The historical or the contemporary context: which of the two ensures a deeper understanding of gas properties?†
Vesna D.
Milanovic
 * and
Dragica D.
Trivic
* and
Dragica D.
Trivic

University of Belgrade, Faculty of Chemistry, Studentski trg 12-16, Belgrade, Serbia. E-mail: vesnamilanovic@chem.bg.ac.rs; Tel: +381 113336854
First published on 28th March 2017
Abstract
The aim of this research was to explore the effects of two approaches, designated as the historical and the contemporary one, on the level of students' understanding of the properties and the practical use of gases. Our research hypothesis was that the historical context of the discovery of gases and the study of their properties would deepen students' understanding of the properties and the practical use of gases more than the contemporary context. A total of 129 students attending the eighth grade of primary school, aged 14, took part in the research project. After taking a pre-test, the students were divided into two groups (A and B respectively), equal in terms of their test scores. Group A (63 students) was exposed to the historical approach, whereas group B (66 students) was exposed to the contemporary approach. The students from group A individually dealt with a text that presented various episodes from the scientific-research work of Joseph Priestley, whereas the students from group B dealt with a text pertaining to the properties and the use of gases in contemporary society. Having been exposed to different approaches, all the students did a post-test. No statistically significant difference was established between the overall results of the students who had been exposed to the historical approach and those exposed to the contemporary approach, and the research hypothesis was rejected. However, one statistically significant better score in one of the post-test items in group A may be connected with the influence of the corresponding episode from the history of science.
Introduction
Reviewing documents that are created within the framework of contemporary educational policies and scientific papers pertaining to the domain of science education, one can observe common notions such as scientific literacy, nature of science, as well as big ideas (for example: Harlen, 2010; ACS Guidelines, 2012; Murphy et al., 2012; Allchin et al., 2014; Sjostrom and Talanquer, 2014; De Jong and Talanquer, 2015; Leyh et al., 2015). How do those notions relate and how do they shape the process of teaching and learning in the sphere of natural sciences?Scientific education should enable each individual to make informed decisions and to undertake corresponding activities that contribute to personal welfare, the welfare of society and the preservation of the environment (Harlen, 2010). In other words, it should enable the implementation of knowledge and skills, logical reasoning and reaching conclusions about personally and socially relevant issues (Rasmussen, 2007; ACS Guidelines, 2012).
The outcome of the traditional teaching of science, focused on facts, is memorising a package of science facts without establishing any connections between them. Such a way of teaching does not provide an integral image of science to the students and does not manage to engage them in activities and discussions wherein scientific ideas could be used for decision-making and problem solving (Rasmussen, 2007; Sjostrom and Talanquer, 2014). In order to overcome this problem, scientific education should develop a set of big ideas that include scientific ideas about objects, phenomena, substances and relations in the natural world, as well as ideas about science and the role of science in society (Harlen, 2010).
In the case of students who, due to their ability to memorise a great amount of decontextualised material, achieve good results at school, changes that involve expecting of them to synthesise, evaluate, reason and connect fundamental ideas in chemistry could be too demanding. However, a model advocating big ideas, long-term understanding, essential knowledge and scientific practice could shift the level of the students' scientific literacy in the desired direction in the future (Rushton, 2012).
Two categories of big ideas of significance for education in the sphere of chemistry have been identified:
(1) Contextual big ideas within a discipline that could be both specific and general – these point to an understanding of chemistry that is directly relevant to the individual and to society.
(2) Conceptual big ideas – these include big ideas of chemistry and big ideas about chemistry, i.e. they encompass a fundamental understanding of the structure of matter and its properties, and of the nature and practice of chemistry.
Contextual and conceptual ideas are interlinked in various ways. For example, an individual contextual idea may encompass several different conceptual ideas, whereas a specific conceptual idea may support the understanding of several contextual ideas (De Jong and Talanquer, 2015). Even though the reforms of the 1980s shifted the focus of the curriculum from conceptual towards contextual big ideas, conceptual ideas remained predominant in the sphere of chemical education at all levels (Eilks et al., 2013; Talanquer, 2013).
Generally, educators in the sphere of chemistry recognise the advantages of teaching/learning organised in accordance with big ideas in: (i) preparing students to understand other chemical concepts as well, (ii) understanding chemistry as a special way of exploring the natural world. In doing so, they consider conceptual big ideas to be more relevant than contextual big ideas. On the other hand, students are more interested in learning contextual big ideas that are directly connected with their personal experience (De Jong and Talanquer, 2015).
The central ideas of science are necessary for acquiring the basic competencies that serve as building blocks for a further and deeper understanding of science (Talanquer, 2016). The correspondence of big ideas, recognised in various spheres by various educators, points to the power of these ideas when it comes to shedding light on the main concepts in the sphere of chemistry (Holme, 2014).
Clearly defined big ideas can be of great help to teachers when it comes to planning activities that lead towards conceptual understanding, and also for the purpose of monitoring students' progress in learning (Stains et al., 2011). If central ideas are given in the curricula in the form of descriptive statements, without pointing much to the type of problem for which they are needed in order to solve it, teaching and verification of the acquired knowledge may be reduced to mere knowledge acquisition, instead of supporting the development of understanding through the application of knowledge in authentic contexts (Talanquer, 2016). A chemistry curriculum should not be limited to chemical concepts only, but the students should be confronted with the nature of the science of chemistry and its relations with other sciences (ACS Guidelines, 2012).
Examination of scientific literacy, which is usually considered to encompass knowledge and understanding of the most important ideas of science and understanding the nature of science, points to a situation that is culturally alarming. A major part of the youth population does not understand the basic scientific concepts, nor do they have any idea of how nature or technology functions (Rasmussen, 2007; Leyh et al., 2015).
The nature of science (NOS) has a prominent place within the framework of education standards in science. Understanding how science functions and a critical evaluation of the validity of scientific claims are clearly relevant when it comes to making decisions on a personal and a collective level (Mamlok-Naaman et al., 2005; Giunta, 2014).
The concept of NOS has changed within the scientific-educational community: from a simple understanding of the scientific method in the early 20th century, through the inclusion of science process skills in the 1960s, to subsequently introducing a series of characteristics of scientific knowledge, psychological and sociocultural factors. The operational definition of NOS given by Lederman (1992) represents a kind of consensus among educators concerning what NOS presupposes. According to this definition, NOS refers to the epistemology of science, to science as a way of knowing or to values and assumptions inseparably linked to scientific knowledge and its development. The key aspects of NOS proposed by Lederman (2007) indicate that scientific knowledge: (1) is tentative (subject to changes), (2) is empirically based (based upon or derived from observation of the natural world), (3) is subjective (includes a personal background, prejudices, and is theory-laden), (4) necessarily involves human inference, imagination and creativity (involves the invention of explanations), and (5) contains in-built social and cultural aspects. Two additional significant aspects are the difference between observation and conclusions, and the functions of and relationships between theories and laws. Lederman's aspects of NOS are close to constructivist epistemology, while the stress is on humane construction, socio-cultural involvement and the changeable nature of scientific knowledge.
Learning NOS may be viewed as a continuum whose one side contains a decontextualised and the other side a highly contextualised NOS (Clough, 2006). Each side of the continuum has its advantages and limitations, and which side teachers will opt for depends on the learning goal that they wish to achieve. If the goal is acquisition of functional scientific literacy, lessons on NOS that are decontextualised have a limited value and are in themselves insufficient for achieving this goal.
Researchers in the sphere of education recognise three contextual approaches through which students can learn about NOS: historical cases, contemporary cases and the students' inquiry activities. The approaches may be used independently, but as each one of them has certain defects, it is best to take into consideration the possibility of their complementary implementation. Also, it is necessary to develop detailed strategies for monitoring the efficiency of each approach (Allchin et al., 2014). The goals that may be realised through the implementation of the contemporary, inquiry-based and historical approach to learning about NOS according to Allchin and collaborators (Allchin et al., 2014) are presented in Table 1.
| Goals/approach | Contemporary | Inquiry-based | Historical |
|---|---|---|---|
| Increasing motivation for learning | + | + | + |
| Understanding the cultural, political and economic context of science | + | − | + |
| Developing experimental competencies | − | + | − |
| Understanding a broad scale of characteristics of NOS | − | − | + |
| Understanding specific characteristics of NOS | + | + | + |
All three approaches may increase the students' motivation for learning about science, but in different ways. The contemporary approach does so through the relevance and the topical character of the issues that it deals with, the inquiry-based approach does so through the students' personal engagement in the work, and the historical approach does so by providing insights into the development of science.
Learning about NOS through the contemporary approach develops the skills of analysing social-scientific problems and leads to the acquisition of functional scientific literacy. This approach focuses on unresolved problems and science-in-the-making. Working on them, the students may become aware of the aspect of science-in-the-making, but they are deprived of insights into aspects of NOS that are connected with the historical development of science or with research activities (Allchin et al., 2014).
The focus of historical cases is not just on presenting historical figures who could serve as role models, on stories about well-known discoveries, or on reconstructions through which science is idealised in accordance with a methodology determined in advance, but on science as a process (Giunta, 1998; Allchin et al., 2014). The most important aspect of NOS that is learned through the history of science is that in science everything is subject to being reviewed (Harlen, 2010; Allchin et al., 2014). As this characteristic of NOS has been the central component of the goals of NOS for half a century, the role of historical episodes is of great importance for realising such goals. Apart from the tentative nature of science, through the historical approach to learning about NOS one can observe a broad range of characteristics of NOS and illustrate the roles of: criticism and debate, theoretical prejudices, the cultural or biographical perspective, general cognitive prejudices, motivation, chance, cooperation, interdisciplinary connections, funding, expertise and credibility, the conflict of interest. This can influence a citizen's or a consumer's evaluation of the reliability of scientific claims made in public (Allchin et al., 2014).
The students may attain a broader overall understanding of the nature of science if given the opportunity to learn about scientific discoveries from a historical perspective (Olsson et al., 2015). The history of science provides an overview of the road from the coming into being, re-examining and changing of scientific knowledge to it being accepted and included in textbooks (Giunta, 2014). The historical approach to science learning may be organised in such a way to enable the students to perceive the similarities between their own ideas and the ideas of scientists in the past (Monk and Osborne, 1997). In historically informed research processes, the students may assume the position of scientists from the past, thus experiencing their mistakes. In doing so, the students, as opposed to scientists, are not burdened by the possibility of failure, so that mistakes can be analysed from a greater critical distance (Allchin et al., 2014). The historical contents humanize the nature of science, which makes it less abstract and more engaging to the students (Rasmussen, 2007).
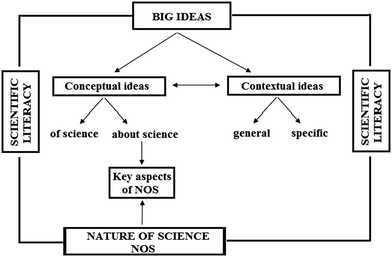
Taking into consideration the meaning of the terms scientific literacy, big ideas, the nature of science and their specific characteristics that have been reviewed previously, the relations between these terms may be presented schematically in the following way (Fig. 1). Each of the said terms affords the teacher a framework for reviewing the purpose of education in the sphere of natural sciences, planning their lessons accordingly and monitoring the students' progress. Reviewing the mutual links between them and their relations enables a deeper understanding of how approaches guided by those concepts mutually complement one another and support the developing of a more functional scientific literacy in the young.
The purpose of the study and the research hypothesis
In comparison with the number of studies which point to the contribution of historical context to NOS understanding (Solomon et al., 1992, 1994; Irwin, 2000; Galili and Hazan, 2001; Faria et al., 2010; Kalman, 2010; Kim and Irving, 2010) as well as to the development of positive attitudes toward science and students' motivations for learning science (Mamlok-Naaman et al., 2005; Faria et al., 2010; Dibattista and Morgese, 2013), the number of studies related to the effects of historical context on the learning of science concepts is lower (Lin, 1998; Seroglou et al., 1998; Sneider and Ohadi, 1998; Galili and Hazan, 2000). Because of that we decided to examine to what extent the historical approach contributes to the acceptance of the conceptual and contextual ideas related to gases in comparison with the context of real life, i.e. the contemporary approach.The purpose of this research was to explore the effects of two approaches to the systematisation of knowledge about gases, which are studied within the framework of the teaching topic Non-metals, non-metal oxides and acids in the eighth grade of primary school (at the age of 14), on the students' level of understanding of the properties and the use of gases in practice. The two approaches to the systematisation of knowledge were designated as: the historical approach and the contemporary approach.
Gases were selected as the object of research because they are an integral part of big ideas and a part of the chemistry curriculum for primary school in our country. For example, the following big ideas comprise gases: Behavior and properties of matter (ACS Guidelines, 2012); Structure/function, Reaction (Murphy et al., 2012); Chemical reactions, Chemistry products in everyday life, Structure–property relations, Reaction energy (De Jong and Talanquer, 2015), as well as Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions or molecules and the forces between them (The College Board, 2011 – AP Chemistry Curriculum Framework for 2013–2014). In the literature review we have found a lot of research studies in which the understanding of concepts such as an ideal gas, kinetic molecular theory, and the gas laws were examined (for example: Benson et al., 1993; Kautz et al., 2005a, 2005b; Senocak et al., 2007; Cetin et al., 2009; Wiebe and Stinner, 2010; Aydeniz et al., 2012). However, these concepts are planned for learning in secondary schools in our country. The primary school students learn only about the properties and the practical use of particular gases, without considering the previously mentioned concepts. Because of that we decided to examine the influence of historical and contemporary contexts on the level of students' understanding of the properties and the practical use of gases at primary school.
Taking into consideration the length of the period in which gases were in the focus of scientists' interest (the period of pneumatic chemistry), the episodes from the history of chemistry about gas discoveries have potential for perceiving different aspects of NOS. Also, for planned research it was important that the historical approach encompasses contents which, in keeping with the aim of the research, can be adapted to the level of students aged 14. At this point, it can be observed that the introduction of the historical context in the teaching of chemistry is not equally suitable for each topic, bearing in mind the students' age and the level of their previous knowledge. Through the practical use of gases in different purposes in everyday life, one can perceive the relevance of gases for individuals and society.
Our hypothesis in this research was that the historical context related to the discovery of gases and to studying their properties would deepen the students' understanding of the properties and the practical use of gases more than the contemporary context.
Methodology
Participants
The sample encompassed 129 students attending the eighth grade, aged 14, from five primary schools in Belgrade, which is 3% of the overall number of primary schools in the territory of the city of Belgrade. The schools making up the sample were selected in such a way that the conditions in them, the state of equipment that they possess and the level of qualification of their teachers should correspond to the average conditions under which chemistry teaching in this country is conducted (Matijašević et al., 2013). Before commencing our research, we addressed the principal of these five schools, asking them whether their school would participate in it, explaining its object, goal and methodology. After receiving the agreement of the principals and the teachers from the natural sciences section, a contract of cooperation was concluded between the Faculty of Chemistry and each school, signed by the Faculty Dean and the school principals. In each school, two eighth-grade classes were selected for the research sample by the random choice method. At the beginning of the research, the students were told its goal and what activities they were expected to participate in during its course. Their participation was voluntary, in the sense that they could opt out of the activities envisaged by the research plan, because they were an addition to the school plan established at the beginning of the school year. Also, it was explained to the students that their scores would be used solely for the research and that they would in no way influence their chemistry marks.Research design
According to the purpose of the study and research hypothesis, the experiment with parallel groups of students (A and B) was conducted. Work with students from both groups encompassed a total of three regular classroom periods, each lasting 45 minutes, two such periods per week. The research was conducted after dealing with the topic Non-metals, non-metal oxides and acids, the first topic of the chemistry curriculum for the eighth grade of primary school. This is the students' second year of studying chemistry as a separate subject within the framework of compulsory eight-year education. In the seventh grade, the students were taught general chemical concepts relating to types of substances (elements and compounds), mixtures and solutions, the structure of substances, and the properties and physical and chemical changes of substances.The schedule of activities in the two groups of students is presented in Table 2. In all the schools constituting our sample the classroom periods were conducted by the same teacher, one of the researchers conducting this study.
| Classroom | The historical approach | The contemporary approach |
|---|---|---|
| Period no. | Group A | Group B |
| 1. | Pre-test | Pre-test |
| 2. |
Dealing with a text containing information on:
• Priestley exploring the influence of CO2 on combustion. (Story about investigation of CO2 properties in the local brewery.) • Priestley discovering soda water. (Story about discovery of the refreshing taste of water impregnated with CO2.) • Priestley getting oxygen by heating mercury(II) oxide. (Story about experiment in which Priestley heated a small quantity of mercury(II) oxide by focusing the rays of the Sun on it.) • Priestley exploring the influence of oxygen on combustion and breathing. (Stories about how Priestley discovered that a candle burned in oxygen and how he breathed pure oxygen after he noticed a positive influence of this gas on the health of a mouse.) • Priestley observing that plants produce oxygen. (Story of how Priestley noticed that plants purified the air in which an animal had breathed or a candle had burned, and story about experiments with water plants.) |
Dealing with a text containing information on:
• The role of carbon dioxide in the process of producing carbonated drinks. (Story about steps in the productions of carbonated drinks and the role of carbon dioxide in it.) • The use of carbon dioxide for extinguishing fires. (Story about fires, the role of oxygen in combustion, and about the use of carbon dioxide for filling fire extinguishers and extinguishing fires.) • The use of oxygen for inhalation in medicine. (Story about the use of oxygen as a therapy for people who have problems breathing.) •The process of photosynthesis. (Story about the role of oxygen and carbon dioxide in the process of photosynthesis.) |
| 3. | Post-test | Post-test |
In the course of the first classroom period, all the students did a pre-test (Appendix 1, ESI†). Based on the pre-test results, two balanced groups of students in terms of achievement (A and B) were formed within the framework of each class. During the second classroom period, the students within each group dealt individually with texts prepared in accordance with the purpose of the study and the posited hypothesis. The students in group A dealt with a text presenting the scientific-research work of Joseph Priestley (1733–1804). The text was compiled on the basis of the researched literature on the history of chemistry and on the life and work of this scientist (Thore, 1924; Holmyard, 1928; Partington, 1937; Jaffe, 1957; Asimov, 1966; Neville and Engineers, 1974). The following episodes were selected from Priestley's eventful biography:
• The discovery of soda water and the observation of the properties of carbon dioxide.
• The discovery of oxygen and the investigation of its properties.
• Experiments wherein it was observed that plants make the air good for breathing by producing oxygen.
Group B was given a text about the use of carbon dioxide in the process of producing carbonated drinks and for filling fire extinguishers, about using oxygen for medical purposes and about the process of photosynthesis. Apart from the texts, the material that the students worked with also included questions which directed the students' attention to the essential bits of information in the texts and checked their understanding of them. The printed material for both groups A and B corresponded to the content of the curriculum for the eighth grade of primary school and was directed towards the same goal: developing the students' ability to describe, explain and draw conclusions about the properties and practical use of gases. At the beginning of the second classroom period, the students were instructed to read the texts carefully and to answer the attendant questions.
The relations among the big ideas, nature of science and scientific literacy according to the historical approach and the contemporary approach in this research are shown in Fig. 2 and 3.
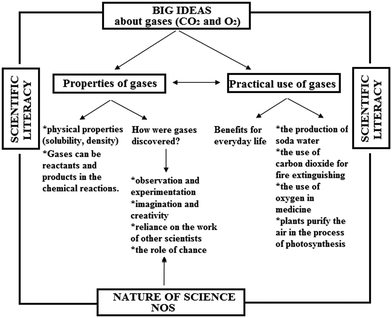 | ||
| Fig. 2 The relations of the big ideas, nature of science and scientific literacy according to the historical approach (group A). | ||
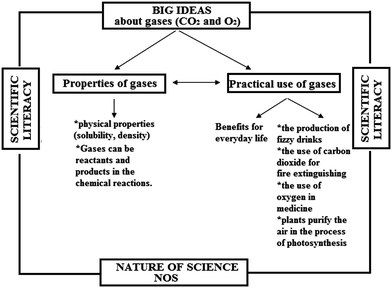 | ||
| Fig. 3 The relations of the big ideas, nature of science and scientific literacy according to the contemporary approach (group B). | ||
As Fig. 2 and 3 show, the both approaches are characterized by the intention to establish the big ideas related to gases and corresponding scientific literacy. The difference between the approaches is associated with the characteristics of NOS (observation and experimentation, imagination and creativity, reliance on the work of other scientists, the role of chance) that are incorporated in the historical approach, i.e. in the stories about the historical period in which Priestley lived and the circumstances under which he performed experiments.
In the course of the third classroom period, the students did a post-test (Appendix 2, ESI†).
Data collection
A pre-test and a post-test were used as research instruments. Both tests contain questions that correspond to the chemistry curriculum for the eighth grade of primary school, and serve to check the students' understanding of the properties of gases (O2 and CO2) and their use.Given that the control and the experimental group should be as similar as possible before the experimental intervention is introduced (Shadish et al., 2002), the results of the pre-test serve as an estimation of how well the students in the two groups are matched when it comes to the previously acquired knowledge concerning the topic Non-metals, non-metal oxides and acids, at the beginning of the experiment. Taking the pre-test may influence the outcome of the subsequent identical post-test for students in both groups (Martella et al., 2013). In order to overcome these obstacles, we applied one of the guidelines for devising the two tests (Cohen et al., 2007), which states that the pre-test and the post-test may differ in form or wording, as long as they refer to the same content. Therefore, in our experiment the pre-test and the post-test were two different tests.
Through a survey of the approved eighth-grade chemistry textbooks currently in use, it was established what content related to the properties of oxygen and carbon dioxide and the use of these gases was available to all the students in the sample before the realisation of the research. Based on that, the pre-test was created. The pre-test contains four questions comprising a total of 15 tasks, 12 closed and three open-ended types. The students had previous experience in dealing with tasks of that type.
The post-test was created with a view to examine the effects of the two approaches on the students' ability to describe, explain and draw conclusions concerning the properties and the practical use of gases. This test contains eight questions comprising a total of 16 tasks, 9 closed and 7 open-ended types. The printed material that corresponded to each approach provided an equal basis to each student for answering the post-test questions, thus preventing favouring either approach.
Both the tests contain the tasks related to the conceptual big ideas of science and specific contextual big ideas presented in Fig. 2 and 3. The big ideas defined by De Jong and Talanquer (2015), conceptual and contextual ideas about gases that are part of these big ideas, as well as the tasks of both tests related to these ideas are presented in Table 3.
| Big ideas (De Jong and Talanquer, 2015) | Part of big ideas | Type of big ideas in the pre-test | Items in the pre-test | Type of big ideas in the post-test | Items in the post-test |
|---|---|---|---|---|---|
| a Item 3 is open-ended type, and encourages divergent thinking, so students' answers could be associated with two big ideas (chemical reactions and structure–property relations). b Item 8B is open-ended type, so a complete answer encompasses two big ideas (chemical reactions and reaction energy). | |||||
| Chemical reactions | There is a need for oxygen in common oxidation reactions (combustion) unlike carbon dioxide. | Conceptual | 1a1, 1b1 | Conceptual | 1A, 1B |
| Contextual | 2A, 3a | ||||
| Some gases react with water and form acids. | Conceptual | 2d | Contextual | 3a | |
| Gases can be reactants and products in chemical reactions. | Conceptual | 3a, 3b, 3c, 3d | Conceptual | 7A, 7B, 7C, 7D | |
| Plants use carbon dioxide and produce oxygen in the process of photosynthesis. | Conceptual | 4a, 4b | Contextual | 8A, 8Bb | |
| Structure–property relations | The density of a particular gas is different from air density. | Conceptual | 1a2, 1b2 | Contextual | 2B, 3a |
| Particular gases have different solubilities in water. | Conceptual | 2c | Conceptual | 5A, 5B | |
| Reaction energy | Sunlight is the source of energy for the process of photosynthesis. | Conceptual | 4c | Contextual | 8Bb |
| Chemistry products in everyday life | Gases are used in the production of fizzy drinks. | Contextual | 2a | Contextual | 4A, 4B |
| Gases are used for medical purposes. | Contextual | 2b | Contextual | 6 | |
The validity of the tests in accordance with the curriculum and the research goal was verified by six chemistry teachers working in primary schools. They evaluated whether both the approaches gave equal chance to students from groups A and B to answer the post-test tasks. Also, these teachers participated in the coding of answers to open-ended questions in the post-test (Appendix 3, ESI†). The applied instruments in this study as well as the coding system are the result of consensus among the teachers. The students' answers to the pre-test and post-test questions were statistically processed using the statistics program SPSS Statistics 17.0.
Results and discussion
Table 4 presents the characteristics of the score distribution in both groups of respondents in the pre-test and the post-test: the number of respondents (N), the minimum (Min) and maximum (Max) scores in the test, the arithmetic mean (Mean), the standard deviation (SD), the percentage of correct answers in each group (p) and the skewness and kurtosis values.| Group | N | Min | Max | Mean | SD | p | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-test | (A) The historical approach | 63 | 4 | 15 | 11.3 | 2.4 | 75.3 | −1.027 | 1.116 |
| (B) The contemporary approach | 66 | 5 | 15 | 10.8 | 2.4 | 72.0 | −0.822 | 0.286 | |
| Post-test | (A) The historical approach | 63 | 5 | 16 | 12.2 | 2.5 | 76.1 | −0.608 | 0.370 |
| (B) The contemporary approach | 66 | 4 | 16 | 12.0 | 2.5 | 75.1 | −0.900 | 0.918 | |
The skewness values for the results distribution in both groups of respondents on both tests are negative and suggest a negatively skewed (left-tailed) distribution with an asymmetric tail extending toward more negative values. The absolute values are less than 1, which means that the skewness of both distributions is slight. The positive kurtosis values for the results distribution in the pre-test and the post-test in both groups point to distributions with a peak.
The statistical significance of the difference in the achievements of group A and group B was investigated by means of the Kruskal–Wallis test. As the level of statistical significance for the obtained values of Chi square exceeds 0.05 (Table 5), it may be concluded that there is no statistically significant difference in the achievements of students from groups A and B in either the pre-test or the post-test.
| Group | N | Mean rank | Chi-square | df | Asymp. sig. | |
|---|---|---|---|---|---|---|
| Pre-test | (A) The historical approach | 63 | 69.46 | 1.795 | 1 | 0.180 |
| (B) The contemporary approach | 66 | 60.74 | ||||
| Post-test | (A) The historical approach | 63 | 66.02 | 0.093 | 1 | 0.761 |
| (B) The contemporary approach | 66 | 64.03 | ||||
Table 6 presents the number and the percentage of students in both groups who accurately fulfilled the tasks in the pre-test, as well as the values of the t-test, which was used to explore the statistical significance of the difference in the percentage of correct answers in groups A and B. The only statistically significant difference between the percentage of correct answers in group A and group B was established concerning the task checking whether the students know that CO2 cannot be a reactant in a combustion reaction (it does not support combustion). The students in group A gave a statistically significant higher percentage of correct answers.
| Items | Correct answers in group A | Correct answers in group B | t | ||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | p A − pB | |
| *The difference in the percentage of correct answers is statistically significant at the level of 0.05. | |||||
| 1a1 | 59 | 93.7 | 55 | 83.3 | 1.84 |
| 1a2 | 25 | 39.7 | 22 | 33.3 | 0.76 |
| 1b1 | 52 | 82.5 | 44 | 66.7 | 2.06* |
| 1b2 | 43 | 68.3 | 42 | 63.6 | 0.56 |
| 2a | 42 | 66.7 | 49 | 74.2 | −0.93 |
| 2b | 56 | 88.9 | 63 | 95.5 | −1.40 |
| 2c | 53 | 84.1 | 54 | 81.8 | 0.35 |
| 2d | 41 | 65.1 | 44 | 66.7 | −0.19 |
| 3a | 58 | 92.1 | 58 | 87.9 | 0.79 |
| 3b | 57 | 90.5 | 56 | 84.8 | 0.98 |
| 3c | 58 | 92.1 | 59 | 89.4 | 0.53 |
| 3d | 57 | 90.5 | 60 | 90.9 | −0.08 |
| 4a | 19 | 30.2 | 18 | 27.3 | 0.36 |
| 4b | 50 | 79.4 | 50 | 75.8 | 0.49 |
| 4c | 41 | 65.1 | 36 | 54.5 | 1.23 |
The percentage of correct answers to most tasks in the pre-test is high in both groups of respondents. Of the 15 tasks contained in the pre-test, in the case of two less than half the students in each group gave the correct answer. Approximately one-third of the respondents in both groups correctly stated the density of oxygen in relation to air. The notion of density is taught within the framework of the subject of physics, and various studies have indicated that there is a problem that students in our country face in connection with the transfer of that knowledge in the sphere of chemistry (Martin et al., 2004, 2008). The other case of low achievement is also indicative of a problem connected with the transfer of knowledge acquired within the framework of another subject. It is the notion of photosynthesis, which is dealt with within the framework of biology. Less than one-third of the students in both groups stated that CO2 and H2O are necessary to plants in the process of photosynthesis. This result is in accordance with the results of the studies which indicate the problems of students in the understanding of the process of photosynthesis (Stavy et al., 1987; Eisen and Stavy, 1988).
Table 7 shows the number and the percentage of students in each group who correctly solved the tasks in the post-test, as well as the values of the t-test, which was used to explore the statistical significance of the difference in the percentage of correct answers in groups A and B. Dealing with the 16 tasks in the post-test, group A scored a higher percentage of correct answers than group B in nine of them. However, a statistically significant difference between the percentage of correct answers scored in group A and group B occurred only in the case of one task (4B), in favour of group A. The task was to provide an explanation of why a particular label corresponds to the composition of fizzy mineral water. Such a result can be connected with the influence of the story about Priestley's work that led to the discovery of soda water, which was included in the textual material that the students in group A dealt with. Through this story, the students were able to perceive the different characteristics of scientists' work: curiosity, observation and experimentation, reliance on the work of other scientists, creativity, and acceptance by the scientific community. We could assume that these contributed to the adoption of the information that carbon dioxide is a constituent of soda water and the application of that information in the new situation (during solving task 4B in the post-test). Group B dealt with textual material in which the industrial process of aerating beverages is described without presentation of the role of individuals in that process.
| Items | Correct answers in the group A | Correct answers in the group B | t | ||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | p A − pB | |
| *The difference in the percentage of correct answers is statistically significant at the level of 0.05. | |||||
| 1A | 52 | 82.5 | 52 | 78.8 | 0.53 |
| 1B | 47 | 74.6 | 53 | 80.3 | −0.78 |
| 2A | 26 | 41.3 | 25 | 37.9 | 0.39 |
| 2B | 8 | 12.7 | 8 | 12.1 | 0.10 |
| 3 | 24 | 38.1 | 21 | 31.8 | 0.75 |
| 4A | 62 | 98.4 | 63 | 95.5 | 0.95 |
| 4B | 57 | 90.5 | 51 | 77.3 | 2.03* |
| 5A | 58 | 92.1 | 57 | 86.4 | 1.04 |
| 5B | 58 | 92.1 | 57 | 86.4 | 1.04 |
| 6 | 57 | 90.5 | 62 | 93.9 | −0.72 |
| 7A | 57 | 90.5 | 62 | 93.9 | −0.72 |
| 7B | 57 | 90.5 | 62 | 93.9 | −0.72 |
| 7C | 58 | 92.1 | 64 | 97.0 | −1.23 |
| 7D | 58 | 92.1 | 63 | 95.5 | −0.80 |
| 8A | 53 | 84.1 | 59 | 89.4 | −0.89 |
| 8B | 37 | 58.7 | 35 | 53.0 | 0.65 |
Of the total of 16 post-test tasks, 12 were completed correctly by around three-quarters of the respondents and more in both groups. Two tasks that were completed correctly by around one-third of the students and less pertain to drawing conclusions on the basis of described experiments were about obtaining and the properties of carbon dioxide (2A and 2B). The expected conclusions were about the fact that CO2 does not support combustion, that is to say, that it is not a reactant in the oxidation reaction, and that its density is greater than that of air. According to the pre-test results, those properties of CO2 were known by more than two-thirds of the students in both groups, but in the case of one-third of the students that knowledge is not functional in the sense that they can apply it in some new situation, for example in the context of fire extinguishing. This result indicates the problem of students in linking conceptual and contextual ideas.
This is also indicated by the achievement of the students from both groups in the third post-test question, wherein they were asked to propose an experiment of their choice whereby they would prove some properties of CO2. The students from both groups had the same experience before this research when it came to observing demonstration experiments and laboratory work. The descriptions of Priestley's experimental work in the material that the students in group A dealt with did not result in a greater number of them proposing, based on the model of Priestley's work, experiments for the purpose of proving CO2 properties.
The fourth post-test task which stands out in terms of a lower percentage of correct answers is 8B. In both groups, more than half the students explained the process of photosynthesis. However, the similar level of achievement in both groups of respondents points to the fact that the historical approach, which encompasses a presentation of Priestley's experimental work with water plants has no greater influence on the students' achievements than the contemporary approach. The majority of students in both groups knew the fact about oxygen formation in the process of photosynthesis (something more than 75% on the pre-test), but that fact in the explanation of the described experiment aquarium with water plant was applied by something more than half of the students in each group. Once again, the problem in linking the conceptual and contextual ideas is noticed.
The students who worked in accordance with approach B were more successful in dealing with the four tasks set in item 7, which was repeated from the pre-test but the difference is not statistically significant. Better achievements of the students in group B on the four post-test tasks set in item 7 in comparison with the four pre-test tasks set in item 3 indicate that the contemporary approach could be more effective in the case of classification of chemical reactions as reactions of combination or decomposition than the historical approach.
Based on the results obtained, the hypothesis that we posited at the beginning of the research cannot be accepted. Overall, in this study both approaches, the historical and the contemporary one, proved to be equally effective. A statistically significant difference in one post-test task can be attributed to the influence of the episode from the history of chemistry, so which combination of the historical and the contemporary approach can contribute to improving the understanding of chemical concepts, and also of the nature of science and scientific-research work is open to further research.
Conclusion
The contemporary teaching of chemistry that arises from the concepts of scientific literacy, nature of science and big ideas requires new solutions relating to the relationship between conceptual and contextual learning. Also, an issue that requires further research is which contexts are the most appropriate ones bearing in mind the general and the specific goals of education and the expected outcomes, the level of the students' previous knowledge and their interests.The research that we conducted, wherein we compared the effects of two contextual approaches, the historical and the contemporary one, on the level of the understanding of students aged fourteen of the properties and the use of gases, showed that the effects of both approaches are similar. In other words, no statistically significant difference was found between the overall achievements of the students who worked on the basis of these two approaches. Based on the results obtained, the research hypothesis about the better effects of the historical approach on the understanding of the properties and the use of gases compared to the effects of the contemporary approach cannot be accepted. However, the statistically significant higher achievement in one post-test task in group A, which was working in accordance with the historical approach, indicates that it would be useful to explore the effects that would be achieved by combining the historical and the contemporary context in which chemical concepts are reviewed. The integration of different contexts may help to avoid a deformed image of science and scientists among children and to stimulate increased interest in science among them. This is in accordance with a request addressed to teachers, which states that, in order for their teaching to be valid, it is necessary to have a deep knowledge of the topic, not only the content but also its methodological aspects, the history of science, STS interactions and recent scientific discoveries (Solbes and Traver, 2003).
Based on the students' achievements on the questions which comprised conceptual ideas on the pre-test and contextual ideas on the post-test, it could be concluded that students have difficulties in establishing links between conceptual and contextual ideas. These difficulties are especially expressed if students' previous experience of learning chemistry is predominantly guided by conceptual ideas, which was the case with the participants in our study. According to that, there is a need for involvement of a greater number of contextual ideas in the teaching process, as well as for better connection between the conceptual and contextual ideas in comparison with usual teaching practice. In that case students would have better opportunities to acquire functional scientific literacy.
Limitations
The order of magnitude of the sample constitutes one of the limitations of the research conducted, as well as the duration of intervention (one classroom period), so that conclusions cannot be generalised. The students' motivation for working with textual materials, as well as their personal preferences concerning historical material may also constitute a limitation of this study. According to that, the students' motivation should be considered in further research.Acknowledgements
This paper is the result of work on the project “The Theory and Practice of Science in Society: Multidisciplinary, Educational and Intergenerational Perspectives”, No. 179048, the realization of which is financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia.References
- Allchin D., Anderesen H. M. and Nielsen K., (2014), Complementary Approaches to Teaching Nature of Science: Integrating Student Inquiry, Historical Cases, and Contemporary Cases in Classroom Practice, Sci. Educ., 98(3), 461–486.
- American Chemical Society, (2012), ACS Guidelines and Recommendations for the Teaching of High School Chemistry, pp. 4–7, available at: http://https://www.acs.org/content/dam/acsorg/education/policies/recommendations-for-the-teaching-of-high-school-chemistry.pdf, accessed 15 Aug. 2014.
- Asimov I., (1966), The search for the elements, New York: Fawcett World Library, pp. 40–42.
- Aydeniz M., Pabuccu A., Cetin P. S. and Kaya E., (2012), Argumentation and Students' Conceptual Understanding of Properties and Behaviors of Gases, Int. J. Sci. Math. Educ., 10(6), 1303–1324.
- Benson D. L., Wittrock M. C. and Baur M. E., (1993), Students' preconceptions of the nature of gases, J. Res. Sci. Teach., 30(6), 587–597.
- Cetin P., Kaya E. and Geban O., (2009), Facilitating conceptual change in gases concepts, J. Sci. Educ. Technol., 18(2), 130–137.
- Clough M. P., (2006), Learners' Responses to the Demands of Conceptual Change: Considerations for Effective Nature of Science Instruction, Sci. Educ., 15(5), 463–494.
- Cohen L., Manion L. and Morrison K., (2007), Research Methods in Education, 6th edn, New York: Routledge.
- De Jong O. and Talanquer V., (2015), Why is it relevant to learn the big ideas in chemistry at school? in Eilks I. and Hofstein A. (ed.), Relevant Chemistry Education From Theory to Practice, Rotterdam: Sense Publishers, pp. 11–31.
- Dibattista L. and Morgese F., (2013), Introducing History (and Philosophy) of Science in the Classroom: A Field Research Experience in Italy, Sci. Educ., 22(3), 543–576.
- Eilks I., Rauch F., Ralle B. and Hofstein A., (2013), How to allocate the chemistry curriculum between science and society, in Hofstein A. and Eilks I. (ed.), A Practical Guide and Textbook for Student Teachers, Teacher Trainees and Teachers, Roterdam: Sense Publishers, pp. 1–36.
- Eisen Y. and Stavy R., (1988), Students' Understanding of Photosynthesis, Am. Biol. Teach., 50(4), 208–212.
- Faria C., Pereira G. and Chagas I., (2010), D. Carlos de Braganca, a Pioneer of Experimental Marine Oceanography: Filling the Gap Between Formal and Informal Science Education, Sci. Educ., 21(6), 813–826.
- Galili I. and Hazan A., (2000), The Influence of an Historically Oriented Course on Students' Content Knowledge in Optics Evaluated by Means of Facets-Schemes Analysis, Am. J. Phys., 68(7), 3–15.
- Galili I. and Hazan A., (2001), The Effect of a History-Based Course in Optics on Students' Views about Science, Sci. Educ., 10(1), 7–32.
- Giunta C. J., (1998), Using History To Teach Scientific Method: The Case of Argon, J. Chem. Educ., 75(10), 1322–1325.
- Giunta C. J., (2014), Review of Teaching the Nature of Science: Perspectives and Resources, J. Chem. Educ., 91(1), 15–16.
- Harlen W. (ed.), (2010), Principles and big ideas of science education, Hatfield: The Association for Science Education, pp. 1–60.
- Holme T., (2014), Comparing Recent Organizing Templates for Test Content between ACS Exams in General Chemistry and AP Chemistry, J. Chem. Educ., 91(9), 1352–1356.
- Holmyard J. E., (1928), The great chemists, London: Methuen & Co., pp. 58–64.
- Irwin A. R., (2000), Historical case studies: teaching the nature of science in context, Sci. Educ., 84(1), 5–26.
- Jaffe B., (1957), Crucibles the story of chemistry, New York: Fawcett World Library, pp. 32–47.
- Kalman C., (2010), Enabling Students to Develop a Scientific Mindset, Sci. Educ., 19(2), 147–163.
- Kautz C. H., Heron P. R. L., Loverude M. E. and McDermott L. C., (2005a), Student understanding of the ideal gas law, part I: a macroscopic perspective, Am. J. Phys., 73(11), 1055–1063.
- Kautz C. H., Heron P. R. L., Shaffer, P. S. and McDermott L. C., (2005b), Student understanding of the ideal gas law, part II: a microscopic perspective, Am. J. Phys., 73(11), 1064–1071.
- Kim S. Y. and Irving K. E., (2010), History of Science as an Instructional Context: Student Learning in Genetics and Nature of Science, Sci. Educ., 19(2), 187–215.
- Lederman N. G., (1992), Students' and Teachers' Conceptions of the Nature of Science: A Review of the Research, J. Res. Sci. Teach., 29(4), 331–359.
- Lederman N. G., (2007), Nature of Science: Past, Present, and Future, in Abell K. S. and Lederman N. G. (ed.), Handbook of research on science education, Mahwah, NJ: Erlbaum, pp. 831–880.
- Leyh B., Avitabile G. and Kelly O., (2015), Designing Courses on the Nature and History of Science, in Maciejowska I. and Byers B. (ed.), A Guidebook of Good Practice for the Pre-Service Training of Chemistry Teachers, Krakow: Faculty of Chemistry, Jagiellonian University in Krakow, pp. 223–246.
- Lin H., (1998), The effectiveness of teaching chemistry through the history of science, J. Chem. Educ., 75(10), 1326–1330.
- Mamlok-Naaman R., Ben-Zvi R., Hofstein A., Menis J. and Erduran S., (2005), Learning science through a historical approach: does it affect the attitudes of non-science-oriented students towards science? Int. J. Sci. Math. Educ., 3(3), 485–507.
- Martella R. C., Nelson J. R., Morgan R. L. and Marchand-Martella N. E., (2013), Understanding and Interpreting Educational Research, New York: The Guilford Press.
- Martin M. O., Mullis I. V. S., Gonzalez E. J. and Chrostowski S. J., (2004), TIMSS 2003 International Science Report: Findings from IEA's Trends in International Mathematics and Science Study at the Fourth and Eighth Grades, Chestnut Hill, MA: TIMSS & PIRLS International Study Center, Boston College.
- Martin M. O., Mullis I. V. S. and Foy P., (2008), TIMSS 2007 International Science Report: Findings from IEA's Trends in International Mathematics and Science Study at the Fourth and Eighth Grades, Chestnut Hill, MA: TIMSS & PIRLS International Study Center, Boston College.
- Matijašević I., Stojiljković D., Đorđević Z., Eraković I. and Korolija J., (2013), State and directions for development of material-technical aspects of the environment for teaching/courses of chemistry in Belgrade primary schools, Pedagogy, 68(4), 619–629.
- Monk M. and Osborne J., (1997), Placing the History and Philosophy of Science on the Curriculum: A Model for the Development of Pedagogy, Sci. Educ., 81(4), 405–424.
- Murphy K., Holme T., Zenisky A., Caruthers H. and Knaus K., (2012), Building the ACS Exams Anchoring Concept Content Map for Undergraduate Chemistry, J. Chem. Educ., 89(6), 715–720.
- Neville R. G. and Engineers K., (1974), Steps leading to the discovery of oxygen, 1774: a bicentennial tribute to Joseph Priestly, J. Chem. Educ., 51(7), 428–431.
- Olsson K. A., Balgopal M. M. and Levinger N. E., (2015) How Did We Get Here? Teaching Chemistry with a Historical Perspective, J. Chem. Educ., 92(11), 1773–1776.
- Partington J. R., (1937), A short history of chemistry, London: Macmillan and Co., pp. 110–120.
- Rasmussen S. C., (2007), The History of Science as a Tool To Identify and Confront Pseudoscience, J. Chem. Educ., 84(6), 949–951.
- Rushton G. T., (2012), Improving High School Chemistry Teaching via the “Trickle Up” Effect: A Perspective on the New AP Chemistry Curriculum Framework, J. Chem. Educ., 89(6), 692–693.
- Senocak E., Taskesenligil Y. and Sozbilir M., (2007), A study on teaching gases to prospective primary science teachers through problem-based learning, Res. Sci. Educ., 37(3), 279–290.
- Seroglou F., Panagiotis K. and Tselfes V., (1998), History of Science and Instructional Design: The Case of Electromagnetism, Sci. Educ., 7(3), 261–280.
- Shadish W. R., Cook T. D. and Campbell D. T., (2002), Experimental and quasi-experimental designs for generalized causal inference, Boston: Houghton Mifflin.
- Sjostrom J. and Talanquer V., (2014), Humanizing Chemistry Education: From Simple Contextualization to Multifaceted Problematization, J. Chem. Educ., 91(8), 1125–1131.
- Sneider C. and Ohadi M. M., (1998), Unraveling Students' Misconceptions about the Earth's Shape and Gravity, Sci. Educ., 82(2), 265–284.
- Solbes J. and Traver M., (2003), Against a Negative Image of Science: History of Science and the Teaching of Physics and Chemistry, Sci. Educ., 12(7), 703–717.
- Solomon J., Duveen J. and Scot L., (1992), Teaching About the Nature of Science through History: Action Research in the Classroom, J. Res. Sci. Teach., 29(4), 409–421.
- Solomon J., Duveen J. and Scott L., (1994), Pupil's images of scientific epistemology, Int. J. Sci. Educ., 16(3), 361–373.
- Stains M., Escriu-Sune M., Lisseth, M., Alvarez de Santizo M. and Sevian H., (2011), Assessing Secondary and College Students’ Implicit Assumptions about the Particulate Nature of Matter: Development and Validation of the Structure and Motion of Matter Survey, J. Chem. Educ., 88(10), 1359–1365.
- Stavy R., Eisen Y. and Yaakobi D., (1987), How students aged 13–15 understand photosynthesis, Int. J. Sci. Educ., 9(1), 105–115.
- Talanquer V., (2013), Chemistry Education: Ten Facets To Shape Us, J. Chem. Educ., 90(7), 832–838.
- Talanquer V., (2016), Central Ideas in Chemistry: An Alternative Perspective, J. Chem. Educ., 93(1), 3–8.
- The College Board, (2011), AP Chemistry Curriculum Framework 2013–2014, pp. 4–72.
- Thore E., (1924), History of chemistry, London: Watts & Co., pp. 76–79.
- Wiebe R. and Stinner A., (2010), Using story to help students' understanding of gas behaviour, Interchange, 41(4), 347–361.
Footnote |
| † Electronic supplementary information (ESI) available: Appendixes 1–3 containing the texts of tests for students taken before and after lessons and open-ended questions answered with teacher participation. See DOI: 10.1039/c7rp00027h |
| This journal is © The Royal Society of Chemistry 2017 |