Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photochromic coenzyme Q derivatives: switching redox potentials with light†
Nadja A.
Simeth
 a,
Andrea C.
Kneuttinger
b,
Reinhard
Sterner
*b and
Burkhard
König
a,
Andrea C.
Kneuttinger
b,
Reinhard
Sterner
*b and
Burkhard
König
 *a
*a
aUniversity of Regensburg, Faculty of Chemistry and Pharmacy, Institute of Organic Chemistry, Universitätsstraße 31, 93053 Regensburg, Germany. E-mail: burkhard.koenig@ur.de; Tel: +49-941-943-4575
bUniversity of Regensburg, Faculty of Biology and Preclinical Medicine, Institute of Biophysics and Physical Biochemistry, Universitätsstraße 31, 93053 Regensburg, Germany. E-mail: reinhard.sterner@ur.de
First published on 20th July 2017
Abstract
Coenzyme Q is an important redox cofactor involved in a variety of cellular processes, and is thus found in several cell compartments. We report a photochromic derivative of coenzyme Q that combines the molecular structures of the redox active cofactor and a photochromic dye. Light irradiation triggers an electronic rearrangement reversibly changing the redox potential. We used this effect to control the intermolecular redox reaction of the photochromic coenzyme Q derivative with dihydropyridine in solution by light irradiation. On mitochondria, the altered redox properties showed an effect on the respiratory chain. The experiments demonstrate that the redox reactions can be initiated inside the system of interest through irradiation with light and the accompanied photoisomerization.
1. Introduction
The concept of employing small, light-responsive molecules to gain spatiotemporal control over biological processes has been known for a long time.1 However, in recent years, the field, now often termed as photopharmacology, expanded rapidly.2–5 Photochromic inhibitors based on azo dyes or bridged diarylethenes (DAEs) were used to modulate enzymatic activities.6–11 Moreover, switchable antibiotics12–14 and molecules, which cause light-induced apoptosis of single cells in tissue15 illustrate the potential of the approach. Using light as an external stimulant adds an extrinsic parameter determining the selectivity of the compound.3 Furthermore, light is non-invasive, easy to apply, and offers an inimitable spatiotemporal control over the process of interest.1 Both azobenzenes and DAEs interconvert upon light irradiation between two structural isomers, which differ strongly in their physical properties, and thus also in their activity towards biomolecules.1,2 DAEs generally show good photoconversion, fatigue resistance, and thermal stability. They are thus frequently used as photochromic tools in biological contexts.1,16While several analogues of DNA bases,17,18 fatty acids, and phospholipids19–23 have been reported, the number of photoswitchable derivatives of enzymatic cofactors such as ATP, NAD(P)H, FAD, CoQ, is small. Recently, Wilson et al. presented a photochromic analogue of pyridoxal-5′-phosphate,24 and furthermore ATP has been derivatized into a light-controllable mimetic.25 However, redox cofactors, a likewise widely spread family of coenzymes, have not been considered so far.26 They are involved in many cellular processes, and therefore found in various cellular compartments. They facilitate e.g. transhydrogenation reactions catalyzed by oxidoreductases by either accepting or providing electrons.27 In general, they act as an activated electron carrier for the oxidation of metabolites in aerobic organisms. An important auxiliary molecule involved in this process is Coenzyme Q (CoQ), also called ubiquinone. The core part of the molecule, the benzoquinone ring, facilitates the molecule to serve as a two-electron carrier – interconverting between a ubiquinone and an ubiquinol form. Due to its large, nonpolar poly-isoprenyl chain, the coenzyme is able to diffuse within lipophilic membranes.28 Besides its function as an auxiliary in metabolism,26 it also acts as an antioxidant,29,30 regulates the physiochemical properties of membranes,31 and modulates the amount of β-integrins on the surface of blood monocytes.32
As most of the cofactors, redox cofactors are not only present in one single cellular compartment, but widely distributed. Thus, targeting them with conventional strategies of medicinal chemistry would cause an uncontrollable impact on all cells of the organism and it would be difficult to regulate the effect in the desired fashion. However, using a photopharmacological strategy, we are able to activate the agent specifically at the favored site for a defined time.
For our CoQ mimetic, we merged a redox-active benzoquinone moiety with two substituted thiophene moieties, building a photochromic DAE. Through irradiation with light, the 6π-system formed by the three parts undergoes an electrocyclic ring-closing reaction. The ring-closure changes color, shape, and electronic properties of the molecule. Most applications of photochromic compounds aim at an altered structure with different physiological effect, but only few studies utilized the different electronic properties of both photoisomeres.24,33 However, we focus on changing the redox potentials of the benzoquinone moiety through electronical changes upon irradiation. This specific mode of action may allow in principle to use one cofactor mimetic to target all related enzymes even if the active site differs, as the molecule's mode of action does not rely on its spatial interaction, but its redox state. Moreover, benzoquinone motifs have been considered as a scaffold by Deng et al. and Katsumura et al. and were investigated regarding their switching behavior.34,35
Light- and electronic-modulated switches have already been a subject of physical–electrochemical as well as spectroscopic studies,36–41 but to the best of our knowledge, have never been considered as a the role of a cofactor in biological context. Thus, we present the first photochromic molecule that has the potential to serve as a redox cofactor.
2. Results and discussion
2.1. Design
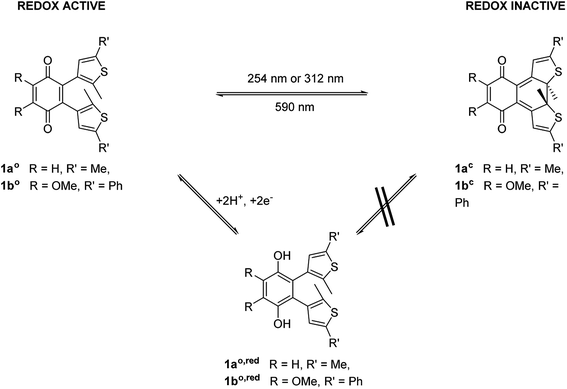
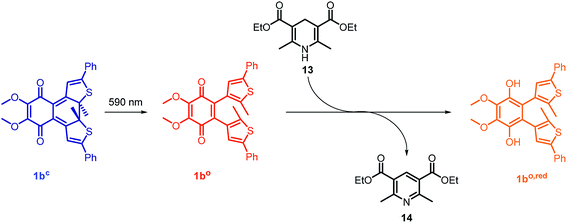
To modulate the redox properties of coenzyme Q by light irradiation, we designed the photochromic derivatives 1ao and 1bo as depicted in Fig. 1 by merging a redox-active benzoquinone with thiophene moieties to build a DAE. This allows the molecule to accept electrons for redox reactions just in one photoisomeric state. Upon irradiation by light of 254 nm, the redox-switch undergoes a 6π-conrotatory electrocyclic ring closure to the closed photoisomer (1ao, 1bo to 1ac, 1bc, Fig. 1). The rearrangement delocalizes one double bond of the redox-active moiety in the conjugated backbone of the closed switch. The “closed photoisomer” should be thus mostly redox-inactive as the quinone system was intercepted. In contrast, the “open form” should be able to undergo oxidation reactions similar to unaltered CoQ (1ao, 1bo to 1ao,red, 1bo,red, Fig. 1). The methoxy groups of the benzoquinone moiety (1bo) are known to be crucial for interaction at the binding site of CoQ-consuming proteins, and are therefore retained in the model compound (Fig. 1).422.2. Synthesis of the photochromic coenzyme Q derivatives
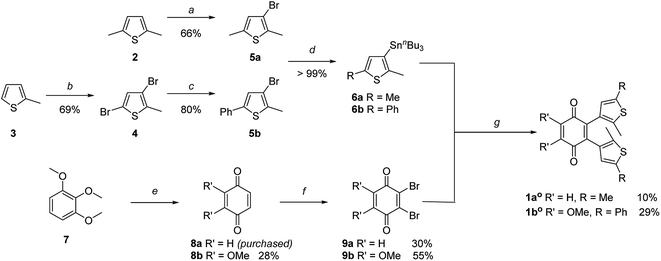
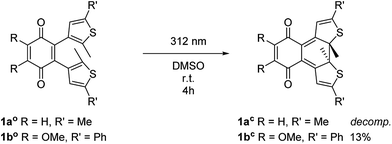
The key step of the synthesis towards compounds 1ao and 1bo was a Stille cross-coupling reaction of 5a or 5b onto quinone 8a or 8b, respectively (Scheme 1). The thiophene precursors (5a and 5b) were easily synthesized from 2,5-dimethyl thiophene (2) or 2-methyl thiophene (3), respectively, partly following known protocols.34,43 After bromination and arylation through Suzuki–Miyaura cross-coupling the pre-functionalized thiophenes 5a and 5b were treated with secBuLi and subsequently SnClBu3 yielding organo stannates 6a and 6b.35 The quinone 8b was synthesized through oxidation of 7 using Fenton's reagent in acetic acid.44 Benzoquinone (8a) as well as 2,3-dimethoxy benzoquinone (8b) were then halogenated utilizing bromine under oxidative conditions.45 The final step was performed employing Pd(PPh3)4 and elevated temperatures for 16 h to give 1ao and 1bo in acceptable yields (10% or 29%, respectively).34The open form of the photochromic quinones (1ao and 1bo) were converted into the corresponding closed isomers (1ac and 1bc) through irradiation with UV light (Scheme 2). Compound 1ao did not tolerate the long-term irradiation and decomposed. However, closed isomer 1bc was isolated from reversed phase column chromatography (13% yield).
2.3. Spectroscopic, electrochemical and spectroelectrochemical studies
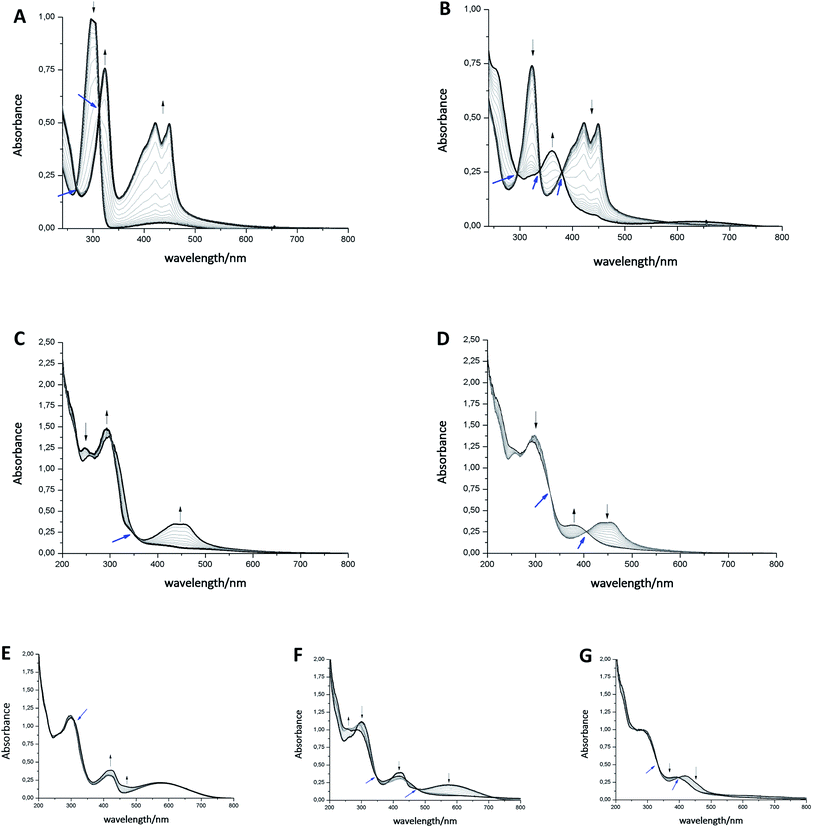
The photoisomerization of compound 1ao and 1bo was investigated in toluene, dimethylsulfoxide (DMSO), dichloromethane (DCM), dioxane and MeCN. Unfortunately, compound 1ao did not tolerate irradiation with UV light (312 nm) and resulted in decomposition into uncharacterized products (for spectra see ESI†). However, compound 1bo switches in polar and non-polar solvents (Fig. 2 and ESI†). Upon irradiation with UV light (312 nm or 254 nm, respectively), the color of the solution changed from bright red to blue. The photoisomerization was monitored by UV/Vis spectroscopy; associated spectra in DMSO are depicted exemplary in Fig. 2 (further spectra are shown in the ESI†). New absorption maxima at 410 nm and 594 nm show the formation of the closed photoisomer; the isosbestic point at 323 nm indicates a distinct two-compound isomerization. Photostationary states (PSSs) were determined by HPLC measurements (ESI†). The PSS for the closing reaction was determined to be 52% in DMSO, whereas the opening reaction was achieved quantitatively through irradiation with orange light (590 nm, 30 μM, 2 min). An intramolecular electron transfer (TICT) as reported for other DAE derivatives bearing an electron-acceptor core moiety rationalizes the generally rather low PSS for the closing reaction.46,47 Thus, we focused on the opening reaction for further experiments, which generates a redox active compound from a redox silent molecule upon irradiation.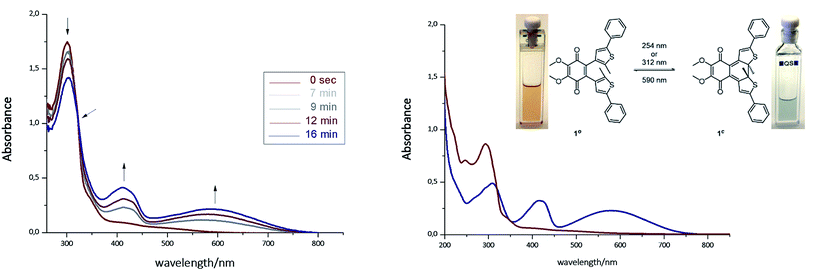 | ||
| Fig. 2 Left: Photoisomerization of 1bo in DMSO (30 μM, irradiation with 312 nm). Right: Spectra of isolated photoisomers 1bo (red line, maxima at 247 nm and 297 nm) and 1bc (blue line, maxima at 307 nm, 415 nm and 583 nm) in MeCN (isosbestic point at 317 nm). For further details see ESI.† | ||
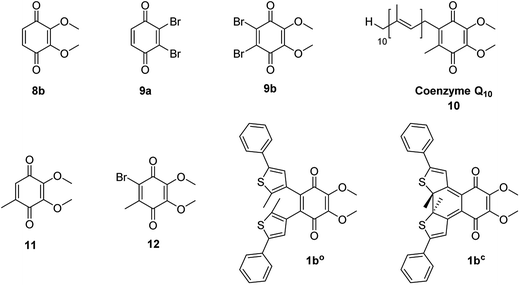
To determine the redox potential of CoQ derivatives 1bo and 1bc, we performed cyclic voltammetric (CV) studies. The compounds included in the study are depicted in Chart 1. Compound 12 was hereby synthesized from commercially available 11 similar to 9b in one step. All 1,4-benzoquinone derivatives (9b, 10: Fig. 3C and D; 8b, 9a, 11, 12, ESI†) including the open photochromic compound 1bo (Fig. 3A) show similar behavior as CoQ10 (Fig. 3D). Specifically, all compounds exhibit two well-defined, fully reversible reduction signals. This finding is in agreement with previous investigations on benzoquinones. The first step corresponds to the formation of a semiquinone radical, while the second step corresponds to the formation of a dianion (or hydroquinone if protons are present).48 The potentials of the molecules are predestined by a substitution pattern of the respective compound and are summarized in Table 1. The substituents of the benzoquinone core affect the reduction potential values. With an increasing number of electron withdrawing bromine substituents the reductions occur at less negative potentials. In contrast to all other investigated para-benzoquinones, the closed isomer 1bc generates three – not two – cathodic waves as well as one anodic one (Fig. 3B), which are reproducible (cf. ESI†). Also, the intensities of the respective waves differ, and hence indicate that redox steps involve a different number of electrons.
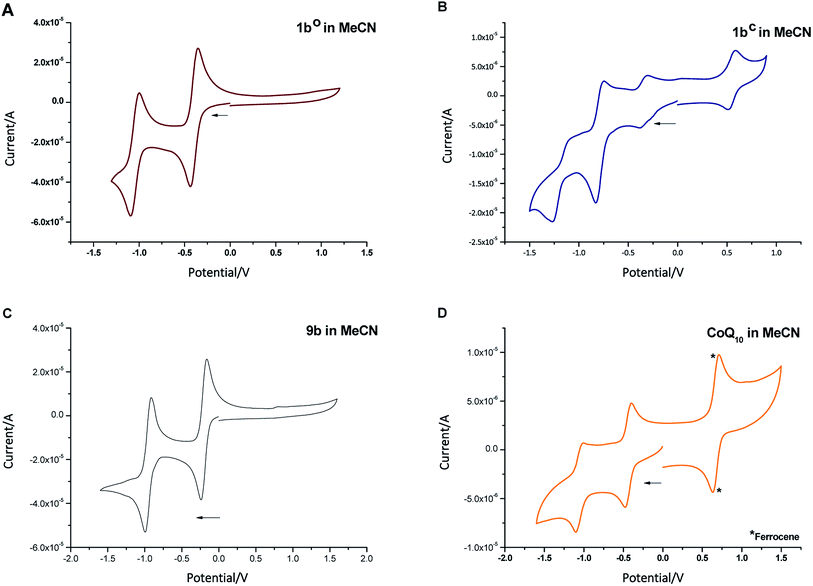 | ||
| Fig. 3 Cyclic voltammogram of compounds 1bo ((A) 30 μM), 1bc ((B) 2 μM), 9b ((C) 30 μM), and CoQ10 ((D) 2 μM, *ferrocene (0.005 M) as internal standard) in MeCN + 0.1 M Bu4NBF4. | ||
| Compound | Potentials vs. SCN49 |
|---|---|
| 1bo | −0.58; −1.23 |
| 1bc | +0.83; −0.24; −0.55; −1.39 |
| 8b | −0.66; −1.37 |
| 9a | −0.24; −0.97 |
| 9b | −0.35; −1.1 |
| CoQ10 (10) | −0.77; −1.39 |
| 11 | −0.69; −1.34 |
| 12 | −0.54; −1.27 |
Next, we investigated the redox states by spectroelectrochemistry, using several different 1,4-benzoquinone derivatives for comparison (Chart 1).
Exemplary, changes of the UV/Vis spectra of compounds 1bo, 1bc, and 9b during one cathodic potential sweep are depicted in Fig. 4 (difference spectra of 1bo, 1bc, and 9b as well as UV/Vis spectra of 9a, 10, 11 and 12 can be found in ESI†). All para-benzoquinones – besides the closed photoswitch 1bc – show similar absorption bands with the changing potential; without applying an external potential, the benzoquinones show an absorption band at around 280–300 nm depending on their substitution pattern. During the first reduction step corresponding to the formation of the semiquinone intermediate,48 this band decreases to give rise to two new bands – one at around 310 nm and a broader one at around 410 nm exhibiting a well-defined double maximum, which is also present in the case of 1bo and CoQ10, but not so well-defined.50 For CoQ10, this might be due to solubility issues in MeCN. The second cathodic wave is the formation of the dianion,48 generating a novel band at around 370 nm, whereas the two bands of the semiquinone radical decrease again. All spectra show clear isosbestic points indicating a distinct two-compound process. The reduction process is fully reversible and can be repeated over several cycles.
In contrast to the other investigated benzoquinones, the closed photoswitch 1bc showed a slightly different spectrum in the beginning due to the attached chromophore. The molecule exhibits maxima at 307 nm, 415 nm, and 583 nm. During the first reduction step, the maximum at 415 nm broadens, while the other maxima remain unchanged. Changes are more drastic as the second reduction occurs; the long-wave band around 580 nm fully decreases, the band at around 410 nm broadens even more and the short-wave band at around 300 nm experiences a hypsochromic shift. While the last reduction step takes place, the band around 410 nm is also hypsochromically shifted.
Although there are similarities to the non-photochromic benzoquinones and to 1bo, the spectroelectrochemical studies clearly reveals a different behavior of 1bc. Especially, the well-pronounced decrease of the long-wave band around 580 nm, which can be assigned to the conjugated thiophene backbone in the chromophore of the photoswitch,16 indicates a redox process in this moiety of the molecule. However, this appears rather counterintuitive, as though impaired the benzoquinone moiety still should be more prompt to reduction. Yet very recently, Saito et al. report EPR and computational studies on a related molecule and could show the formation of a radical anion similar to a semiquinone.51 A charge delocalization over the whole molecule is likely to be allowed through two nearly degenerated LUMO and LUMO+1. Thus, we suggest that the second reductive wave also has semiquinone character but delocalized over the whole molecule (loss of long-wave band around 580 nm, which is associated with the closed ring in DAEs). The first and the third reduction potential show less prominent changes in UV/Vis and are yet to be assigned.
Overall, the cyclovoltammetric and spectroelectrochemical studies revealed a different redox behavior for both photoisomers of 1b. Spectroscopic investigations show that 1bo and 1bc can be in fact interconverted into each another through light, but the PSS values of the closing reaction in all investigated solvents (see ESI†) is rather low (maximum 52% in DMSO). In addition, the time required for the opening and the closing reaction are in the minute-range. However, both characteristics are crucial for many redox mediated reactions, especially in biological systems as e.g. enzymatic reactions take place in the milli-second range or even faster.52 Therefore, compound 1b can only be considered for a limited set of experiments up to now. The investigated redox reactions should be slow or independent of diffusion of the cofactor mimetic during irradiation. The quantitative opening reaction can be used to provide a redox partner within a system upon irradiation similar to caged compounds. However, the reverse process would not be quantitative. Considering these properties, we investigated the applicability of the redox switch as a tool in reactions.
 | ||
| Fig. 5 Photoactivated redox reaction between photochromic CoQ derivative 1bo and an NADH model Hantzsch ester 13. Compound 1bo is generated in situ from closed isomer 1bcvia irradiation. | ||
2.4. Photoactivated oxidation reactions
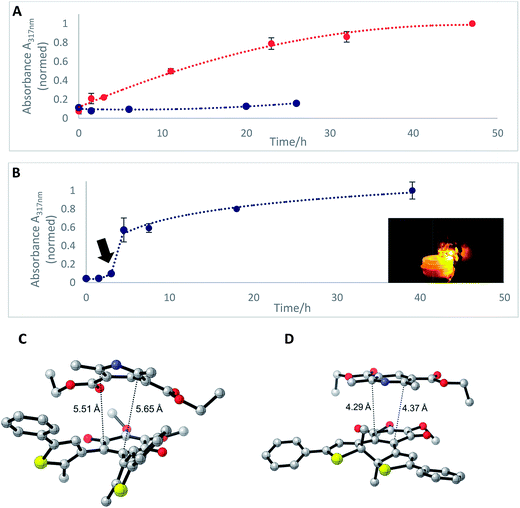
In the mitochondrial respiratory chain, CoQn oxidizes NADH to NAD+ in complex I (NADH-Q oxidoreductase).26 Thus, we decided to let our photochromic CoQ mimetic 1bo react with Hantzsch ester 13, which belongs to the same family of dihydropyridines as NADH, and hence serves as an excellent test substrate for a photoactivated oxidation reaction (Fig. 5).A solution of each photoisomer (5 mM, 1bo, 1bc) was treated with a solution of Hantzsch ester 13 (12 mM) in DMSO and reacted at ambient temperature in the dark. We took samples in certain time intervals and analyzed them through analytical HPLC (UV/Vis detection at 317 nm which equals PSS, HPLC traces see ESI†) to follow the consumption and the generation of the respective redox pairs. The formation of 1bo,red from 1bo and 1bc, respectively, through reduction with 13 is depicted in Fig. 6A (normed areas of HPLC traces). The open photoisomer 1bo is rapidly reduced by Hantzsch ester 13 (red line, Fig. 6A), whereas the closed one 1bc shows almost no conversion to 1bo,red or any other product (blue, Fig. 6B, minor background reaction results from thermal ring-opening). Thus, compound 1bc is unreactive before irradiation.
Then, we investigated whether the formation of 1bo,red can be triggered through light-activation of 1bc. The closed isomer (10 mM, DMSO) was treated with Hantzsch ester 13 (12 mM) and left stirring in the dark for three hours. Subsequently, the solution was irradiated with a 590 nm single-spot LED for 10 min (picture, Fig. 6) to be quantitatively converted into 1bo. HPLC analysis confirmed a full conversion into only the open photoisomer and also an immediately initialized reduction into 1bo,red (Fig. 6B, for HPLC traces see ESI†). The initial slope of the reaction seems to be larger than utilizing 1bo. This could be due to a pre-aggregation of 1bc and the Hantzsch ester. A preliminary computational analysis was carried out in order to unravel the presence of an interaction between both photoisomers and Hantzsch ester 13. The DFT method at the B3LYP/6-31G(d) level of theory (cf. ESI†).53–56 We decided to follow a modification of an approach already found in the literature57 to gain qualitative insight on the presence of a non-covalent π stacking between the two photoisomers of 1b and 13.
From an energetic point of view, the closed form appeared to be more stable (ca. 7 kcal mol−1), cf. ESI† due to the interaction with 13. Moreover, the geometry of the optimized structures clearly shows the Hantzsch ester resides in closer proximity to 1bo than to 1bc (see Fig. 6C and D).
In summary, we can conclude that the intermolecular reaction does relay on the respective redox potentials of the compounds indicating that we are truly able to change redox potentials of 1b by photo-induced ring-opening of the electronically caged closed photoisomer.
2.5. Photoactivated mitochondrial reduction of 2,6-dichlorophenol indophenol
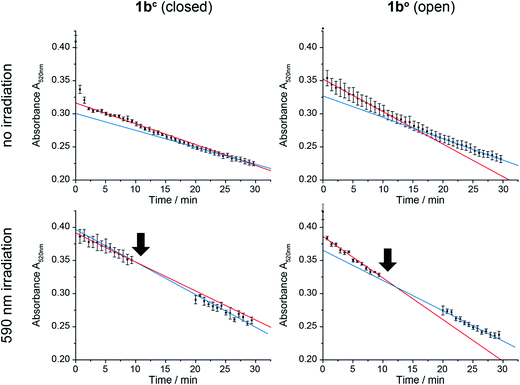
In order to test the effect of 1bc and 1bo on a natural system, mitochondria were isolated from the wildtype yeast strain BY4742.58 CoQn serves as electron transmitter in the respiratory chain of mitochondria from complex II (succinate dehydrogenase) to complex III (cytochrome-C oxidoreductase). By using 2,6-dichlorophenol indophenol (DCIP) as electron acceptor instead of Complex III, CoQ analogues can be tested on isolated mitochondria.59 The reduction of DCIP with 1bc and 1bo was followed spectrophotometrically (Fig. 7), continuously for 30 min or interrupted by irradiation with 590 nm, in order to switch the closed 1bc into the open, more active 1bo isomer.For both isomers of our CoQ mimetic 1b, reduction of DCIP could be observed in the continuous assay (Fig. 7, no irradiation) where a fast initial rate (red) was followed by a lower rate (blue). Comparing the initial rate of DCIP reduction with 1bc and 1bo, the open isomer is only approximately 70% more active than the closed isomer. We assume that this seemingly high activity of the closed isomer is due to residual activity of bound natural CoQn, which we cannot exclude in isolated mitochondria.
To emphasize the activity difference of 1bc and 1bo, we irradiated both isomers after 10 min reaction time (Fig. 7, 590 nm irradiation). The activity for the open isomer decreased as in the non-irradiated assay (blue slope < red slope). However, the rate for the closed isomer after irradiation increased (blue slope > red slope) indicating that the reaction was restarted.
Thus, despite the complex system of mitochondria, in which CoQ is deployed in several reactions, we were able to see an activation of the DCIP reduction after irradiation of 1bc. Consequently, the photochromic CoQ mimetic 1b cannot only replace CoQn in a natural system, but can also activate the same after irradiation.
3. Conclusion
In conclusion, we have developed a photochromic CoQ derivative (1b) combining a redox active and a photochromic moiety in one molecule. We could show by cyclic voltammetry that the redox potential of both photoisomers differs, and thus can be altered through photoisomerization. In addition, the mode of action of cathodic waves in spectroelectrochemical studies was examined indicating the nature of the reductive steps. Furthermore, we could show that the closed form of the switch (1bc) is redox inactive in a reaction with Hantzsch ester, a member of the dihydropyridine family, and has to be photoactivated to its open isomer (1bo) to be redox active. This proves that the reactivity of 1b relies on the altered redox potentials of the two photoisomers, and is thus controllable through electronic changes rather than by conformational and geometry changes as seen in most other studies. Finally, we were able to show that treatment of isolated mitochondria with both photoisomers led to a difference of approximately 70% of the velocity of DCIP reduction. We could further show that the closed isomer 1bc can restart the DCIP reduction upon activation through irradiation. Thus, we present the first photochromic redox cofactor that operates in a complex, biological context. The photochromic compound converts upon irradiation from the formally caged closed form of the mimetic into an active redox probe inside the system. However, reversibility of the redox reaction could not yet be achieved due to low PSS values and insufficiently fast reaction rates. Optimization of both issues should be addressed in further research to finally obtain a tool facilitating the control of redox-dependent biological functions in cells by light.Acknowledgements
We thank Regina Hoheisel for CV measurements, Antonín Králík and Morten Grøtli for fruitful discussions, and Stefano L. Crespi for assistance regarding computational studies. We thank Lisa-Marie Altmann and Lorena Oegl for technical assistance. NAS thanks the Studienstiftung des Deutschen Volkes for a doctoral scholarship.References
- C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed.
- W. Szymański, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema and B. L. Feringa, Chem. Rev., 2013, 113, 6114–6178 CrossRef PubMed.
- W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed.
- M. M. Lerch, M. J. Hansen, G. M. van Dam, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2016, 55, 10978–10999 CrossRef CAS PubMed.
- D. Wilson and N. R. Branda, in Photochromic Materials, ed. H. Tian and J. Zhang, Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim, 2016, ch. 9, pp. 361–391 Search PubMed.
- P. R. Westmark, J. P. Kelly and B. D. Smith, J. Am. Chem. Soc., 1993, 115, 3416–3419 CrossRef CAS.
- C. Falenczyk, M. Schiedel, B. Karaman, T. Rumpf, N. Kuzmanovic, M. Grotli, W. Sippl, M. Jung and B. Konig, Chem. Sci., 2014, 5, 4794–4799 RSC.
- D. Vomasta, C. Högner, N. R. Branda and B. König, Angew. Chem., Int. Ed., 2008, 47, 7644–7647 CrossRef CAS PubMed.
- J. B. Trads, J. Burgstaller, L. Laprell, D. B. Konrad, L. de la Osa de la Rosa, C. D. Weaver, H. Baier, D. Trauner and D. M. Barber, Org. Biomol. Chem., 2017, 15, 76–81 CAS.
- B. Reisinger, N. Kuzmanovic, P. Löffler, R. Merkl, B. König and R. Sterner, Angew. Chem., Int. Ed., 2014, 53, 595–598 CrossRef CAS PubMed.
- W. Szymanski, M. E. Ourailidou, W. A. Velema, F. J. Dekker and B. L. Feringa, Chem.–Eur. J., 2015, 21, 16517–16524 CrossRef CAS PubMed.
- L. Lien, D. C. J. Jaikaran, Z. Zhang and G. A. Woolley, J. Am. Chem. Soc., 1996, 118, 12222–12223 CrossRef CAS.
- O. Babii, S. Afonin, M. Berditsch, S. Reiβer, P. K. Mykhailiuk, V. S. Kubyshkin, T. Steinbrecher, A. S. Ulrich and I. V. Komarov, Angew. Chem., Int. Ed., 2014, 53, 3392–3395 CrossRef CAS PubMed.
- W. A. Velema, J. P. van der Berg, M. J. Hansen, W. Szymanski, A. J. M. Driessen and B. L. Feringa, Nat. Chem., 2013, 5, 924–928 CrossRef CAS PubMed.
- M. Borowiak, W. Nahaboo, M. Reynders, K. Nekolla, P. Jalinot, J. Hasserodt, M. Rehberg, M. Delattre, S. Zahler, A. Vollmar, D. Trauner and O. Thorn-Seshold, Cell, 2015, 162, 403–411 CrossRef CAS PubMed.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- H. Cahová and A. Jäschke, Angew. Chem., Int. Ed., 2013, 52, 3186–3190 CrossRef PubMed.
- M. Singer and A. Jäschke, J. Am. Chem. Soc., 2010, 132, 8372–8377 CrossRef CAS PubMed.
- C. G. Morgan, E. W. Thomas, Y. P. Yianni and S. S. Sandhu, Biochim. Biophys. Acta, Biomembr., 1985, 820, 107–114 CrossRef CAS.
- J. A. Frank, M. Moroni, R. Moshourab, M. Sumser, G. R. Lewin and D. Trauner, Nat. Commun., 2015, 6, 7118 CrossRef PubMed.
- J. A. Frank, D. A. Yushchenko, D. J. Hodson, N. Lipstein, J. Nagpal, G. A. Rutter, J.-S. Rhee, A. Gottschalk, N. Brose, C. Schultz and D. Trauner, Nat. Chem. Biol., 2016, 12, 755–762 CrossRef CAS PubMed.
- C. G. Morgan, Y. P. Yianni, S. S. Sandhu and A. C. Mitchell, Photochem. Photobiol., 1995, 62, 24–29 CrossRef CAS PubMed.
- J. A. Frank, H. G. Franquelim, P. Schwille and D. Trauner, J. Am. Chem. Soc., 2016, 138, 12981–12986 CrossRef CAS PubMed.
- D. Wilson and N. R. Branda, Angew. Chem., Int. Ed., 2012, 51, 5431–5434 CrossRef CAS PubMed.
- T. Kamei, T. Fukaminato and N. Tamaoki, Chem. Commun., 2012, 48, 7625–7627 RSC.
- J. M. Berg, J. L. Tymoczko and L. Stryer, Biochemistry, W.H. Freeman, New York, 2002 Search PubMed.
- C. Cantó and J. Auwerx, Cold Spring Harbor Symp. Quant. Biol., 2011, 76, 291–298 CrossRef PubMed.
- G. Lenaz and M. L. Genova, Biochim. Biophys. Acta, Bioenerg., 2009, 1787, 563–573 CrossRef CAS PubMed.
- M. Bentinger, K. Brismar and G. Dallner, Mitochondrion, 2007, 7(suppl.), S41–S50 CrossRef CAS PubMed.
- R. E. Beyer, J. Bioenerg. Biomembr., 1994, 26, 349–358 CrossRef CAS PubMed.
- B. A. Cornell, M. A. Keniry, A. Post, R. N. Robertson, L. E. Weir and P. W. Westerman, Biochemistry, 1987, 26, 7702–7707 CrossRef CAS PubMed.
- M. Turunen, L. Wehlin, M. Sjöberg, J. Lundahl, G. Dallner, K. Brismar and P. J. Sindelar, Biochem. Biophys. Res. Commun., 2002, 296, 255–260 CrossRef CAS PubMed.
- R. Göstl and S. Hecht, Angew. Chem., Int. Ed., 2014, 53, 8784–8787 CrossRef PubMed.
- X. Deng and L. S. Liebeskind, J. Am. Chem. Soc., 2001, 123, 7703–7704 CrossRef CAS PubMed.
- S. Katsumura, S. Yoshida, H. Kubo and T. Saiga, Jp. Pat., JP09077743A, 1997.
- N. Vilà, G. Royal, F. Loiseau and A. Deronzier, Inorg. Chem., 2011, 50, 10581–10591 CrossRef PubMed.
- V. Goulle, A. Harriman and J.-M. Lehn, J. Chem. Soc., Chem. Commun., 1993, 1034–1036 RSC.
- R. S. Sánchez, R. Gras-Charles, J. L. Bourdelande, G. Guirado and J. Hernando, J. Phys. Chem. C, 2012, 116, 7164–7172 Search PubMed.
- A. Goulet-Hanssens, M. Utecht, D. Mutruc, E. Titov, J. Schwarz, L. Grubert, D. Bléger, P. Saalfrank and S. Hecht, J. Am. Chem. Soc., 2017, 139, 335–341 CrossRef CAS PubMed.
- U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 15966–15967 CrossRef CAS PubMed.
- M. Herder, M. Utecht, N. Manicke, L. Grubert, M. Patzel, P. Saalfrank and S. Hecht, Chem. Sci., 2013, 4, 1028–1040 RSC.
- X. Gao, X. Wen, L. Esser, B. Quinn, L. Yu, C.-A. Yu and D. Xia, Biochemistry, 2003, 42, 9067–9080 CrossRef CAS PubMed.
- J. Ma, X. Cui, F. Wang, X. Wu, J. Zhao and X. Li, J. Org. Chem., 2014, 79, 10855–10866 CrossRef CAS PubMed.
- M. Matsumoto and H. Kobayashi, J. Org. Chem., 1985, 50, 1766–1768 CrossRef CAS.
- D. Yu and D. L. Mattern, Synth. Commun., 1999, 29, 821–825 CrossRef CAS.
- T. Yamaguchi, K. Uchida and M. Irie, J. Am. Chem. Soc., 1997, 119, 6066–6071 CrossRef CAS.
- T. Yamaguchi and M. Irie, Chem. Lett., 2004, 33, 1398–1399 CrossRef CAS.
- P. S. Guin, S. Das and P. C. Mandal, Int. J. Electrochem., 2011, 2011, 816222 Search PubMed.
- V. V. Pavlishchuk and A. W. Addison, Inorg. Chim. Acta, 2000, 298, 97–102 CrossRef CAS.
- Y.-B. Shim and S.-M. Park, J. Electroanal. Chem., 1997, 425, 201–207 CrossRef CAS.
- E. Saito, T. Ako, Y. Kobori and A. Tsuda, RSC Adv., 2017, 7, 2403–2406 RSC.
- W. W. Chen, M. Niepel and P. K. Sorger, Genes Dev., 2010, 24, 1861–1875 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- P. J. Stephens, J. F. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- B. Kuhn and P. A. Kollman, J. Am. Chem. Soc., 2000, 122, 3909–3916 CrossRef CAS.
- C. Gregg, P. Kyryakov and V. I. Titorenko, J. Visualized Exp., 2009, 30, e1417.
- C. A. Reddy, T. J. Beveridge, J. A. Breznak, G. A. Marzluf, T. M. Schmidt and L. R. Snyder, Methods for General and Molecular Microbiology, American Society of Microbiology, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data are available. CCDC 1533545. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc00781g |
| This journal is © The Royal Society of Chemistry 2017 |