Open Access Article
Open Access ArticleTriflimide-catalyzed allylsilane annulations of benzylic alcohols for the divergent synthesis of indanes and tetralins†
Jordan C. T.
Reddel‡
,
Weiwei
Wang‡
,
Kalli
Koukounas
and
Regan J.
Thomson
 *
*
Department of Chemistry, Northwestern University, 2145 Sheridan Rd., Evanston, IL 60208, USA. E-mail: r-thomson@northwestern.edu
First published on 9th December 2016
Abstract
The development of a triflimide-catalyzed annulation of benzylic alcohols with allylsilanes for the synthesis of indane or tetralin structures is reported. In this fragment coupling reaction, complexity is built rapidly from readily available starting materials to yield diverse sets of products with up to three contiguous stereocenters. Indanes or tetralins can be generated from common precursors depending on the structure of the allylsilane reagent used. The concise synthesis of several lignan natural products highlights the utility of this newly devised methodology.
Introduction
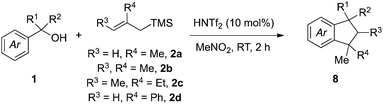
Fragment coupling reactions that assemble complex products convergently from two or more reaction partners are exceptionally useful in synthesis.1 This utility is amplified greatly when new stereochemical elements and rings are formed as a consequence of the reaction, allowing the synthesis practitioner to transform simple starting materials into complex cyclic or polycyclic products directly. Within this context, allylsilane reagents have received significant attention due to their capacity to participate in a wide range of complexity-generating annulations leading to the development of efficient methods for the concise synthesis of numerous chemical architectures,2 including heterocycles3 and carbocycles.3a–c,4We saw an opportunity to contribute to this field through the development of a Brønsted acid-catalyzed allylsilane annulation that transforms simple benzylic alcohols into a range of indanes5,6 or tetralins5c,d,7 depending upon the choice of silane reagent used (Scheme 1). For the case of simple allylsilanes of type A (i.e., 2) we envisioned a scenario where formation of a carbocation from alcohol 1 followed by allylsilane addition would lead to intermediate 5. An ensuing Friedel–Crafts alkylation would then deliver indane 8, whose structure maps onto a variety of useful compounds such as 11. Under the same reaction conditions, type B silanes (i.e., 3) would give rise to an intermediate allylic alcohol 6, which would then undergo an acid-catalyzed 5-exo-trig cyclization to afford indane 9, a structure reminiscent of essential oil component 12. Use of the isomeric type C silane (i.e., 4) would yield intermediate 7 in a similar manner, allowing for a subsequent 6-endo-trig cyclization/alkene isomerization en route to 10 and representing a convergent approach to tetralin lignans (i.e., 13).
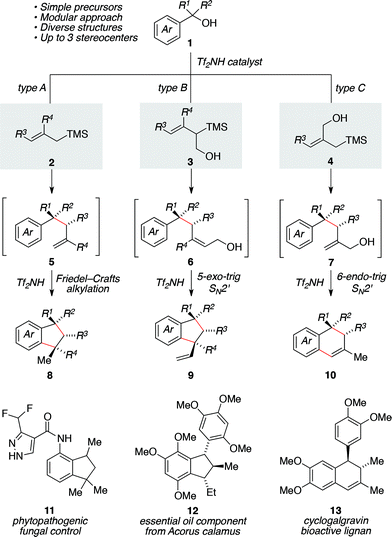 | ||
| Scheme 1 Divergent access to indanes or tetralin products by way of a tandem allylation/ring-closure sequence catalyzed by triflimide. | ||
Results and discussion
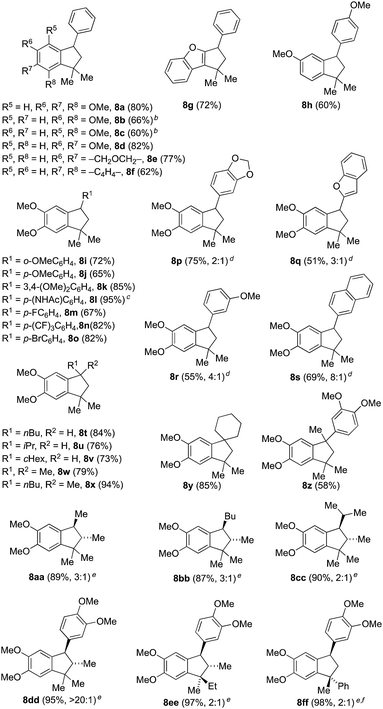
We initiated our investigations (Table 1) using methallylsilane (2a), a commercially available type A silane, and quickly identified bis(trifluoromethanesulfonyl)imide (triflimide) as the best catalyst, with nitromethane as the optimal solvent (see ESI for more details†). Using 10 mol% of triflimide, the reaction proceeded over the course of two hours at room temperature and proved tolerant to a range of benzylic alcohol precursors, thus delivering a diverse set of indane products in good to excellent yields (Table 1). In no cases did we observe products derived from silyl migration. For the case of benzhydryl alcohols where one aromatic ring was a simple phenyl group (8a–8g), the reaction proceeded such that cyclization took place on the more electron-rich ring. In some cases, such as 8b or 8c, yields were improved by running reactions at a higher temperature (60 °C) to overcome the steric encumbrance of cyclization onto aromatic rings with ortho-substituents. Only cyclization to the more electron-rich 1-position, rather than the 3-position, is observed for 8f. In product 8g, cyclization has occurred onto a benzofuran ring system to yield a 5,5,6-fused ring system, which while not technically an indane still represents a useful chemotype.8Complete regioselectivity is observed for the cyclization leading to indanes 8h–8o (Table 1), in line with the expectation that the Friedel–Crafts alkylation would take place para to the most electron releasing substituent. For cases where both rings are activated for cyclization, mixtures of regioisomeric products were obtained with modest to good selectivity (Table 1, 8p–8s). A number of benzyl alcohols with alkyl substituents were employed using the same reaction conditions (Table 1, 8t–8y). Indanes with two quaternary centers are formed in good yields (8w–8z). The efficient construction of spiroindane 8y is particularly notable since this tricyclic core is found in many bioactive compounds and natural products.9
Use of alternative type A allylsilanes allowed access to a range of stereochemically complex indanes (Table 1, 8aa–8ff). For example, silane 2b allows for the introduction of two stereocenters within the indane products (8aa–8ee) with modest to excellent diastereoselectivity. For the three alkyl substituted products (8aa–8cc), the observed anti selectivity was modest as a consequence of low stereoselectivity in the initial addition of silane 2b into the unsymmetrical benzyl cation intermediate derived from 1 (R1 = Me, Bu or iPr, R2 = H). Cyclization in these cases does not generate a new stereocenter. On the other hand, indane 8dd was generated in >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr due to preferential cyclization on one of the two diastereotopic electron-rich aromatic rings of the intermediate following initial silane addition. This outcome is consistent with related observations we had made during our previously reported synthesis of lignan natural products.10 Use of silane 2c gave rise to compound 8ee that contains three contiguous stereocenters in high yield, but as a 2
1 dr due to preferential cyclization on one of the two diastereotopic electron-rich aromatic rings of the intermediate following initial silane addition. This outcome is consistent with related observations we had made during our previously reported synthesis of lignan natural products.10 Use of silane 2c gave rise to compound 8ee that contains three contiguous stereocenters in high yield, but as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers due to poor selectivity during cyclization to form the new quaternary stereocenter. A similar outcome was observed utilizing silane 2d to produce indane 8ff. In this last example, it was necessary to reduce the number of equivalents of allylsilane in order to suppress the competitive formation of a 7-membered ring analog formed by double addition of the silane (see ESI†).
1 mixture of diastereomers due to poor selectivity during cyclization to form the new quaternary stereocenter. A similar outcome was observed utilizing silane 2d to produce indane 8ff. In this last example, it was necessary to reduce the number of equivalents of allylsilane in order to suppress the competitive formation of a 7-membered ring analog formed by double addition of the silane (see ESI†).
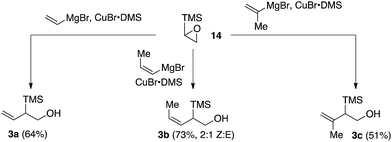
We next sought to explore the utility of type B and type C silanes that would give rise to indanes or tetralins, respectively (see Scheme 1). While the desired type C silanes (i.e., 4) have been reported in the literature,11 general protocols to access the requisite type B silanes (i.e., 3) were less well developed.12 We therefore developed an effective protocol for the synthesis of silanes 3a–3c based on the regioselective addition of Grignard reagent-derived vinylcuprates to trimethylsilylepoxide 14 (Scheme 2).13
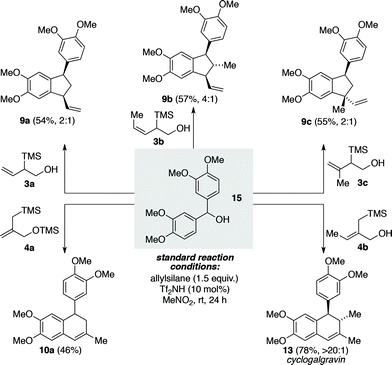
Utilizing the symmetrical benzhydryl alcohol 15 as a common precursor we next demonstrated the capacity of type B and C allylsilanes to undergo the desired triflimide-catalyzed annulation processes (Scheme 3). The type B silanes 3a–3c gave rise to three different vinyl substituted indane products (9a–9c) in reasonable yield and with modest levels of diastereoselectivity, as observed for the corresponding type A silanes. Type C silane 4a was found to be superior to the corresponding free alcohol derivative, undergoing the desired addition/6-endo-trig cyclization/alkene isomerization to provide tetralin 10a in 46% yield. Type C silane 4b was more effective than silane 4a, providing tetralin 13 in 78% yield and with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selectivity for the anti stereochemistry. In this instance the product of the new annulation process is the natural product cyclogalgravin (13), thus establishing a highly efficient two-step total synthesis of this lignan from simple starting materials.14
1 selectivity for the anti stereochemistry. In this instance the product of the new annulation process is the natural product cyclogalgravin (13), thus establishing a highly efficient two-step total synthesis of this lignan from simple starting materials.14
Recognizing the power of this annulation process in accessing cyclolignan natural products,15 we targeted the synthesis of several other related natural products in order to further demonstrate the usefulness of this methodology (Scheme 4). For example, symmetrical benzhydryl species 16 underwent the triflimide-catalyzed annulation with silane 4b to deliver dihydronaphthalene 10b in 49% yield (Scheme 4A). Selective removal of the two isopropyl ethers from 10b delivered the natural product 4′,5-O-didemethylcyclogalgravin (17)16 in 81% yield, while simple oxidation of 10b with DDQ allowed access to cinnamophilin A (18)17 after isopropyl group cleavage (81%, 2 steps). Conducting the same reaction sequences on the more highly substituted benzhydryl starting material 19 provided access to the higher oxidation state analogs, sacidumlignan B (20) and A (21) with similar levels of efficiency (Scheme 4B).18 The unsymmetrical benzhydryl 22 delivered the annulated product 10d as a mixture of cis and trans stereoisomers, along with some of the naphthalene product 23. Cyclization only occurred on the more electron-rich aromatic ring, and this mixture could be treated with DDQ without prior purification to deliver pycnanthuligene C (23) in 73% yield over two steps from 22.19
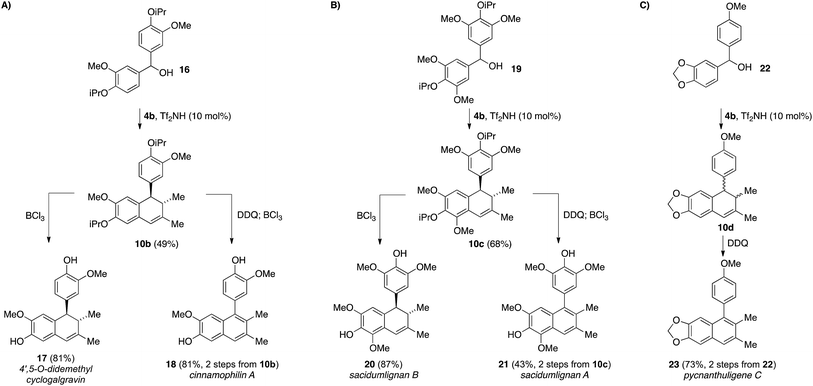 | ||
| Scheme 4 A general approach for accessing tetralin lignan natural products utilizing a triflimide-catalyzed allylsilane annulation. | ||
Conclusions
In summary, we have developed a Brønsted acid-catalyzed annulation for the divergent synthesis of substituted indanes and tetralins from a set of common precursors. The strategic ability to rapidly build complexity from commercially available or easily prepared starting materials makes this methodology amenable to the concise preparation of various chemotypes, including bioactive natural products. Future work will focus on the development of enantioselective variants of these reactions using chiral strong Brønsted acids, which is an exciting prospect given the recent advancements in that field.20Acknowledgements
We thank support in part from the NSF (CHE1361173) and Northwestern University. We gratefully acknowledge the award of a CLP Lambert Fellowship to KK.Notes and references
- E. J. Corey and X.-M. Cheng, The Logic of Chemical Synthesis, Wiley, New York, 1989 Search PubMed.
- For useful reviews, see: (a) C. E. Masse and J. S. Panek, Chem. Rev., 1995, 95, 1293–1316 CrossRef CAS; (b) L. Chabaud, P. James and Y. Landais, Eur. J. Org. Chem., 2004, 3173–3199 CrossRef CAS.
- For representative examples, see: (a) H. J. Knolker, P. G. Jones and R. Graf, Synlett, 1996, 1155–1158 CrossRef; (b) H. J. Knolker, N. Foitzik, H. Goesmann, R. Graf, P. G. Jones and G. Wanzl, Chem.–Eur. J., 1997, 3, 538–551 CrossRef CAS; (c) H. J. Knolker, N. Foitzik and O. Schmitt, Tetrahedron Lett., 1999, 40, 3557–3560 CrossRef CAS; (d) M. G. Organ, V. Dragan, M. Miller, R. D. J. Froese and J. D. Goddard, J. Org. Chem., 2000, 65, 3666–3678 CrossRef CAS PubMed; (e) Z. H. Peng and K. A. Woerpel, Org. Lett., 2000, 2, 1379–1381 CrossRef CAS PubMed; (f) Z. H. Peng and K. A. Woerpel, Org. Lett., 2001, 3, 675–678 CrossRef CAS PubMed; (g) N. V. Hanhan, N. R. Ball-Jones, N. T. Tran and A. K. Franz, Angew. Chem., Int. Ed., 2012, 51, 989–992 CrossRef CAS PubMed; (h) B. H. Shupe, E. E. Allen, J. P. MacDonald, S. O. Wilson and A. K. Franz, Org. Lett., 2013, 15, 3218–3221 CrossRef CAS PubMed; (i) Y. Matsumura, T. Suzuki, A. Sakakura and K. Ishihara, Angew. Chem., Int. Ed., 2014, 53, 6131–6134 CrossRef CAS PubMed; (j) J. D. Rainier, Synthesis of Saturated Oxygenated Heterocycles I: 5- and 6-Membered Rings, ed. J. Cossy, Springer, 2014, vol. 35, pp. 1–41 Search PubMed.
- For representative examples, see: (a) R. L. Danheiser, B. R. Dixon and R. W. Gleason, J. Org. Chem., 1992, 57, 6094–6097 CrossRef CAS; (b) R. L. Danheiser, T. Takahashi, B. Bertók and B. R. Dixon, Tetrahedron Lett., 1993, 34, 3845–3848 CrossRef CAS; (c) J. S. Panek and N. F. Jain, J. Org. Chem., 1993, 58, 2345–2348 CrossRef CAS; (d) B. M. Trost, N. Cramer and S. M. Silverman, J. Am. Chem. Soc., 2007, 129, 12396–12397 CrossRef CAS PubMed; (e) M. S. Dowling and C. D. Vanderwal, J. Am. Chem. Soc., 2009, 131, 15090–15091 CrossRef CAS PubMed; (f) A. W. Schmidt and H.-J. Knoelker, Synlett, 2010, 2207–2239 CAS; (g) N. R. Ball-Jones, J. J. Badillo, N. T. Tran and A. K. Franz, Angew. Chem., Int. Ed., 2014, 53, 9462–9465 CrossRef CAS PubMed.
- For some recent examples of methodology for indane synthesis, see: (a) B. Lantano, J. M. Aguirre, E. A. Ugliarolo, R. Torviso, N. Pomilio and G. Y. Moltrasio, Tetrahedron, 2012, 68, 913–921 CrossRef CAS; (b) A. L. Auvinet, V. Michelet and V. Ratovelomanana-Vidal, Synthesis, 2013, 45, 2003–2008 CrossRef CAS; (c) J. M. Begouin, F. Capitta, X. Wu and M. Niggemann, Org. Lett., 2013, 15, 1370–1373 CrossRef CAS PubMed; (d) M. W. Grafton, L. J. Farrugia and A. Sutherland, J. Org. Chem., 2013, 78, 7199–7207 CrossRef CAS PubMed; (e) Y. C. Fan and O. Kwon, Org. Lett., 2015, 17, 2058–2061 CrossRef CAS PubMed; (f) C. P. Johnston, A. Kothari, T. Sergeieva, S. I. Okovytyy, K. E. Jackson, R. S. Paton and M. D. Smith, Nat. Chem., 2015, 7, 171–177 CrossRef CAS PubMed; (g) A. N. Kazakoya, R. O. Iakovenko, I. A. Boyarskaya, V. G. Nenajdenko and A. V. Vasilyev, J. Org. Chem., 2015, 80, 9506–9517 CrossRef PubMed; (h) W. Q. Kong, N. Fuentes, A. Garcia-Dominguez, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2015, 54, 2487–2491 CrossRef CAS PubMed; (i) A. Ahmad and L. F. Silva, J. Org. Chem., 2016, 81, 2174–2181 CrossRef CAS PubMed; (j) Y. L. Li, L. J. Zhang, Z. Y. Zhang, J. B. Xu, Y. X. Pan, C. H. Xu, L. X. Liu, Z. G. Li, Z. Y. Yu, H. R. Li and L. J. Xu, Adv. Synth. Catal., 2016, 358, 2148–2155 CrossRef CAS.
- For a recent review, see: B. Gabriele, R. Mancuso and L. Veltri, Chem.–Eur. J., 2016, 22, 5056–5094 CrossRef CAS PubMed.
- For recent examples, see: (a) N. Ishida, N. Ishikawa, S. Sawano, Y. Masuda and M. Murakami, Chem. Commun., 2015, 51, 1882–1885 RSC; (b) M. Y. Chang and Y. C. Cheng, Org. Lett., 2016, 18, 608–611 CrossRef CAS PubMed.
- For an example, see: L. Santana, H. González-Díaz, E. Quezada, E. Uriarte, M. Yáñez, D. Viña and F. Orallo, J. Med. Chem., 2008, 51, 6740–6751 CrossRef CAS PubMed.
- (a) M. M. Radwan, M. A. ElSohly, D. Slade, S. A. Ahmed, L. Wilson, A. T. El-Alfy, I. A. Khan and S. A. Ross, Phytochemistry, 2008, 69, 2627–2633 CrossRef CAS PubMed; (b) B. M. Fox, K. Sugimoto, K. Iio, A. Yoshida, J. Zhang, K. Li, X. Hao, M. Labelle, M.-L. Smith, S. M. Rubenstein, G. Ye, D. McMinn, S. Jackson, R. Choi, B. Shan, J. Ma, S. Miao, T. Matsui, N. Ogawa, M. Suzuki, A. Kobayashi, H. Ozeki, C. Okuma, Y. Ishii, D. Tomimoto, N. Furakawa, M. Tanaka, M. Matsushita, M. Takahashi, T. Inaba, S. Sagawa and F. Kayser, J. Med. Chem., 2014, 57, 3464–3483 CrossRef CAS PubMed.
- J. C. T. Reddel, K. E. Lutz, A. B. Diagne and R. J. Thomson, Angew. Chem., Int. Ed., 2014, 53, 1395–1398 CrossRef CAS PubMed.
- (a) B. M. Trost and D. M. T. Chan, J. Am. Chem. Soc., 1981, 103, 5972–5974 CrossRef CAS; (b) B. M. Trost and P. Renaut, J. Am. Chem. Soc., 1982, 104, 6668–6672 CrossRef CAS; (c) B. M. Trost and M. C. Matelich, Synthesis, 1992, 1–2, 151–156 CrossRef.
- (a) K. Fujii, O. Hara and Y. Sakagami, Biosci., Biotechnol., Biochem., 1997, 61, 1394–1396 CrossRef CAS; (b) M. Schlosser and L. Franzini, Synthesis, 1998, 707–709 CrossRef CAS; (c) P. H. Dussault, C. T. Eary, R. J. Lee and U. R. Zope, J. Chem. Soc., Perkin Trans. 1, 1999, 2189–2204 CAS; (d) H. Huang and J. S. Panek, J. Am. Chem. Soc., 2000, 122, 9836–9837 CrossRef CAS; (e) M. Suginome, T. Iwanami, A. Yamamoto and Y. Ito, Synlett, 2001, 1042–1045 CrossRef CAS.
- Y.-L. Chen and D. Hoppe, Tetrahedron: Asymmetry, 2009, 20, 1561–1567 CrossRef CAS.
- T. da Silva and L. M. X. Lopes, Phytochemistry, 2006, 67, 929–937 CrossRef CAS PubMed.
- For a review covering aspects of lignan synthesis, see: J.-Y. Pan, S.-L. Chen, M.-H. Yang, J. Wu, J. Sinkkonen and K. Zou, Nat. Prod. Rep., 2009, 26, 1251–1292 RSC.
- (a) G. B. Messiano, E. M. K. Wijeratne, L. M. X. Lopes and A. A. L. Gunatilaka, J. Nat. Prod., 2010, 73, 1933–1937 CrossRef CAS PubMed; (b) H.-Y. Yu, Z.-Y. Chen, B. Sun, J. Liu, F.-Y. Meng, Y. Liu, T. Tian, A. Jin and H.-L. Ruan, J. Nat. Prod., 2014, 77, 1311–1320 CrossRef CAS PubMed.
- (a) N. Rangkaew, R. Suttisri, M. Moriyasu and K. Kawanishi, Fitoterapia, 2009, 80, 377–379 CrossRef CAS PubMed; (b) C.-Y. Chen, Y.-T. Yeh and Y.-R. Hsui, Chem. Nat. Compd., 2011, 47, 519–520 CrossRef CAS.
- L.-S. Gan, S.-P. Yang, C.-Q. Fan and J.-M. Yue, J. Nat. Prod., 2005, 68, 221–225 CrossRef CAS PubMed.
- E. C. N. Nono, P. Mkounga, V. Kuete, K. Marat, P. G. Hultin and A. E. Nkengfack, J. Nat. Prod., 2010, 73, 213–216 CrossRef CAS PubMed.
- For a recent review of methodology using strong Brønsted acids, including enantioselective catalytic methods, see: T. Akiyama and K. Mori, Chem. Rev., 2015, 115, 9277–9306 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data for all new compounds. See DOI: 10.1039/c6sc04762a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |