Open Access Article
Open Access ArticleNorborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs†
Jui Thiang Brian
Kueh
 a,
Nathan J.
Stanley
a,
Nathan J.
Stanley
 a,
Russell J.
Hewitt
a,
Laura M.
Woods
a,
Lesley
Larsen
a,
Joanne C.
Harrison
a,
Russell J.
Hewitt
a,
Laura M.
Woods
a,
Lesley
Larsen
a,
Joanne C.
Harrison
 b,
David
Rennison
c,
Margaret A.
Brimble
b,
David
Rennison
c,
Margaret A.
Brimble
 c,
Ivan A.
Sammut
c,
Ivan A.
Sammut
 *b and
David S.
Larsen
*b and
David S.
Larsen
 *a
*a
aDepartment of Chemistry, University of Otago, Dunedin, New Zealand. E-mail: david.larsen@otago.ac.nz
bDepartment of Pharmacology, University of Otago, Dunedin, New Zealand. E-mail: ivan.sammut@otago.ac.nz
cSchool of Chemical Sciences, University of Auckland, Auckland, New Zealand
First published on 30th May 2017
Abstract
A prodrug strategy for the release of the gasotransmitter CO at physiological pH, based upon 3a-bromo-norborn-2-en-7-one Diels–Alder cycloadducts of 2-bromomaleimides and 2,5-dimethyl-3,4-diphenylcyclopentadienone has been developed. Examples possessing protonated amine and diamine groups showed good water solubility and thermal stability. Half-lives for CO-release in TRIS-sucrose buffer at pH 7.4 ranged from 19 to 75 min at 37 °C and 31 to 32 h at 4 °C. Bioavailability in rats was demonstrated by oral gavage and oCOm-21 showed a dose dependent vasorelaxant effect in pre-contracted rat aortic rings with an EC50 of 1.6 ± 0.9 μM. Increased intracellular CO levels following oCOm-21 exposure were confirmed using a CO specific fluorescent probe.
Introduction
Carbon monoxide (CO) is a key player in heme oxygenase mediated cellular regulation and protection in health and disease. Evidence, by our group and others,1,2 has established the endogenous existence of critical, CO-activated protective signaling pathways (vasodilatory, anti-apoptotic, anti-thrombotic and anti-inflammatory). For example, delivery of CO gas at low concentrations protects organs from ischaemic injury by decreasing cell death and most significantly inducing a preconditioning response.3,4 Carbon monoxide binds to hemeproteins, to modulate the activity or level of expression of key cellular targets such as soluble guanylate cyclase (sGC), and inducible nitric oxide synthase to produce a plethora of downstream signaling events.5,6 The anti-inflammatory effects of CO are mediated by p38 MAPK signaling and inhibition of the translocation of the potent damage-associated molecular pattern recognition molecule HMBG1. This results in a reduction of pro-inflammatory cytokines, such as TNF-α, and an increase in the expression of the anti-inflammatory IL-10.7,8The protective and physiological effects of low levels of CO have been studied in a number of both animal and clinical models with the literature extensively reviewed.5,8–12 The results of these studies have been so compelling that the FDA has granted approval for the application of low dose CO gas in a range of clinical application trials such as CO gas delivery by inhalation in heart valve replacement surgery13 and in renal transplant procedure recipients.14 Inhalation of CO (100–125 ppm) in chronic obstructive pulmonary disease (COPD) patients, reduced sputum neutrophils and improved bronchial responsiveness.15 In renal transplant procedures low dose CO gas during surgery improves post-transplant kidney function.16 However, the difficulty of controlling gas delivery in a clinical setting, combined with the hazardous consequences of any gas leak, have been acknowledged as significant impediments in the use of CO in gaseous form.6,17 CO-releasing molecules (CORMs) have been developed as an alternative CO delivery system allowing a greater ease of administration and control, and potentially enabling a safer, tightly regulated method of low dose CO delivery. These molecules have been extensively studied and their beneficial effects18 have been demonstrated in transplant and disease models.19–22 A large proportion of existing CORMs are based on transition-metal carbonyl complexes [MxLy(CO)z, where M = Mn, Ru, Fe, W, Mo, Cr, Ir or Re].23–25 Early metal carbonyl complexes suffered from low water solubility and high toxicity. The attachment of organic ligands to the metal centre resulted in a newer generation of metal carbonyl complexes, such as CORM-3,26 with increased water solubility. However, an issue with metal carbonyl complexes is that the residual by-products (iCORMs) obtained after CO release contain transition metals which is undesirable and could contribute to toxicity.27–29
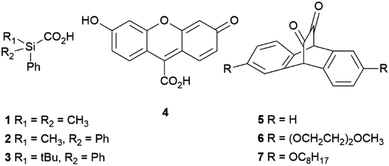
Several CORMs that do not contain transition metals have been reported.30 One example is sodium boranocarbonate (CORM-A1),31 which is water soluble, capable of liberating CO at pH 7.4 and was found to improve the renal function of rabbit kidney transplants upon reperfusion.32 More recent organic examples (Fig. 1) include the silacarboxylic acids 1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 the fluorescein analogue 4
33 the fluorescein analogue 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 and the cyclic α-diketones 5–7.35
34 and the cyclic α-diketones 5–7.35
Silacarboxylic acids 1–3 have only been employed as a source of CO gas for carbonylation reactions and require the addition of fluoride-based desilylating reagents to induce CO release.33,36 The fluorescein analogue 4 requires exposure to visible light (500 nm) for CO release,34 while the cyclic α-diketones 5–7 need to be encapsulated within pluronic micelles so that photoactivation (400–550 nm) can result in effective CO release. However, these examples have yet to show clinical relevance because of issues associated with the mechanism of CO release, solubility and cellular toxicity.
The focus of the current work is the development of organic CORMs that would release CO on physiological stimulus. Furthermore, the formation of an easily characterized organic by-product after CO release would allow determination of the CO-release kinetics whilst potentially reducing residual compound toxicity. Our approach is based upon the bridged carbonyl compound norbornadien-7-one (8)37 which undergoes cheletropic loss of carbon monoxide (CO) giving benzene as a by-product (Fig. 2A).
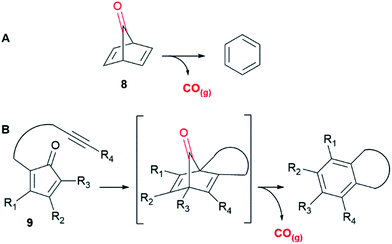 | ||
| Fig. 2 (A) Cheletropic loss of carbon monoxide from norbornadien-7-one (8). (B) Strategy reported by Wang et al.38 | ||
It is well known that compound 8 is remarkably prone to fragmentation with loss of CO and several computation studies37,39 have been carried out on this reaction where the activation energy for this process was calculated to be between 11 to 12 kcal mol−1 with the corresponding rate constant at 300 K (27 °C) of 4.2 × 105 s−1 (t1/2 = 1.6 × 10−5 s).
With this in mind, Wang et al. have recently developed an elegant “click and release” CO prodrug utilising intermolecular Diels–Alder reactions of functionalised tetraphenylcyclopentadienones with strained alkynes to generate unstable norbornadienone intermediates with concomitant loss of CO.40 An intramolecular variant, where the alkyne was tethered to the diene (Fig. 2B, 9) provided a series of CORMs with CO release half-lives, controlled by the rate of the Diels–Alder reaction, ranging from 2 minutes to one week.38 These compounds demonstrated possible therapeutic applications in an anti-inflammatory assay and a colitis animal model, however DMSO was required to solubilize them.
We sought an alternative approach where physiological pH controls the release of CO using bridged carbonyl compounds based upon a norbornenone substructure. These well-known compounds are generally prepared using Diels–Alder reactions of substituted cyclopentadienones and activated dienophiles, such as maleic anhydride, giving stable cycloadducts. Early work on these compounds carried out by Allen et al.41 and Fuch et al.42,43 showed that compounds such as 10 could decompose under thermal and/or photochemical conditions to release carbon monoxide (Scheme 1).
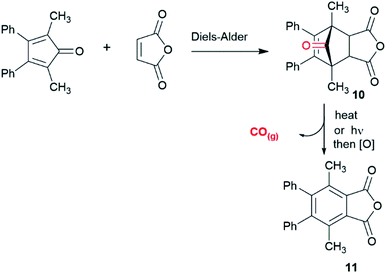 | ||
| Scheme 1 Formation and aromatisation of cycloadduct 10.41,42 | ||
However, the conditions for CO release by heating (200 °C)40 or photochemical activation42,43 as reported for 10 preclude the use of such compounds as CORMs in a biological setting. Given this we have developed a strategy that utilises the properties of both the norbornenone (stability) and norbornadienone (rapid decomposition releasing CO) class of compound.
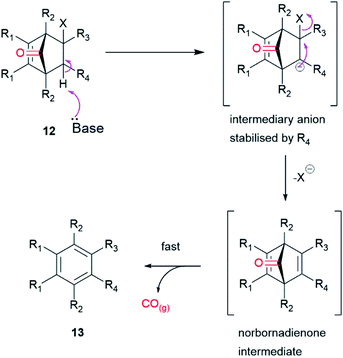
This approach provides a prodrug for an organic CO donor molecule, the general strategy of which utilizes compounds with the functionalised norbornenone structure represented by 12 (Fig. 3). Elimination of HX would produce the transient norbornadienone which would undergo rapid/spontaneous cheletropic loss of CO and concomitant aromatisation to by-product 13. X would be a halogen (Cl, Br or I) and R4 an electron withdrawing group. The rate determining step, an E1cB process, would be affected by the substituents R3 and R4 and thus would control the rate of CO release. This could be achieved when R3/R4 was a cyclic imide, which would be less susceptible to hydrolysis than an anhydride and also provide a handle for further functionalization. This structural motif would allow the process to be triggered by raising the pH to give an intermediary anion, stabilized by the imide, followed by loss of halide to give a norbornadienone intermediate.
Discussion
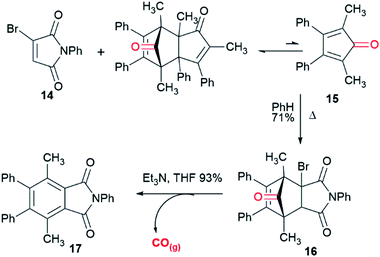
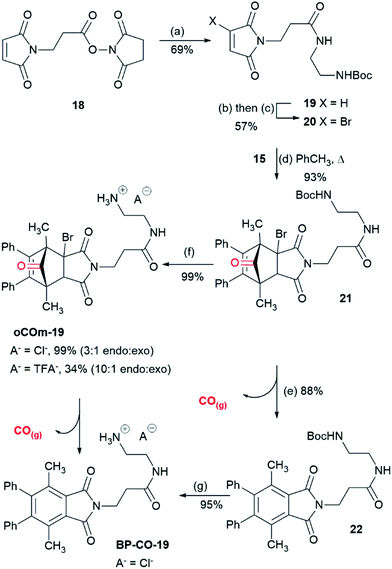
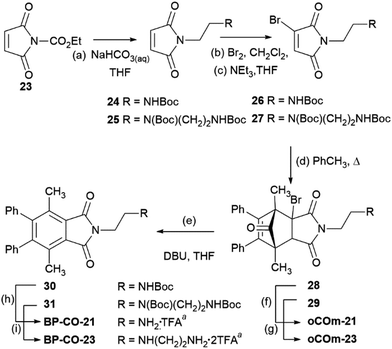
Einhorn et al.44 reported the synthesis of polyarylphthalimides from the Diels–Alder reaction of tetraarylcyclopentadienones and 2-bromomaleimide in refluxing bromobenzene which occurred with concomitant decarbonylation. Our attempts to carry out the reaction of tetraphenylcyclopentadienone and 2-bromo-N-phenylmaleimide (14) at lower temperatures in order to isolate the Diels–Alder cycloadduct were unsuccessful due to the slow rate of reaction in refluxing toluene. We surmised that this was due to steric interactions between the 2- and 5- phenyl groups and the bromine substituent of the maleimide. Pleasingly, the less sterically-encumbered 2,5-dimethyl-3,4-diphenylcyclopentadienone (15), which exists as a reversible dimer, reacted smoothly with 14 to give endo-16 in 71% yield after crystallisation from diethyl ether (Scheme 2). To test whether this compound could release CO it was treated with triethylamine in THF. Effervescence occurred and phthalimide 17 was isolated in 93% yield. GC/MS headspace analysis of a solution of 16 in DMSO in a sealed tube after treatment with DBU confirmed that the gas released was CO. 1H NMR analysis of the solution indicated the complete conversion of 16 into 17.With the proof of principle that cycloadducts like 16 can release CO on treatment with base, attention turned towards making water soluble analogues. To this end a series of CORMs functionalized with amine salts were prepared. Maleimide 18,45 with a pendant activated ester was reacted with mono-Boc protected ethylene diamine giving 19 which was subjected to a bromination–dehydrobromination sequence to give bromomaleimide 20 in 57% yield over the two steps (Scheme 3). Cycloaddition of diene 15 and 20 gave adduct 21 as a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of endo and exo-isomers in 93% yield. Adduct 21 was sensitive to base and on treatment with DBU in THF gave phthalimide 22 (88%). Removal of the Boc-group of adduct 21 was achieved on reaction with 6 M HCl in dioxane to give oCOm-19 as its HCl salt (99%, 3
1 mixture of endo and exo-isomers in 93% yield. Adduct 21 was sensitive to base and on treatment with DBU in THF gave phthalimide 22 (88%). Removal of the Boc-group of adduct 21 was achieved on reaction with 6 M HCl in dioxane to give oCOm-19 as its HCl salt (99%, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of endo and exo isomers). In a separate reaction, the reaction product was subjected to reverse phase chromatography on a C-18 solid phase extraction cartridge using 1% aqueous TFA and acetonitrile which resulted in partial separation of the endo- and exo-isomers (10
1 mixture of endo and exo isomers). In a separate reaction, the reaction product was subjected to reverse phase chromatography on a C-18 solid phase extraction cartridge using 1% aqueous TFA and acetonitrile which resulted in partial separation of the endo- and exo-isomers (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 34%) of oCOm-19 as the TFA salt, with the remainder of the material as a mixture of oCOm-19 and aromatised product, iCORM BP-CO-19. The latter compound was also prepared in 95% yield by treatment of 22 with 6 M HCl in dioxane.
1, 34%) of oCOm-19 as the TFA salt, with the remainder of the material as a mixture of oCOm-19 and aromatised product, iCORM BP-CO-19. The latter compound was also prepared in 95% yield by treatment of 22 with 6 M HCl in dioxane.
Using a similar process ethylenediamine and diethylenetriamine derived CORMs were prepared from carboethoxymaleimide 23 (Scheme 4).
Reaction of bromomaleimide 26 with diene dimer 15 gave cycloadduct 28 (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo = 2.5
exo = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 84% yield after purification by column chromatography. Partial separation was achieved with a sample of endo-28 isolated in 32% yield with the remaining 52% a 1
1) in 84% yield after purification by column chromatography. Partial separation was achieved with a sample of endo-28 isolated in 32% yield with the remaining 52% a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of endo- and exo-isomers. Similarly, reaction of bromomaleimide 27 with 15 gave a 3
1 mixture of endo- and exo-isomers. Similarly, reaction of bromomaleimide 27 with 15 gave a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
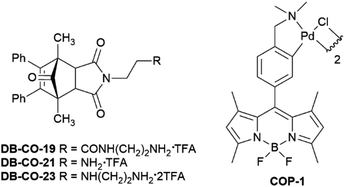
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of endo- and exo-adducts 29 which after column chromatography afforded the endo-isomer (60%) along with a mixture of the isomers in 27% yield. Removal of the Boc-protecting groups of endo-28 and -29 was achieved on treatment with 6 M HCl in dioxane and gave, after purification on reverse-phase solid-phase cartridges and lyophilization, oCOm-21 and -23 both in 63% yield as their mono- and bis-trifluoroacetate salts as white powders. Aromatisation of 28 and 29 on treatment with DBU gave phthalimides 30 and 31 and subsequent removal of the Boc-group provided the respective iCORMs BP-CO-21 and BP-CO-23 in 48 and 62% yield respectively over the two steps. A series of desbromo-analogues of oCOm-19, -21 and -23, DB-CO-19, -21 and -23 respectively, which lack the ability to release CO under physiological conditions, were also synthesised by Diels–Alder reactions of diene 15 and maleimides 19, 24, and 25 and subsequent deprotection of the Boc groups for use as control compounds (Scheme S1, ESI†).
1 mixture of endo- and exo-adducts 29 which after column chromatography afforded the endo-isomer (60%) along with a mixture of the isomers in 27% yield. Removal of the Boc-protecting groups of endo-28 and -29 was achieved on treatment with 6 M HCl in dioxane and gave, after purification on reverse-phase solid-phase cartridges and lyophilization, oCOm-21 and -23 both in 63% yield as their mono- and bis-trifluoroacetate salts as white powders. Aromatisation of 28 and 29 on treatment with DBU gave phthalimides 30 and 31 and subsequent removal of the Boc-group provided the respective iCORMs BP-CO-21 and BP-CO-23 in 48 and 62% yield respectively over the two steps. A series of desbromo-analogues of oCOm-19, -21 and -23, DB-CO-19, -21 and -23 respectively, which lack the ability to release CO under physiological conditions, were also synthesised by Diels–Alder reactions of diene 15 and maleimides 19, 24, and 25 and subsequent deprotection of the Boc groups for use as control compounds (Scheme S1, ESI†).
To assess whether CORMs oCOm-19, -21 and -23 have therapeutic potential their water solubility and CO release profiles were measured at pH 7.4. Pleasingly all three were soluble in tissue culture water at 20 °C at 3.2, 10.8 and 18.2 mg mL−1 respectively. Transplant organ preservation solutions are normally maintained at 8 °C. Unfortunately, the solubility of oCOm-19 at 8 °C was low and could not be determined, whereas those lacking the amide linking group, oCOm-21 and -23, were measured as 3.9 and 17.6 mg mL−1 respectively. The CO release profiles for the more soluble CORMs oCOm-21 and -23 were assessed by monitoring the formation of the corresponding iCORMs, BP-CO-21 and BP-CO-23 in TRIS-sucrose buffer at pH 7.4 using an HPLC method. oCOm-21 showed half-lives for CO release of 19 min at 37 °C (Fig. S1, ESI†) and 31 h at 4 to 5 °C. oCOm-23 half-lives at the same temperatures were 75 min and 32 h, respectively. The CO release was also supported by monitoring via a CO-selective electrode over a range of pH and myoglobin assays (Fig. S2a to c, ESI†). There was no sign of the formation of the corresponding iCORMs from solutions of oCOm-19, -21 and -23 in aqueous media at pH < 7 after prolonged periods of time (>1 week). Samples showed no deterioration after being left at room temperature exposed to ambient light for over one month. The thermal stability of oCOM-21 was investigated by thermogravimetric analysis and was shown to undergo decomposition at 125 °C (Fig. S3a, ESI†). Further analysis showed that heating a sample of oCOM-21 to 90 °C and holding it at that temperature for 30 min resulted in a 4% loss of mass (Fig. S3b, ESI†). HPLC analysis of this sample showed that it was a 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mixture of oCOm-21 and its corresponding iCORM (Fig. S3c, ESI†).
4 mixture of oCOm-21 and its corresponding iCORM (Fig. S3c, ESI†).
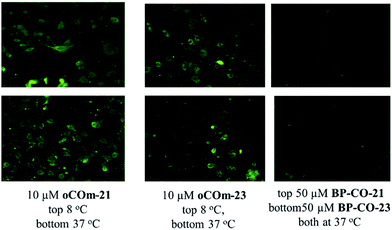
In context to applications such as organ transplantation, the toxicity of oCOm-19, -21 and -23 was assessed using sensitive MDCK cells at 37 °C and EC50 values were measured as 86, 11.2 and 10.1 μM, respectively (Fig. S4, ESI†). iCORMs BP-CO-21 and -23 gave EC50 values of 21 and 100 μM, respectively. The toxicity of oCOm-21 was also measured against this cell line at 8 °C and gave an EC50 > 100 μM.
CO delivery to MDCK cells by oCOm-21 and -23 at 8 and 37 °C was confirmed using the CO-selective fluorescent probe COP-1 developed by Chang et al.46 (Fig. 4).
Oral bioavailability in rats was demonstrated by carboxyhaemoglobin analysis following administration of oCOm-19 and -21 by oral gavage. Moderate but significant increases in carboxyhaemoglobin levels in the plasma were obtained after 3 h of administration for the less soluble oCOm-19 and within 1 h for oCOm-21 (Table S1, ESI†). BP-CO-19, which cannot release CO, showed no increase in carboxyhaemoglobin at the same time points.
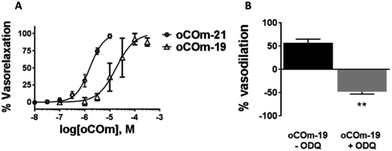
CO relaxes vascular tissues by acting on the cGMP pathway and high conductance calcium-activated K+ (KCa) channels. To assess the vasorelaxant effect of our compounds, pre-contracted rat aortic rings were treated with oCOm-19 and -21. A dose-dependent relationship was observed for both compounds with EC50 values of 18.0 ± 0.8 μM and 1.6 ± 0.9 μM respectively (Fig. 5). This vasodilatory effect was reversed by the addition of 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of soluble guanylate cyclase (sGC).
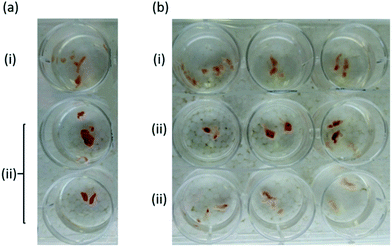
In a preliminary experiment, the preservation of tissue viability was demonstrated for rat myocardium. Tissue samples treated with oCOm-21 stored in Dulbecco's modified Eagle medium appeared to be more viable after 48 h compared to an untreated control based upon dehydrogenase activity indicated by conversion of tetrazolium chloride (TTC, white) into the corresponding formazan (red) (Fig. 6).
Conclusions
In conclusion, we have developed CO prodrugs based upon norbornenones which function via E1cB elimination of HBr under physiological conditions, producing a reactive norbornadienone intermediate that rapidly undergoes chelotropic loss of CO. The three prodrugs, oCOm-19, -21, and -23 are thermally stable solids that show good water solubilities and CO release profiles. Under physiological conditions they have been shown to deliver CO to cells, have a vasorelaxant effect and have the potential to preserve tissue sample viability. We are developing new generations of these compounds and investigating their applications as additives for organ transplantation preservation solutions.Acknowledgements
This work is supported by a University of Otago Research Grant and by the Ministry of Business, Innovation and Employment grant (CONT-29537-HVMSSI-UOO).Notes and references
- I. A. Sammut, R. Foresti, J. E. Clark, D. J. Exon, M. J. J. Vesely, P. Sarathchandra, C. J. Green and R. Motterlini, Br. J. Pharmacol., 1998, 125, 1437–1444 CrossRef CAS PubMed.
- A. Ayer, A. Zarjou, A. Agarwal and R. Stocker, Physiol. Rev., 2016, 96, 1449–1508 CrossRef PubMed.
- A. Bagul, S. A. Hosgood, M. Kaushik and M. L. Nicholson, Transplantation, 2008, 85, 576–581 CrossRef CAS PubMed.
- I. C. Winburn, J. C. Harrison, R. J. MacGinley, R. J. Walker and I. A. Sammut, Transplantation, 2006, 82, 1043 Search PubMed.
- S. Ghosh, J. Gal and N. Marczin, Ann. Med., 2010, 42, 1–12 CrossRef CAS PubMed.
- S. W. Ryter and L. E. Otterbein, BioEssays, 2004, 26, 270–280 CrossRef CAS PubMed.
- L. E. Otterbein, F. H. Bach, J. Alam, M. Soares, L. H. Tao, M. Wysk, R. J. Davis, R. A. Flavell and A. M. Choi, Nat. Med., 2000, 6, 422–428 CrossRef CAS PubMed.
- Y. Ruan, L. Wang, Y. Zhao, Y. Yao, S. Chen, J. Li, H. Guo, C. Ming, S. Chen, F. Gong and G. Chen, Kidney Int., 2014, 86, 525–537 CrossRef CAS PubMed.
- M. Bilban, A. Haschemi, B. Wegiel, B. Y. Chin, O. Wagner and L. E. Otterbein, J. Mol. Med., 2008, 86, 267–279 CrossRef CAS PubMed.
- S. Brouard, L. E. Otterbein, J. Anrather, E. Tobiasch, F. H. Bach, A. M. Choi and M. P. Soares, J. Exp. Med., 2000, 192, 1015–1026 CrossRef CAS PubMed.
- N. Hill-Kapturczak and A. Agarwal, Am. J. Physiol. Renal. Physiol., 2006, 290, F787–F788 CrossRef CAS PubMed.
- S. W. Ryter, J. Alam and A. M. Choi, Physiol. Rev., 2006, 86, 583–650 CrossRef CAS PubMed.
- J. J. Freiberger, CO as a Stimulant for Mitochondrial Biogenesis in Human Cardiac Muscle, ClinicalTrials.gov Bethesda (MD): National Library of Medicine, US, 2012, https://clinicaltrials.gov/ct2/show/NCT01727167 Search PubMed.
- Mallinckrodt Pharmaceuticals, Safety and Tolerability Study of Inhaled Carbon Monoxide in Kidney Transplant Patients, ClinicalTrials.gov Bethesda (MD): National Library of Medicine, US, 2007, https://clinicaltrials.gov/ct2/show/NCT00531856 Search PubMed.
- E. Bathoorn, D. J. Slebos, D. S. Postma, G. H. Koeter, A. J. van Oosterhout, M. van der Toorn, H. M. Boezen and H. A. Kerstjens, Eur. Respir. J., 2007, 30, 1131–1137 CrossRef CAS PubMed.
- H. H. Usansky and K. Jamil, Dosing regimes and methods of treatment using carbon monoxide, US 2011/0280966, 5/12/2011, 1911.
- S. R. Thom, L. K. Weaver and N. B. Hampson, Am. J. Respir. Crit. Care Med., 2005, 171, 1318 CrossRef PubMed.
- C. Steiger, C. Hermann and L. Meinel, Eur. J. Pharm. Biopharm., 2016 DOI:10.1016/j.ejpb.2016.11.002 , ahead of print.
- A. Vannacci, C. Marzocca, L. Giannini, L. Mazzetti, S. Franchi-Micheli, P. Failli, E. Masini, R. Motterlini and P. F. Mannaioni, Inflammation Res., 2006, 55, S05–S06 CrossRef PubMed.
- Y. Caumartin, J. Stephen, J. P. Deng, D. Lian, Z. Lan, W. Liu, B. Garcia, A. M. Jevnikar, H. Wang, G. Cepinskas and P. P. Luke, Kidney Int., 2011, 79, 1080–1089 CrossRef CAS PubMed.
- A. Sener, K. C. Tran, J. P. Deng, B. Garcia, Z. Lan, W. Liu, T. Sun, J. Arp, M. Salna, P. Acott, G. Cepinskas, A. M. Jevnikar and P. P. Luke, J. Urol., 2013, 190, 772–778 CrossRef CAS PubMed.
- M. D. Pizarro, J. V. Rodriguez, M. E. Mamprin, B. J. Fuller, B. E. Mann, R. Motterlini and E. E. Guibert, Cryobiology, 2009, 58, 248–255 CrossRef CAS PubMed.
- S. H. Heinemann, T. Hoshi, M. Westerhausen and A. Schiller, Chem. Commun., 2014, 50, 3644–3660 RSC.
- R. Motterlini and L. E. Otterbein, Nat. Rev. Drug Discovery, 2010, 9, 728–743 CrossRef CAS PubMed.
- A. C. Kautz, P. C. Kunz and C. Janiak, Dalton Trans., 2016, 45, 18045–18063 RSC.
- J. E. Clark, P. Naughton, S. Shurey, C. J. Green, T. R. Johnson, B. E. Mann, R. Foresti and R. Motterlini, Circ. Res., 2003, 93, e2–e8 CrossRef CAS PubMed.
- M. Vadori, M. Seveso, F. Besenzon, E. Bosio, E. Tognato, F. Fante, M. Boldrin, S. Gavasso, L. Ravarotto, B. E. Mann, P. Simioni, E. Ancona, R. Motterlini and E. Cozzi, Xenotransplantation, 2009, 16, 99–114 CrossRef PubMed.
- I. C. Winburn, K. Gunatunga, R. D. McKernan, R. J. Walker, I. A. Sammut and J. C. Harrison, Basic Clin. Pharmacol. Toxicol., 2012, 111, 31–41 CAS.
- S. Romanski, E. Stamellou, J. T. Jaraba, D. Storz, B. K. Kramer, M. Hafner, S. Amslinger, H. G. Schmalz and B. A. Yard, Free Radical Biol. Med., 2013, 65, 78–88 CrossRef CAS PubMed.
- S. H. Heinemann, T. Hoshi, M. Westerhausen and A. Schiller, Chem. Commun., 2014, 50, 3644–3660 RSC.
- R. Motterlini, P. Sawle, S. Bains, J. Hammad, R. Alberto, R. Foresti and C. J. Green, FASEB J., 2005, 19, 284–286 CAS.
- A. Sandouka, B. J. Fuller, B. E. Mann, C. J. Green, R. Foresti and R. Motterlini, Kidney Int., 2006, 69, 239–247 CrossRef CAS PubMed.
- S. D. Friis, R. H. Taaning, A. T. Lindhardt and T. Skrydstrup, J. Am. Chem. Soc., 2011, 133, 18114–18117 CrossRef CAS PubMed.
- L. A. P. Antony, T. Slanina, P. Sebej, T. Solomek and P. Klan, Org. Lett., 2013, 15, 4552–4555 CrossRef CAS PubMed.
- P. Peng, C. Wang, Z. Shi, V. K. Johns, L. Ma, J. Oyer, A. Copik, R. Igarashi and Y. Liao, Org. Biomol. Chem., 2013, 11, 6671–6674 CAS.
- S. D. Friis, T. Skrydstrup and S. L. Buchwald, Org. Lett., 2014, 16, 4296–4299 CrossRef CAS PubMed.
- J. Zhang, D. M. Ho and R. A. Pascal Jr, J. Am. Chem. Soc., 2001, 123, 10919–10926 CrossRef CAS PubMed.
- X. Ji, C. Zhou, K. Ji, R. E. Aghoghovbia, Z. Pan, V. Chittavong, B. Ke and B. Wang, Angew. Chem., Int. Ed., 2016, 55, 15846–15851 CrossRef CAS PubMed.
- C.-H. Lai, E. Y. Li, K.-Y. Chen, T. J. Chow and P.-T. Chou, J. Chem. Theory Comput., 2006, 2, 1078–1084 CrossRef CAS.
- D. Wang, E. Viennois, K. Ji, K. Damera, A. Draganov, Y. Zheng, C. Dai, D. Merlin and B. Wang, Chem. Commun., 2014, 50, 15890–15893 RSC.
- C. F. H. Allen and J. Van Allan, J. Am. Chem. Soc., 1942, 64, 1260–1267 CrossRef CAS.
- B. Fuchs, J. Chem. Soc. C, 1968, 68–71, 10.1039/j39680000068.
- B. Fuchs and G. Scharf, J. Org. Chem., 1981, 46, 5395–5398 CrossRef CAS.
- R. Vanel, F. Berthiol, B. Bessieres, C. Einhorn and J. Einhorn, Synlett, 2011, 1293–1295 CAS.
- Y.-C. Su, Y.-L. Lo, C.-C. Hwang, L.-F. Wang, M. H. Wu, E.-C. Wang, Y.-M. Wang and T.-P. Wang, Org. Biomol. Chem., 2014, 12, 6624–6633 CAS.
- B. W. Michel, A. R. Lippert and C. J. Chang, J. Am. Chem. Soc., 2012, 134, 15668–15671 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for the synthesis of compounds, supporting figures and schemes, and NMR spectra. See DOI: 10.1039/c7sc01647f |
| This journal is © The Royal Society of Chemistry 2017 |