Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation and properties of room temperature vulcanized silicone rubber based on rosin-grafted polydimethylsiloxane†
Qiaoguang Lia,
Xujuan Huanga,
He Liu *a,
Shibin Shang
*a,
Shibin Shang *ab,
Zhanqian Song*ab and
Jie Songc
*ab,
Zhanqian Song*ab and
Jie Songc
aInstitute of Chemical Industry of Forestry Products, Chinese Academy of Forestry, Key Laboratory of Biomass Energy and Material, National Engineering Laboratory for Biomass Chemical Utilization, Key and Laboratory on Forest Chemical Engineering, State Forestry Administration, Nanjing 210042, Jiangsu Province, China. E-mail: liuheicifp@caf.ac.cn; Fax: +86-25-85482499; Tel: +86-25-85482452
bInstitute of New Technology of Forestry, Chinese Academy of Forestry, Beijing 100091, China. E-mail: shangsb@hotmail.com; songzq@hotmail.com
cDepartment of Chemistry and Biochemistry, University of Michigan-Flint, Flint, Michigan 48502, USA
First published on 19th April 2018
Abstract
Rosin-grafted polydimethylsiloxane (RGSO) was prepared via ring-opening reaction of glycidyl ester of rosin acid (ER) with hydroxy-terminated amino polydimethylsiloxane (PDMS). The structure of RGSO was confirmed by 1H and 13C NMR spectroscopy. The effects of ER on relative molecular weight and rheological properties of RGSO were studied by gel permeation chromatography and rotational rheometry. Then, room temperature vulcanized (RTV) silicone rubber modified with rosin was prepared using RGSO, hydroxy-terminated PDMS, tetraethoxysilane, and organotin catalyst. The structures and properties of RTV silicone rubbers were studied by scanning electron microscopy, thermogravimetric analysis, a universal testing machine and dynamic mechanical analysis. The rosin-modified silicone rubber showed remarkably improved thermal and mechanical properties. Temperatures corresponding to 10% weight loss and maximum rate of weight loss increased by 66 °C and 177 °C, respectively. Moreover, the tensile strength and elongation at break increased by 138% and 113%. The role of rosin structure in improvement of properties is discussed.
Introduction
Silicone rubber has gained a lot of attention, due to its unique physical and chemical properties and its special structure, consisting of alternating Si–O backbone chains with organic side chains.1 The unique physical and chemical properties of silicone rubber include: remarkable heat resistance, ozone resistance, weather resistance, etc.2 Because of these merits, silicone rubber has important roles in the construction, healthcare, and electronics industries.3 However, the relatively low mechanical strength of silicone rubber, due to low cohesion energy density, affects its practical applications.4,5 Physical or chemical modification is carried out by adding fillers or hard phases in order to improve the mechanical properties of silicone rubber.6Chemical modification, such as copolymerization, crosslinking, or grafting, is an effective way to significantly alter, optimize, and improve the properties of polymers.1 Liu et al. suggested that grafting of room temperature vulcanized (RTV) silicone rubber with polyhedral oligomeric silsesquioxanes (POSS) to obtain RTV silicone rubber-g-POSS can significantly enhance the thermal stability, because of chemical incorporation of POSS into polydimethylsiloxane (PDMS) chains.7 Meng et al. reported improvement in the thermal stability of poly(methylvinylsiloxane) and found that the grafted POSS inhibited the rate of thermal degradation of silicone rubber.8 Not only POSS with their combined inorganic–organic properties, but also rigid cyclic aliphatic or aromatic petrochemical products have been used as hard phases to modify silicone rubber by chemical bonding. Ardhyananta et al. prepared three types of polybenzoxazine–polysiloxane hybrids and inferred that the pendant group of polysiloxanes strongly affects and controls the thermal and mechanical properties.9 Zhang et al. reported PDMS-g-azobenzene dielectric elastomers with remarkably enhanced dielectric constants and high dielectric strengths, owing to the high dipole moments of azobenzenes.10 Magennis et al. devised a simple method to prepare silicone rubber-g-acrylates.11 Deshpande et al. researched the thermal degradation behaviors of PDMS, poly(dimethyldiphenylsiloxane) and poly(dimethyldiphenyl)siloxane copolymers and reported that the activation energies decreased on incorporation of phenyl groups.12,13 In recent years, biopolymers, based on renewable biomass feedstock, such as lignin, cellulose and rosin, have attracted more attention, due to the decline of fossil reserves and serious environmental pollution.14–17
Rosin, which is an abundantly and widely distributed renewable natural resin, is composed of 90% resin acid and 10% neutral compounds.18,19 Owing to its unique structure, which includes a large hydrogenated phenanthrene ring, rosin is similar to cyclic aliphatic or aromatic compounds in terms of molecular rigidity.20 On account of a carboxylic acid group and a conjugated carbon–carbon double bond as active functional groups, rosin acid can be modified through esterification, Diels–Alder addition, and amidation reactions.20,21 The obtained rosin-based products, which may serve as alternatives to petroleum-based cyclic aliphatic or aromatic monomers, can be introduced into polymers, such as epoxy resins and polyurethanes, and silicone rubber to impart excellent performance.18,22,23 Deng et al. prepared a series of novel rosin-based siloxane epoxy resins with better mechanical and thermal properties by increasing the cross-linking density and forming a protective residue.24 Xu et al. prepared a series of novel rosin-based, waterborne polyurethanes with good mechanical properties, thermal stability, and water resistance with a great potential for applications.25 In our previous study, a series of high temperature vulcanized silicone rubber containing side-chain rosin and its derivatives were prepared.22,26,27 The incorporation of rosin and its derivatives into silicone rubber systems remarkably improves the mechanical and thermal properties. However, to the best of our knowledge, modified RTV silicone rubber derivatized with rosin has seldom been studied.
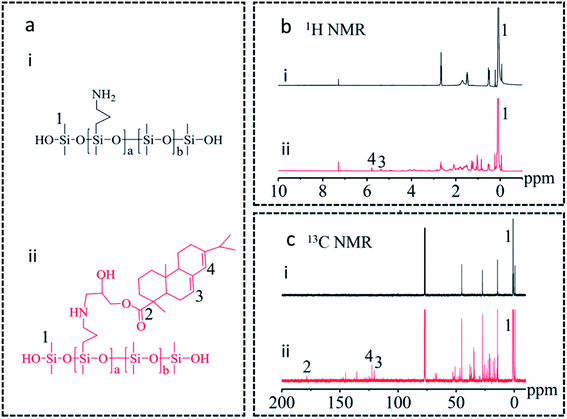
In this work, rosin-grafted polydimethylsiloxane (RGSO) was prepared via ring-opening reaction of epoxy and amino groups under relatively mild conditions (Fig. 1(iii)). The effects of glycidyl ester of rosin acid (ER) on the relative molecular weight and rheological properties of RGSO were investigated. PDMS was grafted with rosin, followed by condensation reaction with PDMS matrix, to obtain rosin-modified RTV silicone rubber (Fig. 1(iv)). The effects of ER on the morphology, thermal properties, mechanical properties, and dynamic mechanical properties of RTV silicone rubber were all explored.
Materials and methods
Materials
Purified rosin (ca. ≧ 95%) was supplied by Hunan Pine Forest Technologies Co. Ltd. Hydroxy-terminated PDMS (5000 MPa s) was purchased from Hubei New Universal Chemical Co. Ltd. Octamethylcyclotetrasiloxane (D4) and 3-aminopropyl(diethoxy)methylsilane were purchased from Wanda Chemical Co. Ltd. Tetraethoxysilane (TEOS), potassium hydroxide (KOH), toluene, sodium hydroxide, calcium oxide, acetic acid, celite, epichlorohydrin, benzyltriethylammonium chloride and dibutyltin dilaurate were supplied by Aladdin Chemical Reagent Co. Ltd. All chemicals were used without further purification.Synthesis of ER
ER with an epoxide equivalent weight of 352 g mol−1 (theoretical 358 g mol−1) was prepared by the reaction of rosin and epichlorohydrin, according to a literature procedure.28 Rosin (50.0 g), epichlorohydrin (157.3 g) and benzyltriethylammonium chloride (0.381 g) were charged into a 500 mL flask. The mixture was maintained at 117 °C for 2 h under nitrogen atmosphere and then cooled to 60 °C. Sodium hydroxide (6.62 g) and calcium oxide (9.272 g) were added to the flask and the mixture was held at around 60 °C for another 3 h. The mixture was filtered through celite and filter paper. The target product was obtained after distillation of the filtrate under vacuum at 100 °C to remove excess epichlorohydrin (Fig. 1(i)).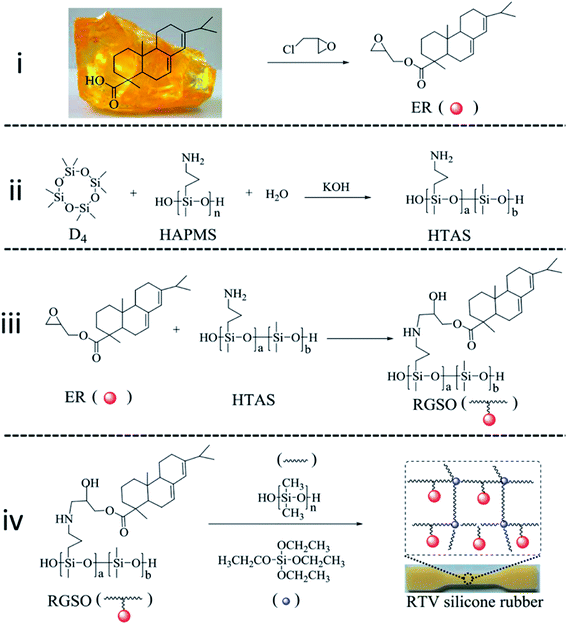 | ||
| Fig. 1 Route for the synthesis of RTV silicone rubber. (i) Synthetic route for ER. (ii) Synthetic route for HTAS. (iii) Synthetic route for RGSO. (iv) Synthetic route for RTV silicone rubber. | ||
Synthesis of hydrolyzate of 3-aminopropyl(diethoxy)methylsilane (HAPMS)
HAPMS was obtained by hydrolyzation of 3-aminopropyl(diethoxy)methylsilane, according to a literature procedure.22 A flask was filled with equal volumes of water and 3-aminopropyl(diethoxy)methylsilane. The mixture was maintained at 80 °C for 4 h. The target product was obtained after removal of excess water under vacuum at 100 °C.Synthesis of hydroxy-terminated amino polydimethylsiloxane (HTAS)
A 1000 mL flask, equipped with a stirrer, thermometer and condenser, was charged with HAPMS (50 g), D4 (450 g), KOH aqueous solution (10 wt%, 2000 μL) and a certain amount of water under nitrogen atmosphere. The mixture was heated at 140 °C for 6 h, after which it turned into a transparent and viscous liquid. After the mixture was cooled to 60 °C, aqueous acetic acid (10 wt%, 2143 μL) was added. The volatiles were removed under vacuum at high temperature. Finally, HTAS (450 g, 90% yield, 5000 MPa s, Fig. 1(ii)) with an amino content of 0.84 mmol g−1 (theoretical 0.85 mmol g−1) was obtained.Preparation of RGSO
HTAS (10 g) and ER (1.5 g, 3 g, 4.5 g or 6 g) were charged into a flask equipped with a stirrer, inert gas inlet, thermometer, and reflux condenser, and the mixture was heated at 80 °C for about 1 h. The molar ratios of the amino groups and epoxide groups were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively (Table S3†). The RGSO products obtained were designated as RGSO-1, RGSO-2, RGSO-3, and RGSO-4, respectively (Fig. 1(iii)).
2, respectively (Table S3†). The RGSO products obtained were designated as RGSO-1, RGSO-2, RGSO-3, and RGSO-4, respectively (Fig. 1(iii)).
Preparation of modified RTV silicone rubber
To a flask were added the products RGSO-1, RGSO-2, RGSO-3, or RGSO-4, obtained as described above, and PDMS (20 g) under vigorous stirring and dry nitrogen. Then the crosslinking agent TEOS (4.5 g) and dibutyltin dilaurate catalyst (100 μL) were added and mixed homogeneously. Finally, the mixture was quickly poured into a mould and cast after removing the air bubbles. The RTV silicone rubber sheets with smooth surfaces were obtained after curing for 7 days at room temperature (Fig. 1(iv)). They were designated correspondingly as SRER-2, SRER-3, SRER-4, and SRER-5. As a reference material, the RTV silicone rubber using PDMS matrix as the primary polymer was also prepared under the same conditions (Table S1, ESI†).Characterizations and measurements
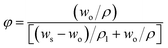 | (1) |
| γe = ρ/MC = −[ln(1 − φ) + φ + X1φ2]/(voφ1/3) | (2) |
In these equations, φ is the volume fraction, wo is the weight of the original sample, ρ is the density of RTV silicone rubber before swelling, ws is the weight of swollen RTV silicone rubber, ρ1 is the density of toluene whose value is 0.87 g cm−3, γe is the crosslinking density, MC is the average molecular weight between the crosslinking points, X1 is the interaction parameter of polymer and solvent whose value is 0.465, and vo is the molar volume of toluene whose value is 106.54 × 10−3 L mol−1.
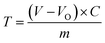 | (3) |
Results and discussion
Characterization of RGSO
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765, 12
765, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195, 12
195, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 262, 13
262, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654, and 14
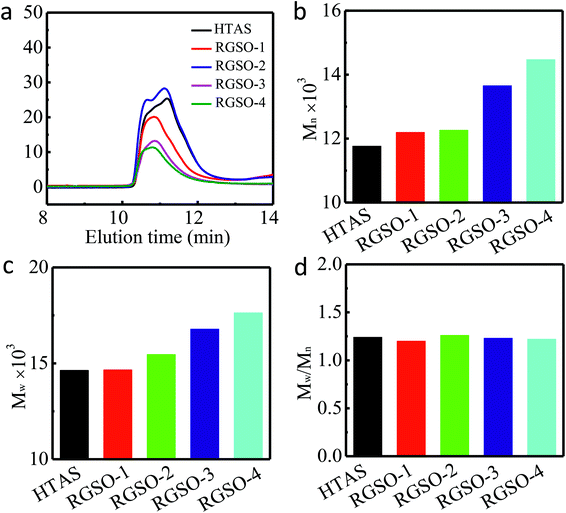
654, and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472 Da, respectively (Fig. 3b). The weight average molecular weights (Mw) of HTAS, RGSO-1, RGSO-2, RGSO-3, and RGSO-4 were 14
472 Da, respectively (Fig. 3b). The weight average molecular weights (Mw) of HTAS, RGSO-1, RGSO-2, RGSO-3, and RGSO-4 were 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635, 14
635, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660, 15
660, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462, 16
462, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 787, and 17
787, and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 Da, respectively, with low dispersities of 1.24, 1.20, 1.26, 1.23, and 1.22, respectively (Fig. 3c and d). The GPC curves for the number average molecular weight (Mn) of HTAS shifted clearly towards higher molecular weights of RGSO because of the chain extension. These results further support the successful grafting of ER onto the side chains of HTAS.20
638 Da, respectively, with low dispersities of 1.24, 1.20, 1.26, 1.23, and 1.22, respectively (Fig. 3c and d). The GPC curves for the number average molecular weight (Mn) of HTAS shifted clearly towards higher molecular weights of RGSO because of the chain extension. These results further support the successful grafting of ER onto the side chains of HTAS.20
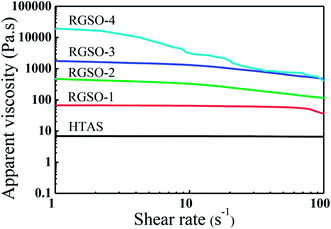
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980 Pa s (RGSO-4) with an increase in grafting of ER. Since the introduction of ER increased the degree of branching in the polysiloxane molecules, which was also consistent with the GPC results, the intermolecular entanglement of chain segments also increased.32 Moreover, compared to the Newtonian property of HTAS, the apparent viscosity of RGSO decreased with an increase in shear rate from 1 to 100 s−1, exhibiting a pseudoplastic property. Due to the separation of the winding structure, under the action of shearing force, it was aligned in the flow direction with increasing shear rate.33
980 Pa s (RGSO-4) with an increase in grafting of ER. Since the introduction of ER increased the degree of branching in the polysiloxane molecules, which was also consistent with the GPC results, the intermolecular entanglement of chain segments also increased.32 Moreover, compared to the Newtonian property of HTAS, the apparent viscosity of RGSO decreased with an increase in shear rate from 1 to 100 s−1, exhibiting a pseudoplastic property. Due to the separation of the winding structure, under the action of shearing force, it was aligned in the flow direction with increasing shear rate.33
| Sample | PDMS (g) | HTAS (g) | ER (g) | TEOS (g) | Catalyst (μL) | ER (wt%) |
|---|---|---|---|---|---|---|
| SRER-1 | 30 | 0 | 0 | 2.1 | 100 | 0 |
| SRER-2 | 20 | 10 | 1.5 | 4.5 | 100 | 5 |
| SRER-3 | 20 | 10 | 3.0 | 4.5 | 100 | 10 |
| SRER-4 | 20 | 10 | 4.5 | 4.5 | 100 | 15 |
| SRER-5 | 20 | 10 | 6.0 | 4.5 | 100 | 20 |
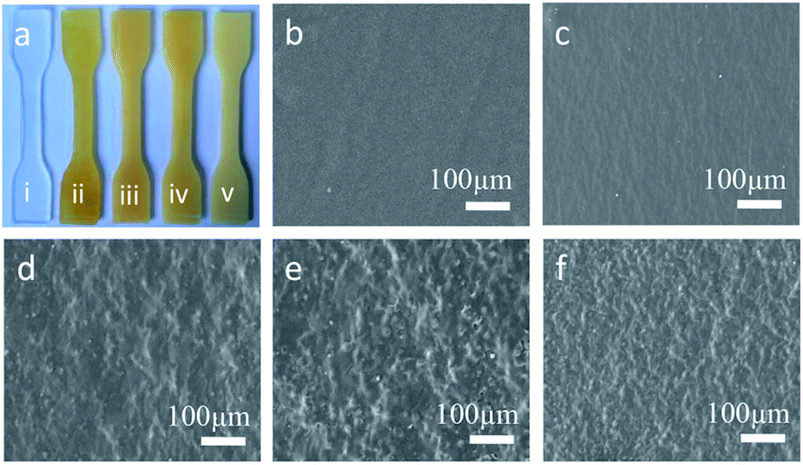
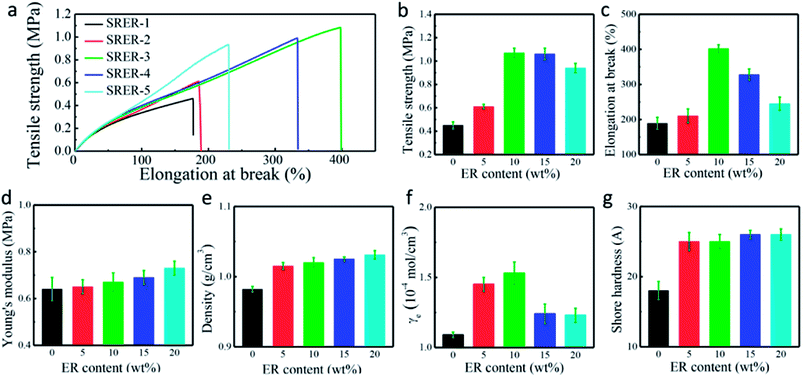
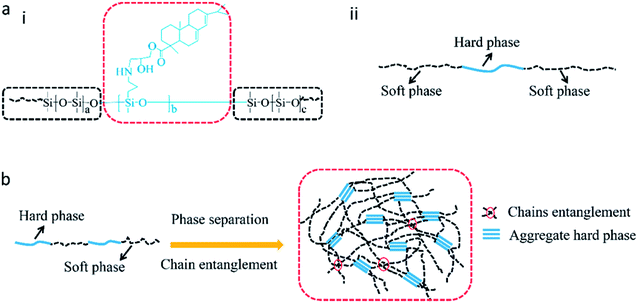
Improvements in tensile strength, elongation at break, and Young's modulus of the modified RTV silicone rubber were observed owing to the chemical incorporation of hydrogenated phenanthrene ring of ER into polysiloxane.35 A hard phase is mainly composed of ER-grafted polysiloxane and a soft phase contains polysiloxane chains (Fig. 7a). The density of hard phase increases with increasing amount of ER-grafted polysiloxane. This leads to an increase in cohesion energy of silicone rubber.22 ER on the side chains of polysiloxane could increase sufficiently the chain entanglement between rosin and polysiloxane chains to achieve good mechanical properties (Fig. 7b).20 Crosslink density is an important factor for the remarkable reinforcement of silicone rubber.36 The crosslinking density increased slightly with an increase in grafted ER from 1.09 × 10−4 mol cm−3 to 1.53 × 10−4 mol cm−3 (Fig. 6f). The mechanical properties of silicone rubber increased obviously due to the synergistic effects of increases in density of hard phase and crosslinking density (Fig. 7b). However, when the amount of grafted ER exceeds 10 wt%, the tensile strength and elongation at break of the ER-modified RTV silicone rubber decreased slightly with the decrease in crosslinking densities in SRER-4 and SRER-5. As the amount of grafted ER exceeded 10 wt%, ER-rich domains were formed in the RTV silicone rubber, because of the more polar and rigid ER. A marked microphasic separation occurred between ER-rich domains and polysiloxane in the structure of silicone rubber, which was evident in the SEM images (Fig. 5e and f).18 Decrease in crosslinking density and a marked microphasic separation could seriously affect the mechanical properties of silicone rubber. Moreover, the density and the Shore hardness increased with increase in ER loading from 0.982 to 1.031 g cm−3 and from 18 to 26 A, respectively (Fig. 6e and g). Therefore, the effective improvement in the mechanical properties of RTV silicone rubber could be attributed to the hard phase of ER, the degree of chain entanglements, and also the uniform distribution of ER in silicone rubber.
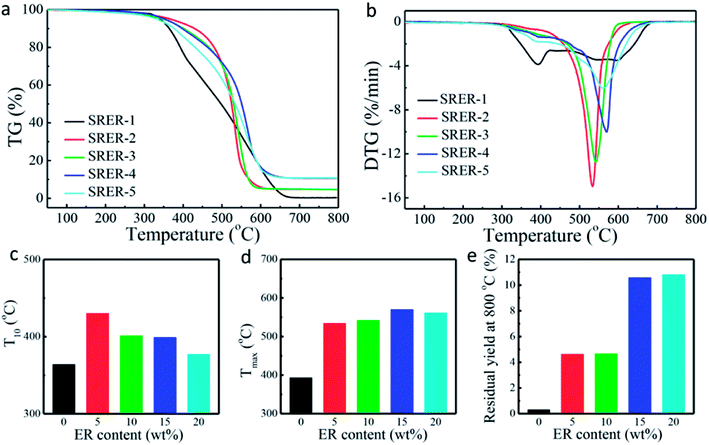
The temperature corresponding to maximum rate of weight loss was also delayed by 177 °C from 393 °C (SRER-1) to 570 °C (SRER-4). Owing to the obvious polarity of the Si–O bond and flexibility of the -(Si–O)n-segments, the traces of residual hydroxyl groups could themselves accelerate the decomposition, leading to rapid degradation at 393 °C (SRER-1).39 However, the ER-modified silicone rubber was prevented from degrading to cyclic oligomers, because of the restricted mobility of polysiloxane chains, which prevented the rearrangement of Si–O bonds in polysiloxane.41 As a result, the decomposition temperature of modified silicone rubber (SRER-4) was delayed up to 570 °C. At these higher temperatures, the obvious increase in mobility of the chain and molecular motion facilitated random degradation.38 Ultimately the unmodified silicone rubber SRER-1 with 0.30% residue at 800 °C was almost entirely degraded into cyclic oligomers. The ER-modified silicone rubber had more residual yield compared to the unmodified silicone rubber SRER-1 (Fig. 8e).42
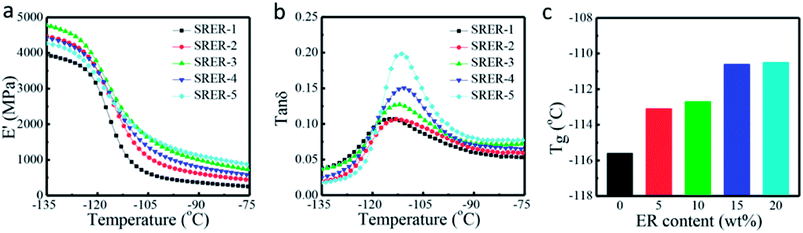 | ||
Fig. 9 Dynamic mechanical properties of silicone rubbers. (a) Curves for storage moduli (E′) of RTV silicone rubbers. (b) Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves of RTV silicone rubbers. (c) Tg values of RTV silicone rubbers. δ curves of RTV silicone rubbers. (c) Tg values of RTV silicone rubbers. | ||
The peak temperature of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ corresponded to the glass transition temperature (Tg). Fig. 9b shows that the tan
δ corresponded to the glass transition temperature (Tg). Fig. 9b shows that the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves of RTV silicone rubber grafted with different weight ratios of ER had only one distinct Tg from −135 °C to −75 °C. The Tg of RTV silicone rubber increased slightly from −115.6 °C to −110.5 °C (Fig. 9c) with an increase in ER loading. This was because of the more rigid hydrogenated phenanthrene ring structure of ER in modified RTV silicone rubber, which restricted the movement of the chains by increasing the rigidity of molecular chains and density due to chain entanglements.43–45
δ curves of RTV silicone rubber grafted with different weight ratios of ER had only one distinct Tg from −135 °C to −75 °C. The Tg of RTV silicone rubber increased slightly from −115.6 °C to −110.5 °C (Fig. 9c) with an increase in ER loading. This was because of the more rigid hydrogenated phenanthrene ring structure of ER in modified RTV silicone rubber, which restricted the movement of the chains by increasing the rigidity of molecular chains and density due to chain entanglements.43–45
Conclusion
In summary, RGSO was prepared via ring-opening reaction and was characterized. The relative molecular weight and viscosity of RGSO increased with increase in ER content. Then, the mixtures of RGSO and PDMS matrix, as primary polymers, were used to prepare rosin-modified RTV silicone rubber by condensation reaction at room temperature. Compared to unmodified silicone rubber SRER-1, rosin significantly enhanced the thermal stabilities and mechanical properties of rosin-modified RTV silicone rubbers. These results could be attributed to the strong rigidity and polar hydrogenated phenanthrene ring structure of ER, the increase in chain entanglement of polysiloxane and the perfect distribution of ER in the rosin-modified RTV silicone rubber. This study provides a strategy for sustainable development and exploration of biomass polymer-containing rosin-modified silicone rubber with superior performance.Funding
The authors express their gratitude for the financial support from National Natural Science Foundation of China (31570562); the Key Laboratory of Biomass Energy and Materials of Jiangsu Province of China (JSBEM-S-201504).Conflicts of interest
The authors declare no competing financial interest.References
- Y. Meng, J. Chu, J. Xue, C. Liu, Z. Wang and L. Zhang, RSC Adv., 2014, 4, 31249–31260 RSC.
- S. C. Shit and P. Shah, Natl. Acad. Sci. Lett., 2013, 36, 355–365 CrossRef CAS.
- A. C. C. Esteves, J. Brokken-Zijp, J. Lavèn, H. P. Huinink, N. J. W. Reuvers, M. P. Van and G. de With, Polymer, 2010, 51, 136–145 CrossRef CAS.
- Z. Sun, Q. Huang, Y. Wang, L. Zhang and Y. Wu, Ind. Eng. Chem. Res., 2017, 56, 1471–1477 CrossRef CAS.
- N. Suzuki, S. Kiba, Y. Kamachi, N. Miyamoto and Y. Yamauchi, J. Mater. Chem., 2011, 21, 5338–5344 RSC.
- A. M. Stricher, R. G. Rinaldi, C. Barrès, F. Ganachaud and L. Chazeau, RSC Adv., 2015, 5, 53713–53725 RSC.
- Y. Liu, Y. Shi, D. Zhang, J. Li and G. Huang, Polymer, 2013, 54, 6140–6149 CrossRef CAS.
- Y. Meng, Z. Wei, L. Liu, L. Liu, L. Zhang, T. Nishi and K. Ito, Polymer, 2013, 54, 3055–3064 CrossRef CAS.
- H. Ardhyananta, T. Kawauchi, H. Ismail and T. Takeichi, Polymer, 2009, 50, 5959–5969 CrossRef CAS.
- L. Zhang, D. Wang, P. Hu, J. W. Zha, F. You, S. T. Li and Z. M. Dang, J. Mater. Chem. C, 2015, 3, 4883–4889 RSC.
- E. P. Magennis, A. L. Hook, P. Williams and M. R. Alexander, ACS Appl. Mater. Interfaces, 2016, 8, 30780–30787 CAS.
- G. Deshpande and M. E. Rezac, Polym. Degrad. Stab., 2002, 76, 17–24 CrossRef CAS.
- G. Deshpande and M. E. Rezac, Polym. Degrad. Stab., 2001, 74, 363–370 CrossRef CAS.
- J. Gutierrez and A. Tercjak, RSC Adv., 2014, 4, 32024–32030 RSC.
- B. S. Kaith, R. Jindal and R. Sharma, RSC Adv., 2015, 5, 43092–43104 RSC.
- J. Varshosaz, S. H. Javanmard, S. Soghrati and B. Behdadfar, RSC Adv., 2016, 6, 12337–12347 RSC.
- J. Yu, Y. Liu, X. Liu, C. Wang, J. Wang, F. Chu and C. Tang, Green Chem., 2014, 16, 1854–1864 RSC.
- M. S. Ganewatta, W. Ding, M. A. Rahman, L. Yuan, Z. Wang, N. Hamidi, M. L. Robertson and C. Tang, Macromolecules, 2016, 49, 7155–7164 CrossRef CAS.
- C. Yu, X. Wang, C. Chen and F. Zhang, Ind. Eng. Chem. Res., 2014, 53, 2244–2250 CrossRef CAS.
- M. A. Rahman, H. N. Lokupitiya, M. S. Ganewatta, L. Yuan, M. Stefik and C. Tang, Macromolecules, 2017, 50, 2069–2077 CrossRef CAS.
- X. Li, M. Li, J. Li, F. Lei, X. Su, M. Liu, P. Li and X. Tan, Anal. Methods, 2014, 6, 6397–6406 RSC.
- T. Xu, H. Liu, J. Song, S. Shang, Z. Song, K. Zou and C. Yang, J. Polym. Sci. Pol. Chem., 2015, 53, 1769–1776 CrossRef CAS.
- S. Lee, K. Lee, Y. W. Kim and J. Shin, ACS Sustainable Chem. Eng., 2015, 3, 2309–2320 CrossRef CAS.
- L. Deng, M. Shen, J. Yu, K. Wu and C. Ha, Ind. Eng. Chem. Res., 2012, 51, 8178–8184 CrossRef CAS.
- X. Xu, Z. Song, S. Shang, S. Cui and X. Rao, Polym. Int., 2011, 60, 1521–1526 CrossRef CAS.
- T. Xu, H. Liu, J. Song, S. B. Shang, Z. Q. Song, X. J. Chen and C. Yang, Chin. Chem. Lett., 2015, 26, 572–574 CrossRef CAS.
- T. Xu, H. Liu, J. Song, S. b. Shang, Z. Song, K. Zou and C. Yang, J. Appl. Polym. Sci., 2015, 132, 41888 Search PubMed.
- K. Huang, J. Zhang, M. Li, J. Xia and Y. Zhou, Ind. Crops Prod., 2013, 49, 497–506 CrossRef CAS.
- W. O. Parker, A. Ferrando, D. Ferri and V. Canepari, Macromolecules, 2007, 40, 5787–5790 CrossRef CAS.
- K. Pal, R. Rajasekar, D. J. Kang, Z. X. Zhang, S. K. Pal, C. K. Das and J. K. Kim, Mater. Des., 2010, 31, 677–686 CrossRef CAS.
- Q. Xu, M. Pang, L. Zhu, Y. Zhang and S. Feng, Mater. Des., 2010, 31, 4083–4087 CrossRef CAS.
- S. Wang, J. Xu, Q. Wang, X. Fan, Y. Yu, P. Wang, Y. Zhang, J. Yuan and A. Cavaco-Paulo, Process Biochem., 2017, 59, 104–110 CrossRef CAS.
- X. Huang, H. Liu, S. Shang, X. Rao and J. Song, J. Agric. Food Chem., 2015, 63, 9062–9068 CrossRef CAS PubMed.
- D. W. Kang and Y. M. Kim, J. Appl. Polym. Sci., 2002, 85, 2867–2874 CrossRef CAS.
- H. Wang, X. Liu, B. Liu, J. Zhang and M. Xian, Polym. Int., 2009, 58, 1435–1441 CrossRef CAS.
- K. Huang, P. Zhang, J. Zhang, S. Li, M. Li, J. Xia and Y. Zhou, Green Chem., 2013, 15, 2466–2475 RSC.
- L. Jiao, H. Xiao, Q. Wang and J. Sun, Polym. Degrad. Stab., 2013, 98, 2687–2696 CrossRef CAS.
- D. Chen, S. Yi, W. Wu, Y. Zhong, J. Liao, C. Huang and W. Shi, Polymer, 2010, 51, 3867–3878 CrossRef CAS.
- Y. Shi, G. Huang, Y. Liu, Y. Qu, D. Zhang and Y. Dang, J. Polym. Res., 2013, 20, 1–11 CAS.
- Y. Shi, X. Gao, D. Zhang, Y. Liu and G. Huang, RSC Adv., 2014, 4, 41453–41460 RSC.
- Y. Han, J. Zhang, L. Shi, S. Qi, J. Cheng and R. Jin, Polym. Degrad. Stab., 2008, 93, 242–251 CrossRef CAS.
- E. Pouget, J. Tonnar, P. Lucas, P. L. Desmazes, F. Ganachaud and B. Boutevin, Chem. Rev., 2010, 110, 1233–1277 CrossRef CAS PubMed.
- X. Liu, W. Xin and J. Zhang, Bioresour. Technol., 2010, 101, 2520–2524 CrossRef CAS.
- X. Xu, L. Chen, J. Guo, X. Cao and S. Wang, RSC Adv., 2017, 7, 30439–30445 RSC.
- C. Liu, Q. Shang, P. Jia, Y. Dai, Y. Zhou and Z. Liu, ACS Sustainable Chem. Eng., 2016, 4, 3437–3449 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Components of pure RTV silicone rubbers. (2) Mechanical properties of RTV silicone rubber with different ER content. (3) The molar ratio of amino groups and epoxide groups in the reactants. (4) The test for mechanical properties of SRER-1 and SRER-3. (5) The aggregation behavior of rosin segment in silicone rubber. See DOI: 10.1039/c7ra13672b |
| This journal is © The Royal Society of Chemistry 2018 |