 2011, 49, 264–269 CrossRef CAS.
- Z. N. Li, X. P. Liu, Z. Huang, L. J. Jiang and S. M. Wang, Rare Met., 2006, 25, 247–251 CrossRef.
- M. Lucaci, A. R. Biris, R. L. Orban, G. B. Sbarcea and V. Tsakiris, J. Alloys Compd., 2009, 488, 163–168 CrossRef CAS.
- T. Yang, Z. M. Yuan, W. G. Bu, Z. C. Jia, Y. Qi and Y. H. Zhang, Int. J. Hydrogen Energy, 2016, 41, 2689–2699 CrossRef CAS.
- Z. M. Yuan, T. Yang, W. G. Bu, H. W. Shang, Y. Qi and Y. H. Zhang, Int. J. Hydrogen Energy, 2016, 41, 5994–6003 CrossRef CAS.
- Y. H. Zhang, G. Huang, Z. M. Yuan, H. W. Shang, Y. Qi and S. H. Guo, Mater. Sci. Eng., B, 2017, 225, 1–9 CrossRef CAS.
- T. Spassov, P. Delchev, P. Madjarov, M. Spassova and T. S. Himitliiska, J. Alloys Compd., 2010, 495, 149–153 CrossRef CAS.
- S. Long, J. X. Zou, X. Chen, X. Q. Zeng and W. J. Ding, J. Alloys Compd., 2014, 615, S684–S688 CrossRef CAS.
- J. Z. Song, S. M. Han and R. D. Fu, Mater. Sci. Eng., B, 2014, 188, 114–118 CrossRef CAS.
- Y. J. Kwak, S. H. Lee, D. R. Mumm and M. Y. Song, Int. J. Hydrogen Energy, 2015, 40, 11908–11916 CrossRef CAS.
- L. P. Ma, P. Wang and H. M. Cheng, J. Alloys Compd., 2007, 432, L1–L4 CrossRef CAS.
- S. K. Peng, X. Z. Xiao, R. J. Xu, L. Li, F. Wu, S. Q. Li, Q. D. Wang and L. X. Chen, Trans. Nonferrous Met. Soc. China, 2010, 20, 1879–1884 CrossRef CAS.
- A. R. Yavari, A. LeMoulec, F. R. de Castro, S. Deledda, O. Friedrichs, W. J. Botta, G. Vaughan, T. Klassen, A. Fernandez and Å. Kvick, Scr. Mater., 2005, 52, 719–724 CrossRef CAS.
- S. H. Lee, Y. J. Kwak, H. R. Park and M. Y. Song, Int. J. Hydrogen Energy, 2014, 39, 16486–16492 CrossRef CAS.
- N. Recham, V. V. Bhat, M. Kandavel, L. Aymard, J. M. Tarascon and A. Rougier, J. Alloys Compd., 2008, 464, 377–382 CrossRef CAS.
- R. K. Singh, T. Sadhasivam, G. I. Sheeja, P. Singh and O. N. Srivastava, Int. J. Hydrogen Energy, 2013, 38, 6221–6225 CrossRef CAS.
- M. Ismail, Y. Zhao, X. B. Yu and S. X. Dou, Int. J. Hydrogen Energy, 2012, 37, 8395–8401 CrossRef CAS.
- S. S. Liu, L. X. Sun, F. Xu, J. Zhang, Z. Cao and Y. L. Liu, Int. J. Hydrogen Energy, 2011, 36, 11785–11793 CrossRef CAS.
- N. S. Mustafa, M. C. Law and M. Ismail, Mater. Today, 2016, 3, S96–S103 CrossRef.
- R. R. Shahi, A. Bhatnagar, S. K. Pandey, V. Dixit and O. N. Srivastava, Int. J. Hydrogen Energy, 2014, 39, 14255–14261 CrossRef CAS.
- A. Grzech, U. Lafont, P. C. M. M. Magusin and F. M. Mulder, J. Phys. Chem. C, 2012, 116, 26027–26035 CrossRef CAS.
- Y. J. Kwak, S. H. lee, D. R. Mumm and M. Y. Song, Int. J. Hydrogen Energy, 2015, 40, 11908–11916 CrossRef CAS.
- M. Pourabdoli, S. Raygan, H. Abdizadeh and D. Uner, Int. J. Hydrogen Energy, 2013, 38, 11910–11919 CrossRef CAS.
- T. Czujko, R. A. Varin, C. Chiu and Z. Wronski, J. Alloys Compd., 2006, 414, 240–247 CrossRef CAS.
- T. Liu, Y. R. Cao, C. G. Qin, W. S. Chou and X. G. Li, J. Power Sources, 2014, 246, 277–282 CrossRef CAS.
- T. Kimura, H. Miyaoka, T. Ichikawa and Y. Kojima, Int. J. Hydrogen Energy, 2013, 38, 13728–13733 CrossRef CAS.
- J. F. Mao, Z. P. Guo, X. B. Yu, H. K. Liu, Z. Wu and J. Ni, Int. J. Hydrogen Energy, 2010, 35, 4569–4575 CrossRef CAS.
- J. G. Yuan, N. Xing and Y. Wu, Int. J. Hydrogen Energy, 2017, 42, 6118–6126 CrossRef CAS.
- H. Falahati and D. P. J. Barz, Int. J. Hydrogen Energy, 2013, 38, 8838–8851 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab
*ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The milling speed was 350 rpm. During ball milling, in order to prevent excess heating, the mill was set to rest for 1 h after every 1 h of working. The samples were milled for a total of 5 h. The as-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) in this research are named as Mg85Zn5Ni10–4C (C = TiF3, NbF5). Phase structures, morphologies and crystalline states of the ball-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) alloys were investigated and observed by X-ray diffraction (XRD) (D/max/2400), SEM and high resolution transmission electron microscopy (HRTEM) (JEM-2100F).
40. The milling speed was 350 rpm. During ball milling, in order to prevent excess heating, the mill was set to rest for 1 h after every 1 h of working. The samples were milled for a total of 5 h. The as-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) in this research are named as Mg85Zn5Ni10–4C (C = TiF3, NbF5). Phase structures, morphologies and crystalline states of the ball-milled Mg85Zn5Ni10 + 4 wt% C (C = TiF3 and NbF5) alloys were investigated and observed by X-ray diffraction (XRD) (D/max/2400), SEM and high resolution transmission electron microscopy (HRTEM) (JEM-2100F).







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + η
k + η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t
t
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t. Evidently, the JMA plots described in Fig. 11 are nearly linear, illustrating that dehydriding reactions of the samples follow instantaneous nucleation followed by interface controlled-three-dimensional growth process.52 According to the slope and intercept of the linear fitting, η and η
t. Evidently, the JMA plots described in Fig. 11 are nearly linear, illustrating that dehydriding reactions of the samples follow instantaneous nucleation followed by interface controlled-three-dimensional growth process.52 According to the slope and intercept of the linear fitting, η and η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values at each temperature can be calculated. On the basis of η and η
k values at each temperature can be calculated. On the basis of η and η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values, the rate constant k is calculated, then the activation energy (Ea) for dehydrogenation can be computed from the following Arrhenius equation:53,54
k values, the rate constant k is calculated, then the activation energy (Ea) for dehydrogenation can be computed from the following Arrhenius equation:53,54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT)
exp(−Ea/RT)







