Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Auxeticity enhancement due to size polydispersity in fcc crystals of hard-core repulsive Yukawa particles†
Paweł M.
Pigłowski
,
Jakub W.
Narojczyk
 ,
Krzysztof W.
Wojciechowski
and
Konstantin V.
Tretiakov
,
Krzysztof W.
Wojciechowski
and
Konstantin V.
Tretiakov
 *
*
Institute of Molecular Physics, Polish Academy of Sciences, Mariana Smoluchowskiego 17, 60-179 Poznań, Poland. E-mail: tretiakov@ifmpan.poznan.pl; Fax: +48 61 86 84 524; Tel: +48 61 86 95 100
First published on 16th October 2017
Abstract
The Poisson's ratio of the fcc hard-core repulsive Yukawa crystals with size polydispersity was determined by Monte Carlo simulations in the isothermal–isobaric ensemble. The effect of size polydispersity on the auxetic properties of Yukawa crystals has been studied. It has been found that an increase of particle size polydispersity causes a decrease of the Poisson's ratio in auxetic directions as well as appearance of a negative Poisson's ratio in formerly non-auxetic directions. A measure of auxeticity was introduced to estimate quantitatively an enhancement of auxetic properties in polydisperse Yukawa crystals. The proposed measure of auxeticity can be applied to appraise the auxeticity of any studied system.
1 Introduction
Interest in colloidal crystals1 comes from their potential applications in optoelectronics,2 photonics,3 and medicine.4 In order to obtain materials with desired physical properties, studies are made on colloids composed of both spherically symmetric and more complex molecules like dumbbells5,6 and further on mixtures of spherical and cylindrical molecules.7 Electric or magnetic fields are also used to control the self-assembly of colloidal crystals,8,9 as well as to obtain desired structures10,11 through the changes of molecular orientation. The electric field can also impact the optical properties of colloids composed from spherically symmetric particles.12 In the case of spherical particles, a large difference in the elastic properties of face-centered-cubic (fcc) and hexagonal-close-packed hard-sphere crystals was also found.13 The latter model molecule (hard sphere) is important for colloids and is the limiting case of Yukawa particles. Spherical particles with hard-core repulsive Yukawa interaction form body-centered-cubic or face-centred-cubic crystals depending on the Debye screening length and the volume fraction, which have been observed in experiments14 and computer simulations.15,16 Recently it has been shown that such fcc Yukawa crystals exhibit interesting elastic properties.17 Namely, in these crystals a negative Poisson's ratio in one of the main crystallographic directions is observed.Systems exhibiting unusual elastic properties such as negative compressibility,18 negative Poisson's ratio19 (known as auxetics20), or both these properties21 have attracted increasing attention22–48 over the past few decades due to their potential applications. Poisson's ratio is defined as the negative ratio of the relative change in the transverse dimension to the relative change in the longitudinal dimension when infinitesimal change of the longitudinal strain is applied. Its value for isotropic materials ranges from −1 (so called ideal auxetic) through 0 (e.g. cork) up to 1/2 (e.g. rubber). In the case of materials that exhibit anisotropy of elastic properties, the value of Poisson's ratio depends on both the direction in which the deformation is applied and the direction in which the response of the system is studied. It has been shown that most metals (with a cubic crystallographic structure) exhibit negative Poisson's ratio in some directions49 – they are called partial auxetics.50 A number of man-made materials also exhibit negative Poisson's ratio, e.g. polyurethane foams,19 composites,51 and other structures based on the rotating rigid units model.52
Studies of two-dimensional Yukawa crystals revealed that this system is not auxetic.53 However, as mentioned before, the three-dimensional Yukawa crystals exhibit negative Poisson's ratio in the [110][1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]-direction and are partial auxetics, supposedly with a similar mechanism of partial auxeticity as discussed for fcc crystals by Milstein and Huang54 and as found in the hard sphere model near melting55 and near close packing56 and as reported by Baughman et al. for some fcc metals.49 Moreover, the value of Poisson's ratio can be modified by changing the screening length – an increase of the screening length causes Poisson's ratio to decrease.17 Furthermore, very recently, other possibilities to control Poisson's ratio in Yukawa systems have been proposed. It has been shown that structural modifications of Yukawa crystals (by introducing nano-slits or nano-channels) decrease the value of the Poisson's ratio in known auxetic directions57 or even induce completely new auxetic directions in the studied crystal.58
0]-direction and are partial auxetics, supposedly with a similar mechanism of partial auxeticity as discussed for fcc crystals by Milstein and Huang54 and as found in the hard sphere model near melting55 and near close packing56 and as reported by Baughman et al. for some fcc metals.49 Moreover, the value of Poisson's ratio can be modified by changing the screening length – an increase of the screening length causes Poisson's ratio to decrease.17 Furthermore, very recently, other possibilities to control Poisson's ratio in Yukawa systems have been proposed. It has been shown that structural modifications of Yukawa crystals (by introducing nano-slits or nano-channels) decrease the value of the Poisson's ratio in known auxetic directions57 or even induce completely new auxetic directions in the studied crystal.58
On the other hand, it is known that the particle size polydispersity, manifested by different diameters of particles in the system, is one of the characteristic features of colloidal systems. The particle size polydispersity is associated with the synthesis of colloidal particles and has a key impact on the thermodynamic properties of hard-particle systems.59,60 The influence of polydispersity on the elastic properties of systems with both hard61 and soft (inverse power)62 interaction potentials was investigated. It has been shown that polydispersity significantly influences the phase diagram of Yukawa systems by shifting the phase transition towards higher packing fractions with increasing size polydispersity.63,64
The main aim of this work is to study the influence of particle size polydispersity on the auxetic properties of Yukawa crystals, which combine the hard and soft interactions.
2 Model and method
We modeled the system of N particles forming a face centred cubic lattice under periodic boundary conditions, where particles exhibit dispersion of size, i.e. their diameters are not identical. It is known from experiments that in real colloidal systems the distribution of particle diameters can be described approximately by Gaussian or log-normal distribution.65 In this study, we used the Gaussian distribution of diameters of particles, as in previous works.60,61 The parameter that describes the polydispersity of particles in the studied system is defined as60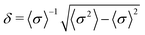 . The particles in the system interact through the hard-core repulsive Yukawa potential15,16 in which the polydispersity has been included:63,64
. The particles in the system interact through the hard-core repulsive Yukawa potential15,16 in which the polydispersity has been included:63,64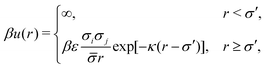 | (1) |
![[small sigma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d2.gif) is the typical particle diameter in the system. In this work the polydispersity varied between 0 and 0.08. For these values, according to the phase diagram obtained by Dijkstra et al.,63,64 the fcc structure is observed in a wide range of dimensionless parameters of Yukawa potential (βε, κσ).
is the typical particle diameter in the system. In this work the polydispersity varied between 0 and 0.08. For these values, according to the phase diagram obtained by Dijkstra et al.,63,64 the fcc structure is observed in a wide range of dimensionless parameters of Yukawa potential (βε, κσ).
The elastic properties of the systems have been determined using Monte Carlo simulations in the isothermal–isobaric ensemble by averaging strain tensor fluctuations based on the idea of Parrinello and Rahman66–68 (more details in the work by Wojciechowski et al.69 and ESI†). In this approach, the components of the tensor of elastic compliances are calculated as follows: Sijkl = βVp〈ΔεijΔεkl〉, where Vp is the average volume of the system under pressure p and Δεij = εij − 〈εij〉. In the following, we will use matrix notation in Voigt's form instead of tensor notation.70 In the case of cubic symmetry we have only three independent elements of elastic compliance tensor S. A knowledge of the elastic compliances (S11, S12, S44) allows one to calculate the Poisson's ratio in any crystallographic direction:
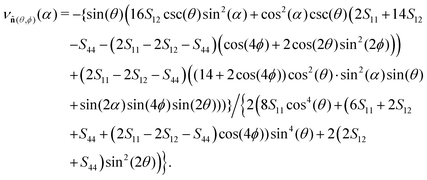 | (2) |
![[n with combining circumflex]](https://www.rsc.org/images/entities/b_char_006e_0302.gif) (θ, ϕ) is the versor (in polar coordinates) representing the direction of applied stress, whereas α is an angle in the plane perpendicular to
(θ, ϕ) is the versor (in polar coordinates) representing the direction of applied stress, whereas α is an angle in the plane perpendicular to ![[n with combining circumflex]](https://www.rsc.org/images/entities/b_char_006e_0302.gif) , which indicates the direction of measurement of Poisson's ratio (for details see ESI†). All other details of simulations are summarised in the ESI.†
, which indicates the direction of measurement of Poisson's ratio (for details see ESI†). All other details of simulations are summarised in the ESI.†
3 Results and discussion
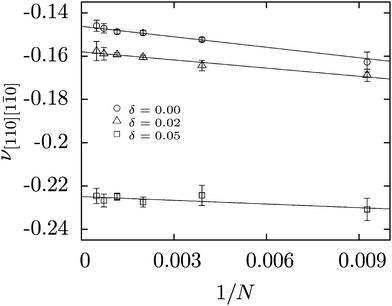
In order to estimate the size effect of Poisson's ratio, the latter has been determined in the thermodynamic limit (N → ∞) for a monodisperse system and two polydisperse systems with δ = 0.02 and δ = 0.05. The obtained results are shown in Fig. 1. Based on the data presented, one can conclude that the Poisson's ratio for a system consisting of N = 256 differs from its value in the thermodynamic limit by about 3%. Similar conclusions regarding the methodology used can also be found in the literature.57,69,71 Therefore, majority of studies in this paper have been performed for systems consisting of 256 particles.Previous studies of the elastic properties of monodisperse hard-core repulsive Yukawa crystals in a wide range of pressures and densities have shown that Poisson's ratio depends weakly on density at a constant value of the contact potential but is sensitive to changes in the screening length.17 So, because this research is about the impact of particle polydispersity on the auxetic properties of the system, here we studied the Poisson's ratio of Yukawa crystals at p* = 60. At this pressure the Yukawa system has a face centred cubic structure over a wide range of screening length (6.7 ≤ κσ ≤ 16.7) and contact potential (20 ≤ βε ≤ 81).63,64
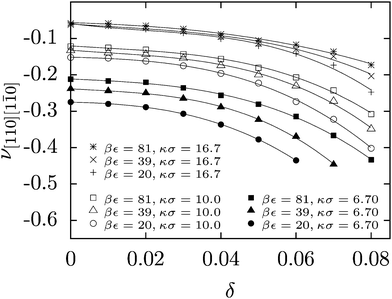
The effect of polydispersity on Poisson's ratio in the auxetic crystallographic direction of [110][1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] is shown in Fig. 2 (for other directions and the effect of temperature on auxeticity see ESI†). We observe here a decrease in the Poisson's ratio with increasing polydispersity for all investigated crystals over a wide range of Yukawa parameters (contact potential and screening length). As mentioned above, in anisotropic systems, the Poisson's ratio depends not only on the direction of applied stress (
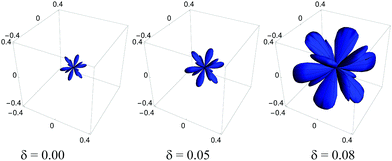
0] is shown in Fig. 2 (for other directions and the effect of temperature on auxeticity see ESI†). We observe here a decrease in the Poisson's ratio with increasing polydispersity for all investigated crystals over a wide range of Yukawa parameters (contact potential and screening length). As mentioned above, in anisotropic systems, the Poisson's ratio depends not only on the direction of applied stress (![[n with combining circumflex]](https://www.rsc.org/images/entities/b_char_006e_0302.gif) (θ, ϕ)) but also on the transverse direction of measurement (see ESI†); thus a question arises whether this is the only auxetic direction in the Yukawa systems. In response, using formula (2), the minimal Poisson's ratio has been calculated in all crystallographic directions. In Fig. 3, the Poisson's ratio for monodisperse (δ = 0.00) and polydisperse systems at δ = 0.05 and δ = 0.08 has been plotted in spherical coordinates. We observe not only a decrease of Poisson's ratio with increasing polydispersity but also an increase in the range of auxetic directions, which is manifested by increasing volume of the obtained shape in Fig. 3. The increase of the range of auxetic directions can be clearly seen by analysis of the Poisson's ratio at some given crystallographic direction as a function of the angle α. In Fig. 4, the dependence of Poisson's ratio on the angle α, which describes the direction of measurement of the response to the applied stress, has been shown. We observe here new auxetic directions (within the range α1 ≤ α ≤ α2) which are induced by increasing particle size polydispersity. So, Fig. 4 shows that an increase of the polydispersity results in an enhancement of auxeticity of the system not only by decreasing the value of Poisson's ratio but also by increasing the range of crystallographic directions for which the Poisson's ratio takes negative values. This effect of enhanced auxeticity due to polydispersity can be explained as follows. As shown by Tretiakov and Wojciechowski,17 the Poisson's ratio in the [110][1
(θ, ϕ)) but also on the transverse direction of measurement (see ESI†); thus a question arises whether this is the only auxetic direction in the Yukawa systems. In response, using formula (2), the minimal Poisson's ratio has been calculated in all crystallographic directions. In Fig. 3, the Poisson's ratio for monodisperse (δ = 0.00) and polydisperse systems at δ = 0.05 and δ = 0.08 has been plotted in spherical coordinates. We observe not only a decrease of Poisson's ratio with increasing polydispersity but also an increase in the range of auxetic directions, which is manifested by increasing volume of the obtained shape in Fig. 3. The increase of the range of auxetic directions can be clearly seen by analysis of the Poisson's ratio at some given crystallographic direction as a function of the angle α. In Fig. 4, the dependence of Poisson's ratio on the angle α, which describes the direction of measurement of the response to the applied stress, has been shown. We observe here new auxetic directions (within the range α1 ≤ α ≤ α2) which are induced by increasing particle size polydispersity. So, Fig. 4 shows that an increase of the polydispersity results in an enhancement of auxeticity of the system not only by decreasing the value of Poisson's ratio but also by increasing the range of crystallographic directions for which the Poisson's ratio takes negative values. This effect of enhanced auxeticity due to polydispersity can be explained as follows. As shown by Tretiakov and Wojciechowski,17 the Poisson's ratio in the [110][1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]-direction decreases with increasing Debye screening length and/or with decreasing density of the system. In the studied system an introduction or an increase of polydispersity in the system is accompanied by a slight decrease of density (with the other thermodynamic parameters kept constant, i.e. pressure and temperature) which is originated from the increase in the effective size of particle's hard-core. This in turn leads to a small increase of the “effective” Debye screening length. Both these mechanisms lead to an enhancement of auxeticity in the [110][1
0]-direction decreases with increasing Debye screening length and/or with decreasing density of the system. In the studied system an introduction or an increase of polydispersity in the system is accompanied by a slight decrease of density (with the other thermodynamic parameters kept constant, i.e. pressure and temperature) which is originated from the increase in the effective size of particle's hard-core. This in turn leads to a small increase of the “effective” Debye screening length. Both these mechanisms lead to an enhancement of auxeticity in the [110][1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]-direction. Taking into account that we are dealing with a continuous process, the directions that are very close to the [110][1
0]-direction. Taking into account that we are dealing with a continuous process, the directions that are very close to the [110][1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]-direction also become auxetic. As a result, we have an enhancement of the auxeticity (in the [110][1
0]-direction also become auxetic. As a result, we have an enhancement of the auxeticity (in the [110][1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]-direction) and an extension of the range of crystallographic directions with increasing polydispersity.
0]-direction) and an extension of the range of crystallographic directions with increasing polydispersity.
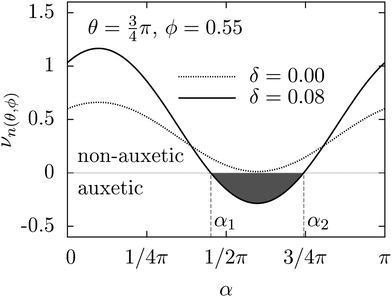 | ||
| Fig. 4 Poisson's ratio as a function of the transverse direction described by α for the following parameters: βε = 20, κσ = 10 (α1 = 1.4193, α2 = 2.3377). | ||
In order to perform a quantitative analysis of the auxeticity enhancement effect, we define the degree of auxeticity of the studied system with respect to the ideal auxetic as (for details see ESI†)
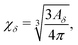 | (3) |
| η = χδ − χ0. | (4) |
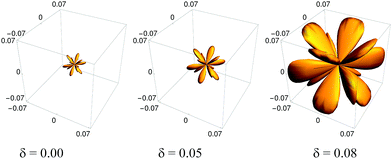 | ||
| Fig. 5 Absolute value of the average negative Poisson's ratio for all crystallographic directions of monodisperse Yukawa crystals (δ = 0) and polydisperse Yukawa crystals with δ = 0.05 and δ = 0.08. | ||
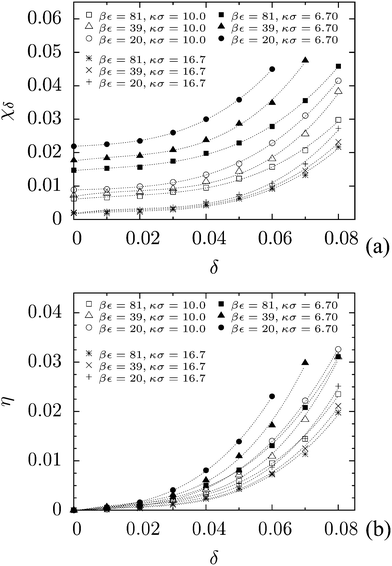 | ||
| Fig. 6 (a) Auxeticity degree as a function of polydispersity. (b) Auxeticity enhancement as a function of polydispersity. In both figures, lines are drawn to guide the eyes. | ||
4 Conclusions
Extensive computer simulations have shown that the increase of size polydispersity of particles in Yukawa crystals leads to enhancement of the auxetic properties of the system, both by reducing Poisson's ratio and by extending the range of auxetic directions. In other words, the fcc hard-core repulsive Yukawa crystals with larger polydispersity of particles exhibit lower negative Poisson's ratio values and have more crystallographic directions in which the value of Poisson's ratio is negative. The introduced measure of auxeticity allows a quantitative evaluation of the enhancement of auxetic properties caused by polydispersity of particles.The auxeticity degree (χ) and the auxeticity enhancement parameter (η) proposed in this paper can be used to assess changes of auxetic properties in various systems.
This work is a part of a project aimed at finding materials with unusual elastic properties and establishing mechanisms leading to them. It is worth noticing that an interesting direction in the search of such properties is the study of systems with various defects, which on the one hand can affect the functionality of the system, and on the other hand can strongly influence the stability of the studied system. It is known, for example, that the value of Poisson's ratio is influenced by point defects in hard-sphere crystals71 or dislocations in colloidal crystals of soft thermosensitive spheres.72 Therefore, it is interesting to investigate the effect of various defects on the elastic properties of polydisperse Yukawa crystals. Another important direction of future research, from the point of view of practical applications, is to investigate the elastic properties of polydisperse Yukawa systems both with and without defects in case of large deformations. Such studies should be conducted with special attention devoted to the stability of the system because large loads may lead to mechanical instability of the systems under consideration.
Finally, we believe that the effect of polydispersity on the auxetic properties of the crystal is not only interesting and important in itself from the cognitive point of view but it may be also useful in future for designing new metamaterials73 and for synthesizing materials with required elastic properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The calculations were partially performed at the Poznań Supercomputing and Networking Center (PCSS).References
- A. van Blaaderen, R. Ruel and P. Wiltzius, Nature, 1997, 385, 321–324 CrossRef CAS.
- D. V. Talapin, J. S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389–458 CrossRef CAS PubMed.
- J. Galisteo-López, M. Ibisate, R. Sapienza, L. Froufe-Peérez, A. Blanco and C. López, Adv. Mater., 2011, 23, 30–69 CrossRef PubMed.
- V. L. Alexeev, D. N. Finegold and S. A. Asher, Clin. Chem., 2004, 50, 2353–2360 CAS.
- A. F. Demirors, P. M. Johnson, C. M. van Kats, A. van Blaaderen and A. Imhof, Langmuir, 2010, 26, 14466–14471 CrossRef CAS PubMed.
- B. Peng, F. Smallenburg, A. Imhof, M. Dijkstra and A. van Blaaderen, Angew. Chem., Int. Ed., 2013, 52, 6709–6712 CrossRef CAS PubMed.
- H. E. Bakker, S. Dussi, B. L. Droste, T. H. Besseling, C. L. Kennedy, E. I. Wiegant, B. Liu, A. Imhof, M. Dijkstra and A. van Blaaderen, Soft Matter, 2016, 12, 9238–9245 RSC.
- F. Smallenburg, H. R. Vutukuri, A. Imhof, A. van Blaaderen and M. Dijkstra, J. Phys.: Condens. Matter, 2012, 24, 464113 CrossRef PubMed.
- A. F. Demirors, P. P. Pillai, B. Kowalczyk and B. A. Grzybowski, Nature, 2013, 503, 99–103 CrossRef PubMed.
- S. H. Lee and C. M. Liddell, Small, 2009, 5, 1957–1962 CrossRef CAS PubMed.
- S. Sacanna, L. Rossi and D. J. Pine, J. Am. Chem. Soc., 2012, 134, 6112–6115 CrossRef CAS PubMed.
- K. Watanabe, H. Ishii, M. Konno, A. Imhof, A. van Blaaderen and D. Nagao, Langmuir, 2017, 33, 296–302 CrossRef CAS PubMed.
- S. Pronk and D. Frenkel, Phys. Rev. Lett., 2003, 90, 255501 CrossRef PubMed.
- C. P. Royall, M. E. Leunissen, A. P. Hynninen, M. Dijkstra and A. van Blaaderen, J. Chem. Phys., 2006, 124, 244706 CrossRef PubMed.
- F. El Azhar, M. Baus and J. P. Ryckaert, J. Chem. Phys., 2000, 112, 5121–5126 CrossRef CAS.
- A. P. Hynninen and M. Dijkstra, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 68, 021407 CrossRef PubMed.
- K. V. Tretiakov and K. W. Wojciechowski, Phys. Status Solidi B, 2014, 251, 383–387 CrossRef CAS.
- R. Baughman, S. Stafstrom, C. Cui and S. Dantas, Science, 1998, 279, 1522 CrossRef CAS PubMed.
- R. Lakes, Science, 1987, 235, 1038–1040 CAS.
- K. E. Evans, Endeavour, 1991, 15, 170–174 CrossRef CAS.
- R. Lakes and K. W. Wojciechowski, Phys. Status Solidi B, 2008, 245, 545 CrossRef CAS.
- K. W. Wojciechowski, Phys. Lett. A, 1989, 137, 60–64 CrossRef.
- R. Lakes, Adv. Mater., 1993, 5, 293–296 CrossRef CAS.
- K. E. Evans and A. Alderson, Adv. Mater., 2000, 12, 617–628 CrossRef CAS.
- W. G. Hoover and C. G. Hoover, Phys. Status Solidi B, 2005, 242, 585–594 CrossRef CAS.
- J. Grima, D. Attard, R. Caruana-Gauci and R. Gatt, Scr. Mater., 2011, 65, 565 CrossRef CAS.
- Y. Prawoto, Comput. Mater. Sci., 2012, 58, 140–153 CrossRef CAS.
- A. B. Cairns, J. Catafesta, C. Levelut, J. Rouquette, A. van der Lee, L. Peters, A. L. Thompson, V. Dmitriev, J. Haines and A. L. Goodwin, Nat. Mater., 2013, 12, 212 CrossRef CAS PubMed.
- R. Gatt, R. Caruana-Gauci, D. Attard, A. R. Casha, W. Wolak, K. Dudek, L. Mizzi and J. N. Grima, Phys. Status Solidi B, 2014, 251, 321–327 CrossRef CAS.
- K. M. Azzopardi, J. P. Brincat, J. N. Grima and R. Gatt, Phys. Status Solidi B, 2015, 252, 1486 CrossRef CAS.
- A. B. Cairns and A. L. Goodwin, Phys. Chem. Chem. Phys., 2015, 17, 20449 RSC.
- P. Verma, M. L. Shofner and A. C. Griffin, Phys. Status Solidi B, 2014, 251, 289–296 CrossRef CAS.
- T. C. Lim, Auxetic Materials and Structures, Springer, 2015 Search PubMed.
- P. Verma, M. L. Shofner, A. Lin, K. B. Wagner and A. C. Griffin, Phys. Status Solidi B, 2015, 252, 1455–1464 CrossRef CAS.
- A. Slann, W. White, F. Scarpa, K. Boba and I. Farrow, Phys. Status Solidi B, 2015, 252, 1533–1539 CrossRef CAS.
- S. Czarnecki and P. Wawruch, Phys. Status Solidi B, 2015, 252, 1620–1630 CrossRef CAS.
- S. Czarnecki, Comput. Methods Sci. Technol., 2015, 21, 49–64 CrossRef.
- J. A. Baimova, L. K. Rysaeva, B. Liu, S. V. Dmitriev and K. Zhou, Phys. Status Solidi B, 2015, 252, 1502–1507 CrossRef CAS.
- D. T. Ho, S. D. Park, S. Y. Kwon, T. S. Han and S. Y. Kim, Phys. Status Solidi B, 2016, 253, 1288–1294 CrossRef CAS.
- C. Huang and L. Chen, Adv. Matter., 2016, 28, 8079–8096 CrossRef CAS PubMed.
- K. K. Saxena, R. Das and E. P. Calius, Adv. Eng. Mater., 2016, 18, 1847–1870 CrossRef CAS.
- V. H. Ho, D. T. Ho, S. Y. Kwon and S. Y. Kim, Phys. Status Solidi B, 2016, 253, 1303–1309 CrossRef CAS.
- T. A. M. Hewage, K. L. Alderson, A. Alderson and F. Scarpa, Adv. Mater., 2016, 28, 10323–10332 CrossRef CAS PubMed.
- E. Pasternak, I. Shufrin and A. V. Dyskin, Compos. Struct., 2016, 138, 313–321 CrossRef.
- D. S. Lisovenko, J. A. Baimova, L. K. Rysaeva, V. A. Gorodtsov, A. I. Rudskoy and S. V. Dmitriev, Phys. Status Solidi B, 2016, 253, 1295–1302 CrossRef CAS.
- I. Shufrin, E. Pasternak and A. V. Dyskin, Phys. Status Solidi B, 2016, 253, 1342–1358 CrossRef CAS.
- K. K. Dudek, R. Gatt, L. Mizzi, M. R. Dudek, D. Attard, K. E. Evans and J. N. Grima, Sci. Rep., 2017, 7, 46529 CrossRef PubMed.
- Yun-Che Wang, Meng-Wei Shen and Si-Min Liao, Phys. Status Solidi B, 2017, 254 DOI:10.1002/pssb.201700024.
- R. H. Baughman, J. M. Shacklette, A. A. Zakhidov and S. Stafstrom, Nature, 1998, 392, 362–365 CrossRef CAS.
- A. C. Brańka, D. M. Heyes and K. W. Wojciechowski, Phys. Status Solidi B, 2011, 248, 96–104 CrossRef.
- K. L. Alderson, V. R. Simkins, V. L. Coenen, P. J. Davies, A. Alderson and K. E. Evans, Phys. Status Solidi B, 2005, 252, 509–518 CrossRef.
- J. N. Grima and K. E. Evans, J. Mater. Sci. Lett., 2000, 19, 1563–1565 CrossRef CAS.
- J. W. Narojczyk, P. M. Piglowski, K. W. Wojciechowski and K. V. Tretiakov, Phys. Status Solidi B, 2015, 252, 1508–1513 CrossRef CAS.
- F. Milstein and K. Huang, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 19, 2030 CrossRef CAS.
- K. J. Runge and G. V. Chester, Phys. Rev. A: At., Mol., Opt. Phys., 1987, 36, 4852 CrossRef.
- K. W. Wojciecowski, Comput. Methods Sci. Technol., 2005, 11, 73–79 CrossRef.
- K. V. Tretiakov, P. M. Piglowski, K. Hyzorek and K. W. Wojciechowski, Smart Mater. Struct., 2016, 25, 054007 CrossRef.
- P. M. Piglowski, K. W. Wojciechowski and K. V. Tretiakov, Phys. Status Solidi RRL, 2016, 10, 566–569 CrossRef CAS.
- S.-E. Phan, W. B. Russel, Z. Cheng, J. Zhu, P. M. Chaikin, J. H. Dunsmuir and R. H. Ottewill, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1996, 54, 6633 CrossRef CAS.
- S.-E. Phan, W. B. Russel, J. Zhu and P. M. Chaikin, J. Chem. Phys., 1998, 108, 9789–9795 CrossRef CAS.
- K. V. Tretiakov and K. W. Wojciechowski, J. Chem. Phys., 2012, 136, 204506 CrossRef PubMed.
- K. V. Tretiakov and K. W. Wojciechowski, Phys. Status Solidi B, 2013, 250, 2020–2029 CAS.
- J. Colombo and M. Dijkstra, J. Chem. Phys., 2011, 134, 154504 CrossRef PubMed.
- M. N. van der Linden, A. van Blaaderen and M. Dijkstra, J. Chem. Phys., 2013, 138, 114903 CrossRef PubMed.
- W. B. Russel, D. A. Saville and W. R. Schowalter, Colloidal Dispersions, Cambridge University Press, Cambridge, 1992 Search PubMed.
- M. Parrinello and A. Rahman, J. Chem. Phys., 1982, 76, 2662–2666 CrossRef CAS.
- J. R. Ray and A. Rahman, J. Chem. Phys., 1984, 80, 4423–4428 CrossRef CAS.
- J. R. Ray and A. Rahman, J. Chem. Phys., 1985, 82, 4243–4247 CrossRef CAS.
- K. W. Wojciechowski, K. V. Tretiakov and M. Kowalik, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 67, 036121 CrossRef CAS PubMed.
- J. F. Nye, Physical Properties of Crystalls, Their Representation by Tensors and Matrices, Clarendon Press, Oxford, 1957 Search PubMed.
- K. V. Tretiakov and K. W. Wojciechowski, J. Chem. Phys., 2005, 123, 074509 CrossRef PubMed.
- J. Hilhorst and A. V. Petukhov, Phys. Rev. Lett., 2011, 107, 095501 CrossRef CAS PubMed.
- H. M. A. Kolken and A. A. Zadpoor, RSC Adv., 2017, 8, 5111 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sm01231d |
| This journal is © The Royal Society of Chemistry 2017 |