Open Access Article
Open Access ArticleSupramolecular assemblies of a nitrogen-embedded buckybowl dimer with C60†
Hiroki
Yokoi
a,
Satoru
Hiroto
 *a,
Daisuke
Sakamaki
*a,
Daisuke
Sakamaki
 b,
Shu
Seki
b,
Shu
Seki
 b and
Hiroshi
Shinokubo
b and
Hiroshi
Shinokubo
 *a
*a
aDepartment of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8603, Japan. E-mail: hiroto@chembio.nagoya-u.ac.jp; hshino@chembio.nagoya-u.ac.jp
bDepartment of Molecular Engineering, Graduate School of Engineering, Kyoto University, Kyoto daigaku Katsura, Nishikyo-ku, Kyoto 615-8510, Japan
First published on 21st November 2017
Abstract
A directly connected azabuckybowl dimer was synthesized via a palladium-catalysed C–H/C–Br coupling. The electron-donating nature of the pyrrolic nitrogen atoms of the azabuckybowl enabled a strong complexation with pristine C60. In the presence of two equivalents of C60, the azabuckybowl dimer formed crystals with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry. Conversely, in diluted solution, complexes with a 1
2 stoichiometry. Conversely, in diluted solution, complexes with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the dimer and C60 were detected predominantly, and these precipitated upon increasing the concentration of C60. Scanning electron microscopy images of the precipitate showed fibre-like aggregates, indicating the formation of supramolecular assemblies with 1D chain structures. A variable-temperature 1H NMR analysis revealed that the precipitate consists of the dimer and C60 in a 1
1 stoichiometry of the dimer and C60 were detected predominantly, and these precipitated upon increasing the concentration of C60. Scanning electron microscopy images of the precipitate showed fibre-like aggregates, indicating the formation of supramolecular assemblies with 1D chain structures. A variable-temperature 1H NMR analysis revealed that the precipitate consists of the dimer and C60 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
Introduction
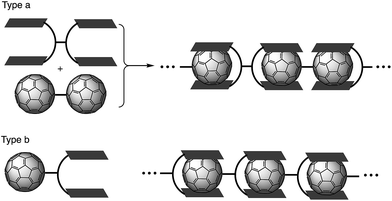
Supramolecular assembly is defined as higher-order aggregates constructed from two or more components, in which the molecules interact by non-covalent interactions such as hydrogen bonding, metal coordination, or hydrophobic interactions.1 As these interactions are much weaker than covalent bonds, cleavage of the aggregates easily occurs by adjusting the temperature or concentration, which regenerates the corresponding monomers. Due to this flexibility, supramolecular polymers are expected to act as stimulus-responsive materials.2 Among all the research in this field, supramolecular polymers with fullerenes based on host–guest interactions have been extensively studied in recent years (Fig. 1).3–6 Previous studies have often employed molecular tweezers hosts to ensure strong binding with C60 derivatives. These strategies require a C60 dimer (type a; Fig. 1) or a functionalized C60 bearing a binding site (type b; Fig. 1). However, supramolecular polymerization with pristine C60 remains a challenge because two binding units of the molecular tweezers would be needed to capture one C60 molecule.7 Consequently, stronger yet sterically less-demanding host molecules are required.Buckybowls are bowl-shaped π-conjugated molecules, for which sumanenes and corannulenes are representative examples.8,9 Such curved polycyclic aromatic hydrocarbons have been used for the recognition of fullerenes, given that the concave surface of the former efficiently overlaps with the convex surface of the latter.10 And it is exactly for this reason that extensive research on assemblies of buckybowls with fullerenes has been carried out.11 However, due to the poor electron-donating nature of these buckybowls,12 their binding ability is usually insufficient to construct large supramolecular assemblies.
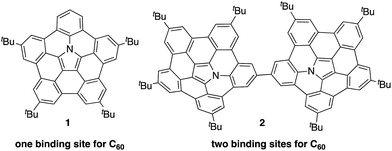
Recently, the group of Nozaki and our own group have independently succeeded in the synthesis of nitrogen-embedded buckybowls such as penta-peri-pentabenzoazacorannulene 1 (Chart 1).13 Due to the electron-donating nature of the pyrrolic nitrogen atom, buckybowl 1 exhibited a large association constant with C60 in solution. The binding constant of 1 was 3800 M−1 in 1,2-dichlorobenzene, which is a top-class value reported for a bowl-shaped molecule. This result suggests that 1 could be used as a new building block for supramolecular assemblies with C60.
Herein, we disclose the synthesis of buckybowl dimer 2 as a host molecule for pristine C60. Owing to its two binding sites, dimer 2 was expected to form complexes with C60 in either a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. We discovered that 2 acts as a concentration-dependent fullerene host, showing drastic morphological changes in the solid state depending on the number of C60 molecules that are contained within the structure. In particular, 1D-chain fibre aggregates consisting of 2 and C60 were obtained.
2 ratio. We discovered that 2 acts as a concentration-dependent fullerene host, showing drastic morphological changes in the solid state depending on the number of C60 molecules that are contained within the structure. In particular, 1D-chain fibre aggregates consisting of 2 and C60 were obtained.
Results and discussion
Synthesis and characterization
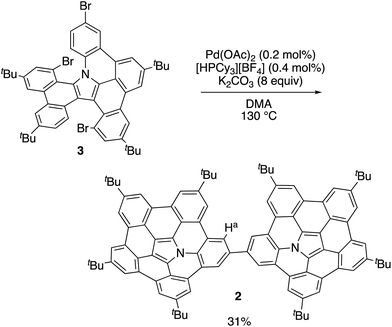
In our previous work, 1 was synthesized via the Pd-mediated C–H/C–Br coupling of tribrominated precursor 3 using an excess of palladium(II) acetate and tricyclohexylphosphonium tetrafluoroborate. Interestingly, the use of catalytic amounts of these reagents provided the linked azabuckybowl dimer 2 in 31% yield (Scheme 1). The structure of 2 was characterized by NMR spectroscopy and mass spectrometry. The parent mass ion peak of 2 was observed at m/z = 1321.7303, which confirms its dimeric structure. The 1H NMR spectrum of 2 exhibited five singlet peaks in the aromatic region, consistent with a symmetric structure for 2 (Fig. S1†). The downfield shifts of the Ha protons (Scheme 1) in 2 compared to those in 1 indicate a deshielding effect by the second azabuckybowl unit. In addition, the 13C NMR spectrum exhibited 18 peaks assignable to sp2-carbons, which suggests a conformation with C2v symmetry (Fig. S2†).Optical and electrochemical properties
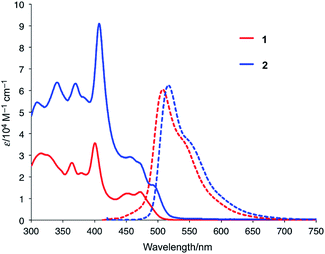
Fig. 2 shows the UV-vis absorption and emission spectra of 1 and 2 in CH2Cl2. Compared to the spectrum of 1, the lowest-energy band of 2 was red-shifted from 472 nm to 495 nm, which indicates the presence of electronic communication between the two azabuckybowl units through the covalent bond. The emission band of 2 was observed at 517 nm with a quantum yield of 0.17, which is almost identical to that of 1. The electrochemical properties of 2 were investigated by cyclic voltammetry (Fig. S3†), where 2 exhibited a lower oxidation potential (0.18 V) than 1 (0.20 V), commensurate with higher electron-donating properties for 2.Titration experiments between 2 and C60
In order to examine the binding ability of 2 toward C60 in solution, we carried out titration experiments under diluted conditions (c = 1.3 × 10−5 M−1) (Fig. 3). The titration was conducted in 1,2-dichlorobenzene and monitored by UV-vis-NIR absorption spectroscopy. Upon addition of a solution of C60 to a solution of 2, an absorption band around 800 nm appeared, which is similar to the behaviour of 1. The Job's plot for the absorbance at 800 nm indicated the predominant formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes in solution (Fig. S4†). A nonlinear curve fitting based on a 1
1 complexes in solution (Fig. S4†). A nonlinear curve fitting based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding afforded an association constant of 7.8 × 103 M−1, which is higher than that of 1 (Ka = 3.8 × 103 M−1). This result corroborates the superior electron-donating nature of 2 relative to that of 1. It should also be noted that the binding constant reached 1.0 × 105 M−1 in toluene (Fig. S5 and S6†). Such solvent-dependent association constants should probably be attributed to the different solvophobicity of the fullerene in each solvent.14
1 binding afforded an association constant of 7.8 × 103 M−1, which is higher than that of 1 (Ka = 3.8 × 103 M−1). This result corroborates the superior electron-donating nature of 2 relative to that of 1. It should also be noted that the binding constant reached 1.0 × 105 M−1 in toluene (Fig. S5 and S6†). Such solvent-dependent association constants should probably be attributed to the different solvophobicity of the fullerene in each solvent.14
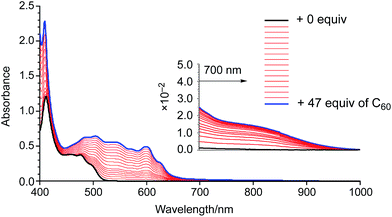 | ||
| Fig. 3 UV-vis-NIR absorption spectra of a 1,2-dichlorobenzene solution of 2 upon addition of 0–47 equiv. of C60. | ||
X-ray crystal structure and charge-carrier mobility
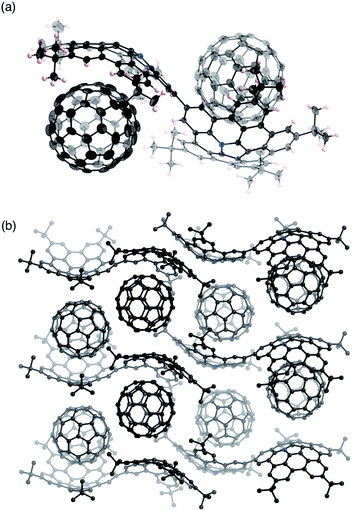
Fortunately, we obtained single co-crystals of 2 and C60 that were suitable for an X-ray diffraction analysis, which were prepared by vapour diffusion of acetonitrile into a toluene solution of a mixture of 2 and C60 (Fig. 4).15 In these crystals, the two azabuckybowl units face in opposite directions, reflected in the tilt angle (50.1°) between the two buckybowl units around the central bond. The C60 molecules are coordinated to the azabuckybowl units in a concave–convex fashion, resulting in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in the crystal. Between the centroid of the pyrrole ring to the closest surface of the C60 molecules, distances of 3.28 and 3.29 Å were measured. Such short distances indicate the existence of strong electronic interactions between 2 and C60 in the solid state. The packing structure is shown in Fig. 4b. Similar to 1·C60, 2 and the C60 molecules present segregate stacking (Fig. S7†). The photo-induced transient conductivity of 2 and 2·C60 was determined by flash-photolysis time-resolved microwave conductivity (FP-TRMC) measurements.16 The carrier mobility of the 2·C60 crystals (2.0 × 10−4 cm2 V−1 S−1) is approximately by one order of magnitude higher than that of 2 (Fig. S8†). The carrier mobility of the 2·C60 crystals was similar to that of 1·C60, indicating a similar charge-separation state between C60 and the azabuckybowl unit in the crystal.
2 ratio in the crystal. Between the centroid of the pyrrole ring to the closest surface of the C60 molecules, distances of 3.28 and 3.29 Å were measured. Such short distances indicate the existence of strong electronic interactions between 2 and C60 in the solid state. The packing structure is shown in Fig. 4b. Similar to 1·C60, 2 and the C60 molecules present segregate stacking (Fig. S7†). The photo-induced transient conductivity of 2 and 2·C60 was determined by flash-photolysis time-resolved microwave conductivity (FP-TRMC) measurements.16 The carrier mobility of the 2·C60 crystals (2.0 × 10−4 cm2 V−1 S−1) is approximately by one order of magnitude higher than that of 2 (Fig. S8†). The carrier mobility of the 2·C60 crystals was similar to that of 1·C60, indicating a similar charge-separation state between C60 and the azabuckybowl unit in the crystal.
Supramolecular assembly of 2 with C60
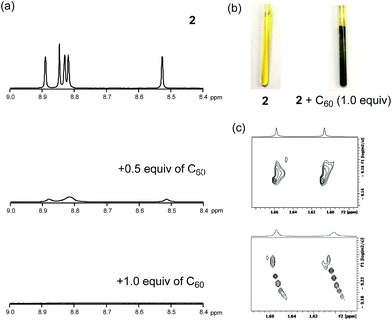
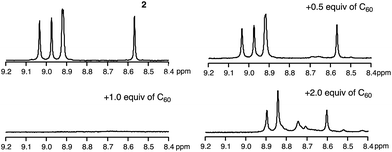
To uncover the different binding ratios of 2 with C60 in solution and the crystalline state, we carried out titration experiments in toluene at 20 °C, which were monitored by 1H NMR spectroscopy (Fig. 5). With increasing amount of C60, the spectral shape of the signal peaks became broader and the peak intensities decreased. Notably, a precipitate was formed in the presence of 1.0 equiv. or less of C60. This phenomenon was not observed in the case of 1, for which a clear solution was obtained in the presence of C60. To clarify this phenomenon, we performed diffusion-ordered two-dimensional NMR spectroscopy (DOSY) experiments.17 The diffusion coefficient (D) determined for 2 (6.83 × 10−10 m2 s−1) decreased by 15% (5.80 × 10−10 m2 s−1) in the presence of 0.5 equiv. of C60. In contrast, a reduction of only 2% was observed in the case of 1 (Fig. S9†). This drop in the D value of 2 indicates the formation of larger structures.The macroscopic structure of the precipitate formed in the presence of C60 was investigated by scanning electron microscopy (SEM). For that purpose, samples were prepared by drop-casting toluene solutions onto silicon wafers. Fig. 6 displays the SEM images of 2 and 2 with 1.0 equiv. of C60. In the precipitate, fibre-like structures were observed, while a film-like morphology was observed for 2, similar to the case of 1 with C60 (Fig. S10†). These results indicate that 2 and C60 assemble into a one-dimensional (1D) supramolecular structure. Based on comparative experiments with 1, it can be concluded that the dimeric structure plays an important role in the formation of supramolecular assemblies.
The stoichiometric ratio between 2 and C60 in the precipitate was determined by variable-temperature 1H NMR measurements in toluene-d8 (Fig. 7). We conducted experiments at low temperature to accelerate the assembly process. At −40 °C, the broad spectrum of 2 in the presence of 0.5 equiv. of C60 became very similar to the spectrum of 2, which exhibited sharp peaks. Using 1,1,2,2-tetrachloroethane as the internal standard revealed that ∼50% the original amount of 2 remained in solution (Fig. S11†). Consequently, we concluded that the precipitate consists of 2 and C60 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Notably, peaks in the aromatic region appeared upon addition of 2.0 equiv. of C60, and these are completely different from those observed for 2 (see also Fig. S12†).
1 ratio. Notably, peaks in the aromatic region appeared upon addition of 2.0 equiv. of C60, and these are completely different from those observed for 2 (see also Fig. S12†).
The composition of the fibres was further analysed by MALDI-TOF mass spectrometry (Fig. 8). The spectrum exhibited several intense peaks at regular intervals. The gaps between the peaks correspond to the molecular weight of 2 or C60. The largest observable peak was at Mw = 13 kDa, corresponding to a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 complex of 2 and C60. In their entirety, the NMR, SEM, and MS analyses allow the conclusion that the fibres consist of a 1D chain-like assembly of 2 and C60 in a 1
6 complex of 2 and C60. In their entirety, the NMR, SEM, and MS analyses allow the conclusion that the fibres consist of a 1D chain-like assembly of 2 and C60 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
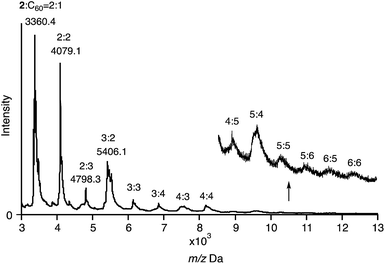 | ||
| Fig. 8 MALDI-TOF MS spectrum of 2 with 1.0 equiv. of C60 (matrix: trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile; DCTB). | ||
To elucidate more structural details of the fibres, we performed a powder X-ray diffraction (XRD) analysis (Fig. S13†), which exhibited two broad peaks at 2θ = 2.44° (36.2 Å) and 5.00° (17.7 Å). The spectral pattern of the fibres is thus inconsistent with that of the single crystal of 2·C60, suggesting the formation of a different packing structure. On the other hand, a powdered sample of 2 showed weak and broad reflections at 2θ = 4.94° (17.9 Å) and 6.18° (14.3 Å), indicating the lack of structural regularity in 2.
Fig. 9 shows the UV-vis-NIR absorption of 2 and its inclusion complexes in the solid state. In contrast to 2, the 2·C60 crystal exhibits a broad absorption band around 850 nm, which was characterized as a charge transfer (CT) band. The fibre aggregates also exhibit an NIR absorption band, indicating similar concave–convex binding between 2 and C60 in the structure. However, the intensity of this band was higher for the fibre than for the crystal, suggesting different packing structures for these two samples.
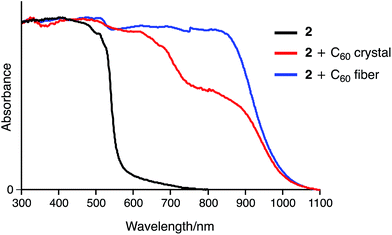 | ||
| Fig. 9 Solid-state UV-vis-NIR absorption spectra of 2, 2 + C60 crystal, and 2 + C60 fibre (spectra were normalized at 300 nm). | ||
Two binding modes are possible for the association of 1 with C60. One is a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 concave–convex complex and the other one involves the formation of a 1
1 concave–convex complex and the other one involves the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 sandwich-type complex. We anticipated that these two binding modes should result in different UV-vis-NIR absorption features in the solid state. Fortunately, by changing the solvents used for recrystallization from methanol/toluene to hexane/chloroform, a 2
2 sandwich-type complex. We anticipated that these two binding modes should result in different UV-vis-NIR absorption features in the solid state. Fortunately, by changing the solvents used for recrystallization from methanol/toluene to hexane/chloroform, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of 1 and C60 was obtained.18 The single-crystal X-ray diffraction analysis of the complex unambiguously revealed a sandwich-type structure, in which two azabuckybowl molecules cooperatively capture a C60 molecule by concave–convex interactions (Fig. 10). In addition, we recorded the solid-state UV-vis-NIR absorption spectra of the crystals for both 1
1 complex of 1 and C60 was obtained.18 The single-crystal X-ray diffraction analysis of the complex unambiguously revealed a sandwich-type structure, in which two azabuckybowl molecules cooperatively capture a C60 molecule by concave–convex interactions (Fig. 10). In addition, we recorded the solid-state UV-vis-NIR absorption spectra of the crystals for both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding modes (Fig. 11). Both 1
1 binding modes (Fig. 11). Both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes exhibit CT absorption bands around 850 nm. Notably, the absorption intensity at this wavelength is higher for the 2
1 complexes exhibit CT absorption bands around 850 nm. Notably, the absorption intensity at this wavelength is higher for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex than for the 1
1 complex than for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. The theoretical calculations by the TD-DFT method also support these experimental results. The simulated absorption bands assigned to the CT transitions are significantly large in the 2
1 complex. The theoretical calculations by the TD-DFT method also support these experimental results. The simulated absorption bands assigned to the CT transitions are significantly large in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex as compared to that in the 1
1 complex as compared to that in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Fig. S14†). Such an enhancement of the CT band was also observed in the absorption spectrum of 2 + C60. This spectral similarity strongly indicates a sandwich-type binding mode in the 2·C60 fibres.
1 complex (Fig. S14†). Such an enhancement of the CT band was also observed in the absorption spectrum of 2 + C60. This spectral similarity strongly indicates a sandwich-type binding mode in the 2·C60 fibres.
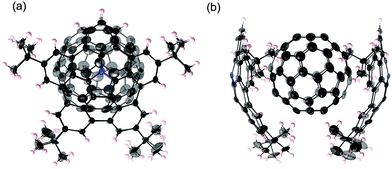 | ||
| Fig. 10 Molecular structure of 12·C60 in the crystal: (a) side view and (b) packing structure. The thermal ellipsoids are scaled at 50% probability level. | ||
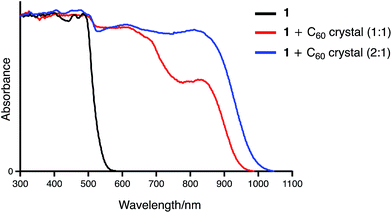 | ||
| Fig. 11 Solid state UV-vis-NIR absorption spectra of 1, 1·C60, and 12·C60 (spectra were normalized at 300 nm). | ||
Plausible structures and mechanism of supramolecular assembly
Scheme 2 illustrates the plausible association mechanism of 2 with C60. In the initial binding step, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between 2 and C60 should be formed. Binding to the second C60 molecule would be weaker due to the reduced electron-donating ability of the other azabuckybowl unit after binding the first electron-deficient C60.19 Consequently, the 1
1 complex between 2 and C60 should be formed. Binding to the second C60 molecule would be weaker due to the reduced electron-donating ability of the other azabuckybowl unit after binding the first electron-deficient C60.19 Consequently, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is obtained predominant in dilution. The 1
1 complex is obtained predominant in dilution. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes interact with each other under more concentrated conditions to form fibres by sandwich-type binding, which are insoluble in organic solvents. We optimized the structure of the 1D chain-like arrangement using PM6 semi-empirical calculations (Fig. S15†). The calculated interplanar spacing (17.8 Å) is in good agreement with the XRD results (17.7 Å). Further addition of C60 to the fibres induces cleavage of the polymer chain to form soluble fragments, as detected by 1H NMR spectroscopy.
1 complexes interact with each other under more concentrated conditions to form fibres by sandwich-type binding, which are insoluble in organic solvents. We optimized the structure of the 1D chain-like arrangement using PM6 semi-empirical calculations (Fig. S15†). The calculated interplanar spacing (17.8 Å) is in good agreement with the XRD results (17.7 Å). Further addition of C60 to the fibres induces cleavage of the polymer chain to form soluble fragments, as detected by 1H NMR spectroscopy.
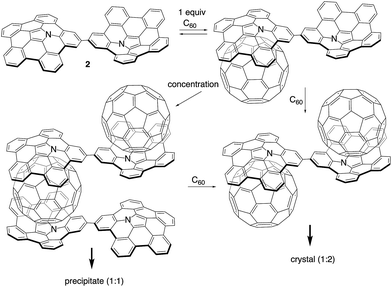 | ||
| Scheme 2 Plausible association mechanism for 2 in the presence of increasing amounts of C60 (tert-butyl groups are omitted for clarity). | ||
Conclusions
In summary, we have synthesized a directly linked azabuckybowl dimer from a tribrominated monomeric precursor. The dimer exhibits strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation with C60 in solution. Segregated stacks of 2 and C60 were observed in the crystalline state, suggesting efficient photo-excited charge-carrier mobility. Under concentrated conditions, 2 and C60 form 1D chain supramolecular assemblies with a fibrous structure. The present results demonstrate that an electron-donating bowl-shaped π-conjugated molecule can serve as a binding motif for pristine C60 for the construction of supramolecular assemblies based on strong donor–acceptor interactions.
1 complexation with C60 in solution. Segregated stacks of 2 and C60 were observed in the crystalline state, suggesting efficient photo-excited charge-carrier mobility. Under concentrated conditions, 2 and C60 form 1D chain supramolecular assemblies with a fibrous structure. The present results demonstrate that an electron-donating bowl-shaped π-conjugated molecule can serve as a binding motif for pristine C60 for the construction of supramolecular assemblies based on strong donor–acceptor interactions.
Conflicts of interest
There are no conflicts for declare.Acknowledgements
We would like to acknowledge Prof. Takahiro Seki and Dr Mituo Hara (Nagoya University) for their help with the powder X-ray diffraction measurements. We also thank Dr Ichiro Hisaki (Osaka University) for his help with the single-crystal X-ray diffraction analysis of 2·C60. This work was supported by JSPS KAKENHI grants JP16H06031, JP26102003, and JP15H00731, as well as the Program for Leading Graduate Schools “Integrative Graduate Education and Research in Green Natural Sciences” from MEXT (Japan). S. H. expresses his gratitude for financial support from the Tokuyama Science Foundation. Y. H. acknowledges a grant-in-aid for JSPS Research Fellows (JP15J10528).Notes and references
- (a) L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS PubMed; (b) T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef CAS PubMed; (c) L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed.
- (a) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC; (b) X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971 CrossRef CAS PubMed.
- F. Giacalone and N. Martín, Chem. Rev., 2006, 106, 5136 CrossRef CAS PubMed.
- Supramolecular Chemistry of Fullerenes and Carbon Nanotubes, ed. N. Martín and J.-F. Nierengarten, Wiley-VCH, Weinheim, 2012 Search PubMed.
- (a) G. Fernández, E. M. Pérez, L. Sánchez and N. Martín, Angew. Chem., Int. Ed., 2008, 47, 1094 CrossRef PubMed; (b) G. Fernández, E. M. Pérez, L. Sánchez and N. Martín, J. Am. Chem. Soc., 2008, 130, 2410 CrossRef PubMed.
- (a) T. Hirao, M. Tosaka, S. Yamago and T. Haino, Chem.–Eur. J., 2014, 20, 16138 CrossRef CAS PubMed; (b) T. Haino, Y. Matsumoto and Y. Fukazawa, J. Am. Chem. Soc., 2005, 127, 8936 CrossRef CAS PubMed; (c) H. Isla, E. M. Pérez and N. Martín, Angew. Chem., Int. Ed., 2014, 53, 5629 CrossRef CAS PubMed.
- (a) Y. Liu, H. Wang, P. Liang and H.-Y. Zhang, Angew. Chem., Int. Ed., 2004, 43, 2690 CrossRef CAS; (b) Y. Liu, Y.-W. Yang, Y. Chen and H.-X. Zou, Macromolecules, 2005, 38, 5838 CrossRef CAS; (c) M. Shirakawa, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2003, 125, 9902 CrossRef CAS PubMed; (d) M. Fathalla, S.-C. Li, U. Diebold, A. Alb and J. Jayawickramarajah, Chem. Commun., 2009, 4209 RSC.
- Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis, Unusual Reactions, and Coordination Chemistry, ed. M. A. Petrukhina and L. T. Scott, Wiley, Hoboken, 2012 Search PubMed.
- (a) Y.-T. Wu and J. S. Siegel, Chem. Rev., 2006, 106, 4843 CrossRef CAS PubMed; (b) H. Sakurai, T. Daiko and T. Hirao, Science, 2003, 301, 1878 CrossRef CAS PubMed; (c) W. E. Barth and R. G. Lawton, J. Am. Chem. Soc., 1966, 88, 380 CrossRef CAS.
- T. Kawase and H. Kurata, Chem. Rev., 2006, 106, 5250 CrossRef CAS PubMed.
- (a) L. N. Dawe, T. A. AlHujran, H.-A. Tran, J. I. Mercer, E. A. Jackson, L. T. Scott and P. E. Georghiou, Chem. Commun., 2012, 48, 5563 RSC; (b) A. S. Filatov, M. V. Ferguson, S. N. Spisak, B. Li, C. F. Campana and M. A. Petrukhina, Cryst. Growth Des., 2014, 14, 756 CrossRef CAS; (c) S. Mizyed, P. E. Georghiou, M. Bancu, B. Cuadra, A. K. Rai, P. Cheng and L. T. Scott, J. Am. Chem. Soc., 2001, 123, 12770 CrossRef CAS PubMed; (d) A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842 CrossRef CAS PubMed; (e) M. Yanney, F. R. Fronczek and A. Sygula, Angew. Chem., Int. Ed., 2015, 54, 11153 CrossRef CAS PubMed; (f) C. M. Álvarez, L. A. García-Escudero, R. García-Rodríguez, J. M. Martín-Álvarez, D. Miguel and V. M. Rayón, Dalton Trans., 2014, 43, 15693 RSC; (g) P. L. A. Kuragama, F. R. Fronczek and A. Sygula, Org. Lett., 2015, 17, 5292 CrossRef PubMed.
- (a) J. Janata, J. Gendell, C.-Y. Ling, W. E. Barth, L. Backes, H. B. Mark and R. G. Lawton, J. Am. Chem. Soc., 1967, 89, 3056 CrossRef CAS; (b) T. J. Seiders, K. K. Baldridge, J. S. Siegel and R. Gleiter, Tetrahedron Lett., 2000, 41, 4519 CrossRef CAS; (c) P. Zanello, S. Fedi, F. F. de Biani, G. Giorgi, T. Amaya, H. Sakane and T. Hirao, Dalton Trans., 2009, 9192 RSC.
- (a) H. Yokoi, Y. Hiraoka, S. Hiroto, D. Sakamaki, S. Seki and H. Shinokubo, Nat. Commun., 2015, 6, 8215 CrossRef PubMed; (b) S. Ito, Y. Tokimaru and K. Nozaki, Angew. Chem., Int. Ed., 2015, 54, 7256 CrossRef CAS PubMed.
- V. H. Le, M. Yanney, M. McGuire, A. Sygula and E. A. Lewis, J. Phys. Chem. B, 2014, 118, 11956 CrossRef CAS PubMed.
- X-ray crystallographic data for 2·C60: formula: C247.73H123.18N2, Mw = 3127.42, monoclinic, space group: P21/c, a = 13.1579(2) Å, b = 37.8588(7) Å, c = 30.9277(5) Å, β = 96.192(2)°, V = 15316.5(4) Å3, Z = 4, Dc = 1.356 g cm−3, R1 = 0.1090(I > 2σ(I)), wR2 = 0.3338 (all data), GOF = 1.085. CCDC number: 1579079.
- S. Seki, A. Saeki, T. Sakurai and D. Sakamaki, Phys. Chem. Chem. Phys., 2014, 16, 11093 RSC.
- C. S. Johnson Jr, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203 CrossRef.
- X-ray crystallographic data for 12·C60: formula: C320H188N4, Mw = 4088.73, monoclinic, space group: P21, a = 17.0175(8) Å, b = 28.9186(13) Å, c = 22.7929(10) Å, β = 100.4840(10), V = 11029.6(9) Å3, Z = 2, Dc = 1.231 g cm−3, R1 = 0.0969(I > 2σ(I)), wR2 = 0.2627 (all data), GOF = 1.077. CCDC number: 1579080.
- A negative allosteric effect of C60 has been reported; see: (a) K. Miki, T. Matsushita, Y. Inoue, Y. Senda, T. Kowada and K. Ohe, Chem. Commun., 2013, 49, 9092 RSC; (b) H. Sato, K. Tashiro, H. Shinmori, A. Osuka, Y. Murata, K. Komatsu and T. Aida, J. Am. Chem. Soc., 2005, 127, 13086 CrossRef CAS PubMed; (c) L. Moreira, J. Calbo, J. Aragó, B. M. Illescas, I. Nierengarten, B. Delavaux-Nicot, E. Ortí, N. Martín and J.-F. Nierengarten, J. Am. Chem. Soc., 2016, 138, 15359 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1579079 and 1579080. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04453d |
| This journal is © The Royal Society of Chemistry 2018 |