Synthesis of a BODIPY–2-(2′-hydroxyphenyl)benzothiazole conjugate with solid state emission and its application as a fluorescent pH probe†
Abstract
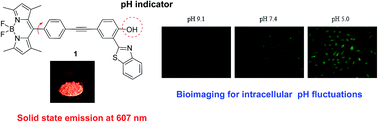
A solid-state red-emitting dye (1) containing 2-(2′-hydroxyphenyl)benzothiazole (HBT) group at the 8-position of BODIPY was designed and synthesized. The emission peak of 1 in powder was located at 607 nm, and it exhibited a 59 nm red-shift than that in THF (548 nm). The increasing solvent viscosity and solution concentration induced fluorescent enhancement. Because of the phenolic hydroxyl group as pH-sensitive moiety, 1 could be used as a fluorescent and colorimetric probe for the determination of pH. When the pH was increased from 4.5 to 9.8, 1 displayed a remarkable emission quenching at 528 nm as well a red-shift from 455 nm to 484 nm, simultaneously exhibiting a significant fluorescence color change from bright green to blackish green. The dye 1 responded linearly to minor pH fluctuations within the range of 6.8–8.5. The pKa value was 7.32. In addition, 1 could be utilized in pH-dependent fluorescence imaging in HeLa cells. The CCK-8 assay showed that the cytotoxicity of 1 was low.


 Please wait while we load your content...
Please wait while we load your content...