Fluorescence sensing of mercury(ii) and melamine in aqueous solutions through microwave-assisted synthesis of egg-white-protected gold nanoclusters†
Abstract
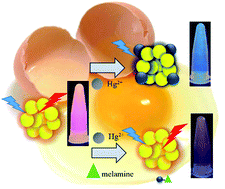
In this study, a microwave-assisted synthesis of fluorescent egg-white-protected gold nanoclusters (ew–AuNCs) for sensing Hg2+ ions and melamine was developed, optimized, and evaluated. By conducting infrared spectrometry and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, we confirmed that clusters consisting of 22 Au atoms were successfully embedded in egg white. Transmission electron microscopy measurements revealed that the ew–AuNCs were well dispersed with an average diameter of 2.7 ± 0.7 nm. The ew–AuNCs exhibited red fluorescence emission (λem 648 nm) with high quantum yield (2.37%). In the presence of Hg2+ ions, the fluorescence of these ew–AuNCs was quenched because of the Hg2+–Au+ interaction. The relative change in fluorescence intensity at 648 nm was dependent on the concentration of Hg2+ ions. Detection of melamine was based on the diminished fluorescence quenching of ew–AuNCs by Hg2+ when melamine was added along with Hg2+. The limit of detection for Hg2+ ions and melamine was 0.89 nM and 0.46 μM, respectively. Determination of Hg2+ ions in a pond water sample and of melamine in milk and dog food samples was performed, and the recoveries from the developed method were >92.9%, confirming its potential for sensor applications.


 Please wait while we load your content...
Please wait while we load your content...