Visual representations of microcosm in textbooks of chemistry: constructing a systemic network for their main conceptual framework
George
Papageorgiou
 *a,
Vasilios
Amariotakis
a and
Vasiliki
Spiliotopoulou
b
*a,
Vasilios
Amariotakis
a and
Vasiliki
Spiliotopoulou
b
aDemocritus University of Thrace, Greece. E-mail: gpapageo@eled.duth.gr
bSchool of Pedagogical and Technological Education (ASPETE), Greece
First published on 24th April 2017
Abstract
The main objective of this work is to analyse the visual representations (VRs) of the microcosm depicted in nine Greek secondary chemistry school textbooks of the last three decades in order to construct a systemic network for their main conceptual framework and to evaluate the contribution of each one of the resulting categories to the network. The sample comprises a total number of 221 VRs of microcosm, 66 of which are VRs of the 8th grade, 92 of the 9th grade and 63 of the 10th grade. For the qualitative analysis of VRs the phenomenographic method was implemented, whereas a basic quantitative analysis followed. Results provide us with a network that can help science teachers and textbooks designers in identifying the plethora of codes employed in these VRs and the plethora of ways in which VRs can be used, as well as, in determining possible causes of relevant students' misconceptions. Quantitative analysis indicates an effect of grade on the content of VRs and relevant implications for science education are discussed.
Introduction
Since students' conceptions and misconceptions have been in the center of science education research over the last decades, the study of their origins is of paramount importance. One of these origins is related to the content and the structure of the textbooks that science teachers use in the classroom during the teaching/learning procedure (e.g.Myers, 1992; Mayer et al., 1993; Tulip and Cook, 1993). Textbooks play a dominant role in this procedure as repositories of what is thought to be true. In many cases a statement in a textbook is considered to be an indisputable fact, just because it is in the textbook. Taking also into account that textbooks have been the main didactic tool in the context of many educational systems worldwide (Valverde et al., 2002), their analysis is critical and it could provide valuable information for a number of factors affecting students' construction of knowledge and the whole teaching and learning procedure as well (Souza and Porto, 2012). This probably explains the increasing interest of researchers for relevant studies concerning the content, the structure and the general characteristics of the school textbooks (Abd-El-Khalick et al., 2008).In a school textbook, scientific knowledge is re-constructed in order to be understandable by the students and it is presented through various modalities, such as texts and visual displays. These are external representations, on the basis of which, the student constructs corresponding internal representations (e.g.Schnotz, 2002; Gilbert, 2008, 2010; Eliam and Poyas, 2010). Among external visual representations (VRs), those of the submicroscopic entities, such as molecules, atoms, ions or sub-atomic particles, are of great importance, since they can shape students' internal representations of these entities, a precondition for the understanding of the real world phenomena. However, representing submicroscopic entities is not an easy procedure. Onwu and Randall (2006) for instance, investigating students' understanding of the particulate nature of matter through particular representations in three different countries (Japan, Nigeria and South Africa), pointed out that it is difficult to find a certain didactically appropriate way in representing entities, such as atoms and molecules, and the whole procedure is quite problematic. They realized that, trying to manipulate certain properties through such representations, it is possible to mislead students in relation to some other properties, since one cannot manage the sum of the properties through such representations.
Consequently, the systematic study and analysis of the characteristics of VRs of submicroscopic entities in school chemistry textbooks could provide us with information concerning the way in which students possibly internalize them, affecting the whole learning procedure. These submicroscopic entities, i.e. molecules, atoms, ions and sub-atomic particles, constitute in fact the ‘microcosm’ of school chemistry. Although in the everyday language the term ‘microcosm’ is used in various ways, it usually implies a small world, something like a small version of the real world. Considering that, such a small world in school chemistry usually brings to the mind entities and behaviours at the submicroscopic level, despite the fact that this small world functions differently without ‘inherited’ characteristic from the real world (Talanquer, 2009), the term ‘microcosm’ will be used in the present study in order to describe this world of the submicroscopic level.
Theoretical background
In the literature of science education, the term ‘Visual Representations’ (VRs) is usually found to attribute visual objects (e.g. photographs, drawings, symbols) with or without verbal information, which are distinct from the main text and they display aspects of concepts or phenomena (e.g.Cook, 2006; Eliam and Poyas, 2010; Eilam and Gilbert, 2014). As Schnotz (2002) suggests, VRs are not simply decorative elements around the text, but they can act as powerful tools for the representation, organization, transformation and storage of information. From teachers and curriculum designers' perspective, school textbooks' VRs are considered to be potential meaning-making resources facilitating the learning procedure. Thus, a significant number of studies advocate their positive contribution to the students' understanding of the main text in various textbooks (Pozzer-Ardenghi and Roth, 2004). For instance, there are indications that, when students interact with appropriate VRs depicted in textbooks, their competence in the relevant topics increases (Carney and Levin, 2002; Ainsworth, 2006; Eliam and Poyas, 2010). Also, VRs can operate as memorizing aids, since there is evidence that, information in a text connected to VRs can be memorized more easily by the students in comparison to those, without any connection to VRs (Schnotz, 2002).VRs can refer to any of the three levels that Johnstone (1991, 1993) has introduced, i.e. the macroscopic, the submicroscopic and the symbolic, or to any of their combination. The representation of chemical phenomena at these three levels, known as ‘chemistry triplet’, is one of the most powerful ideas in chemical education over the last 30 years, despite its multiple interpretation and reconceptualization in diverse ways during this period (Talanquer, 2011; Taber, 2013). However, this kind of multiple representations is not always beneficial for the students. As a number of researchers suggest (e.g.Kirschner, 2002; Johnstone, 2007; Nyachwaya and Gillaspie, 2016), multiple representations could overload students' working memory depending on what it is represented and how it is presented. Especially, when prior knowledge is limited, students cannot easily combine simultaneously more than one representation, since such a process increases cognitive load beyond to what they can elaborate in their working memory (Seufert, 2003; Cook, 2006; Cook et al., 2008). Thus, the role of the science teachers and the instructional material is very important at this point. A science teacher can help students in stimulating their attention to the distinct role of each one of the representations of the ‘chemistry triplet’ and in understanding the meaning of them prior to any use of their combination for explanatory reasons (Johnstone, 2007). However, as Treagust et al. (2003) suggest, science teachers often fail to help students in such an effort using any of the three levels or all of them without any sufficient clarification as for their meaning and explanatory power. On the other hand, VRs are also powerful instructional materials and they can help students towards this direction. As Braga et al. (2012) suggest, when a student is studying a VR, (s)he is trying to understand its meaning by decoding and deploying the entities involved. When a VR is appropriately designed, this decoding can shape student's internal representations for any of the three levels (macro-, submicro- and symbolic) and help them in understanding the relations between them, fulfilling a fundamental precondition for the understanding of phenomena (Ardac and Akaygun, 2004, 2005; Gilbert and Treagust, 2009). As a result, the appropriate design of such VRs can ultimately lead students to the adoption of a more scientific view through the construction of the corresponding internal mental models (Wu et al., 2001; Pozzer and Roth, 2003; Kapici and Savasi-Acikalin, 2015).
Specifically, the ‘VRs of microcosm’ are major didactic tools that can stimulate the imagination of students and help them in seeing the unseen world of particles prior to face more complex concepts and phenomena (Close et al., 2002; Arcavi, 2003; Adadan et al., 2009). This is of great importance since a ‘view’ of this submicroscopic world can provide students with answers for the behavior and the properties of the substances in the real world. Any change in substances properties is due to the corresponding change in the structures and/or the behavior of these submicroscopic entities and thus, any explanation of phenomena presupposes the successful connection of these two worlds (e.g.Johnson and Papageorgiou, 2010; Papageorgiou, 2013). Consequently, VRs providing models of these submicroscopic entities can serve as valuable tools for the development of the corresponding students' internal mental models of these entities (e.g.Akaygun and Jones, 2014; Kapici and Savasi-Acikalin, 2015), contributing to the connection between the submicro- and the macro-worlds, as well as to the interplay between all the three levels of the chemistry triplet, in general. This is the main reason why the role of the VRs of microcosm in the school textbooks has been studied by many researchers, acknowledging their contribution to the teaching/learning procedure (e.g.Mesa, 2004; Cook, 2006; Gkitzia et al., 2011; Mason et al., 2013; Kapici and Savasi-Acikalin, 2015). Although the researchers generally approach the role of this kind of VRs from various perspectives, they all acknowledge explicitly or implicitly the importance of their appropriate design. On the contrary, there is evidence that any designing inattentions can lead students to relevant misconceptions (Han and Roth, 2005; Cook, 2006; Onwu and Randall, 2006). In those cases, the students are not enabled to understand the correspondence between the characteristics of the model in the VR and those of the submicroscopic entities (e.g.Grosslight et al., 1991; Harrison and Treagust, 2000; Coll and Treagust., 2003; Han and Roth, 2005), attributing characteristics such as color or size to the submicroscopic entities (e.g.Griffiths and Preston, 1992; Coll and Treagust, 2003; Talanquer, 2009; Taber and García-Franco, 2010), describing them as entities that they can feel or want (e.g.Taber, 2003; Talanquer, 2013) or describing some of them anthropomorphically as alive (e.g.Griffiths and Preston, 1992; Harrison and Treagust, 1996).
Rationale of the study
Due to the great importance of the VRs of textbooks in the learning process, their analysis has been found many times in the center of the researchers' interest. However, researchers focus on VRs from different perspectives each time, using different tools for their analysis and leading to a variety of conclusions. For instance, Dimopoulos et al. (2003), in order to study textbooks' VRs from pedagogical and sociosemiotic perspectives, focused on their content classification, their social–pedagogical relationships and the degree of abstraction, elaboration and specialization of the expressive codes employed. They found that there was an extended use of VRs in order to help students to be familiar with technical and scientific codes. They also found that the content of VRs varied according to the educational level, as well as the abstraction and elaboration of the visual codes were increasing along with the level. From a different perspective, Souza and Porto (2012) analyzed VRs in 31 chemistry textbooks that had been used in Brazilian universities emphasizing their iconographic elements in relation to the represented conceptions of chemistry. Nine categories of VRs resulted, attributing interactions between the discipline of chemistry, its teaching/learning context, any author's personal characteristics and society. Also differently and in a semiotics framework involving macroscopic and submicroscopic entities, VRs in grade-seven Korean science textbooks were analyzed by Han and Roth (2005) in terms of their function and structure. Their analysis suggests that, students' difficulties in understanding the particulate nature of matter are possibly due to various different processes in interpreting the relation between the inscriptions depicting macroscopic entities and the corresponding models based on submicroscopic particles.Searching the literature of science education, one can find a variety of such methods, tools and criteria for the analysis and categorization of the textbooks' VRs including those of the microcosm. In these efforts, the VRs of microcosm are usually analyzed as a part of the total VRs of textbooks, under the implementation of a single analyzing idea or scheme for all the VRs. However, taking into account the paramount importance of students' understanding of the microcosm for the further understanding of concepts and phenomena in general, it seems imperative to focus systematically on the analysis of exclusively the VRs of microcosm, in a way that could be useful for science teachers and curricula designers. In this context, we launched a project in order to analyze the characteristics of the VRs of microcosm depicted in chemistry school textbooks, which are used in Greek secondary education during the last three decades, generating a relevant systemic network. According to Bliss and Ogborn (1979), a systemic network is a structured pattern of interdependent options that can be created in an environment that offers those options. In the present effort, the environment is formed by the VRs of microcosm and the options are the possible resulting categories. During the construction of this systemic network, four distinct parts of the whole network emerged, which constitute in fact four axes of it, concerning the following:
(a) The main conceptual framework of the VRs.
(b) The didactic characteristics of the VRs, exploring the way in which a VR could didactically be used (e.g. for the presentation of an entity, for the interpretation of a phenomenon, etc.), the topics that could be facilitated in a teaching/learning procedure, etc.
(c) The illustration characteristics of the VRs, exploring whether a VR is a diagram, a photograph, a drawing etc, whether it is 2-D or 3-D, whether it is realistic, symbolic, metaphoric etc.
(d) The relations of a VR to the main text of the corresponding textbook (whether and how it is connected to the text, its position in the page etc.).
The main objective of the construction of the whole systemic network was to help researchers, science teachers and curricula designers in identifying the plethora of codes (symbols, ways/kinds of representations, features, etc.) employed in these VRs and the plethora of ways in which VRs can be used, as well as, in determining possible causes of relevant students’ misconceptions when interpreting these codes. In some axes, a further exploration across grades was found to be interesting, whereas in others, the interest focused on their progress through these three decades. The present paper refers to the analysis of these VRs regarding their main conceptual framework across three grades of Greek secondary education.
In this context, the following research questions are addressed in the present study:
• How can the main conceptual framework of the VRs of microcosm, which are included in the Greek secondary chemistry textbooks over the last thirty years, be categorized in a systemic network, in order to better understand the attributes they include and the ways of their interpretation, as well as to identify possible causes of students' misconceptions?
• How often can the corresponding categories of such a network be found in the VRs of microcosm of Greek secondary education across grades and how could any differences be related to the chemistry curricula design?
Method
The sample
For the needs of the present work, nine textbooks of chemistry, which have been used in Greek secondary education over the last three decades, were studied and their VRs of microcosm were analyzed (see Appendix 1). Three of them have been textbooks of the 8th grade, three of the 9th grade and three of the 10th grade. The sample comprises a total number of 221 VRs of microcosm, 66 of which are VRs of the 8th grade, 92 of the 9th grade and 63 of the 10th grade. In addition, although the textbooks of the sample have some differences in their relevant units comparing to each other, an outline of these textbooks units in each grade can be presented in Table 1.| Unit | 8th grade (66) | 9th grade (92) | 10th grade (63) | Total (221) |
|---|---|---|---|---|
| Structure of matter | 36 | 0 | 11 | 47 |
| Chemical bond | 3 | 0 | 18 | 21 |
| Chemical changes | 7 | 3 | 3 | 13 |
| Changes of states | 0 | 0 | 12 | 12 |
| Elements and their properties | 14 | 13 | 4 | 31 |
| Inorganic compounds and their properties | 5 | 12 | 1 | 18 |
| Organic compounds and their properties | 1 | 60 | 11 | 72 |
| Mixtures | 0 | 4 | 3 | 7 |
Throughout the sample, a ‘VR of microcosm’ was considered to be any visual object representing either (a) entities of the submicro-level, such as atoms, molecules, ions or sub-atomic particles, using various scientific codes for their depiction, such as structural formulas, ball-and-stick models or appropriate symbols/signs, or (b) entities of the macro-level, which analogically or metaphorically refer to corresponding entities or phenomena of the microcosm, such as those of Fig. 1 and 2. The ‘unit of analysis’ was the VR itself and its caption. Any text connected to the VR, although studied, was not analyzed.
 | ||
| Fig. 1 A photo of some (macroscopic) ball-and-stick models made of plastic. The figure is taken from the 10th grade textbook (Liodakis et al., 2011). | ||
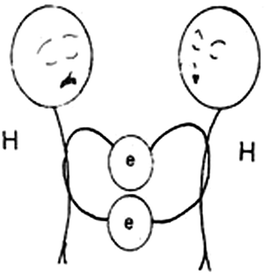 | ||
| Fig. 2 An analogy for the hydrogen covalent bond. The figure is taken from the 8th grade textbook (Frassari and Drouka-Liapati, 1993). | ||
The procedure of analysis
For the analysis of VRs the phenomenographic method was implemented (Marton, 1986; Marton and Booth, 1997; Albertazzi, 2013) that explores the qualitatively different ways in which one can experience and understand a situation. Although a VR expresses the ways in which its designer experiences the situation, in a phenomenographic analysis the exploration is based primarily on the experiences of the researcher and secondarily on those of the designer/author of the textbook. The ‘situation’ in the present study is the microcosm and the ‘exploration’ concerns its visual representations in Greek chemistry textbooks through our experiences.In this context, a qualitative content analysis (Mayring, 2000) was applied for the sample, using an inductive approach for the formation of the categories. For each one of the ‘units analysis’, all the existing data were phenomenographically explored, in order to form the corresponding categories. As this procedure was going on, a common coding system was adopting for each category. On this basis, a systemic network was developed (Bliss and Ogborn, 1979; Bliss et al., 1983) comprising the four distinct parts, i.e. the four axes, reported above. One of these axes concerns the main conceptual framework of the VRs, which is the one presented in this paper. In order to bring this axis in its final form, three researchers independently applied it in parts on the corpus of the sample. During this procedure, a number of VRs were given to the researchers in order to categorize them in the existing categories. When this was not possible, new categories were created or existing categories were modified. The categorizations of the three researchers were compared to each other and any disagreement was discussed in a meeting in order to reach an agreement. This procedure was repeated until the entire sample has been examined and a total agreement has been achieved. Fig. 3 shows the total procedure followed by the researchers during the analysis of the present axis concerning the main conceptual framework of the VRs. As it is presented in Fig. 3, after the formation of the final categorisation scheme, a basic quantitative analysis follows using descriptive statistics. For this analysis, the frequencies of the VRs of each one of the three grades were examined and the corresponding percentages (%) of final categories were calculated separately in each grade, whereas the total percentages of final categories were also calculated on the basis of the entire sample.
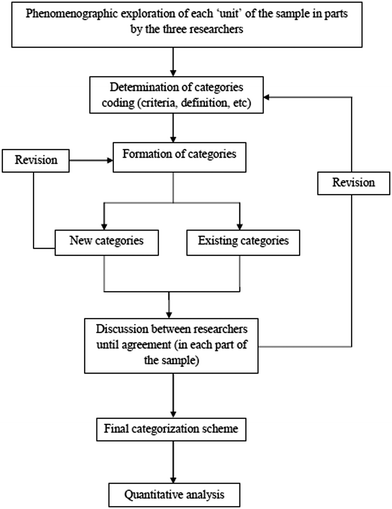 | ||
| Fig. 3 A schematic presentation of the procedure of analysis concerning the axis of the main conceptual framework. | ||
Results and discussion
The systemic network
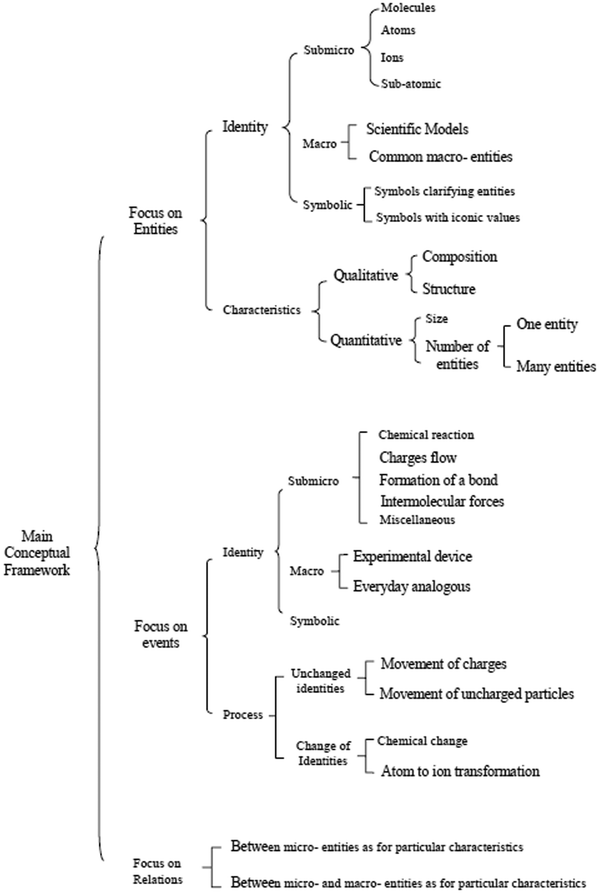
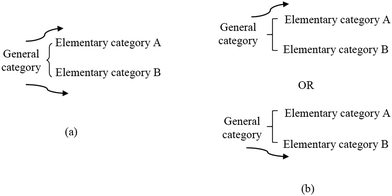
Fig. 4 presents the part of the systemic network resulting from the analysis of the VRs of the present study, which concerns their main conceptual framework. In the network, elementary categories are combined in order to form more general categories or further general categories. Reading the network from the left to the right, a number of paths can be followed from more general categories to more elementary categories passing through one out of two kinds of notation; that is, a curly bracket or a square bracket. In a curly bracket (case a, Fig. 5), one can choose to pass from the general category (on the left) to one or more elementary categories (on the right), whereas in a square bracket (case b, Fig. 5) (s)he has to choose one of the elementary categories excluding any other.On this basis, Fig. 4 shows that the main conceptual framework of a VR of the sample focuses on entities and/or events and/or relations. In the first case, a VR is an attempt to represent one or more entities of the microcosm in order to identify them and/or describe their characteristics. An entity can be identified at the submicro-level as a particle (atom, molecule, etc.) that is depicted as a circle, a sphere or any other appropriate way conventionally accepted by the scientific community. In Fig. 6 for instance, the ions of Na+ and Cl− can be identified in a lattice of NaCl depicted as spheres. An entity could also be identified at the macro-level through a scientific model of the microcosm, which analogically or metaphorically refers to the corresponding entities of the microcosm, (e.g., a photo of a plastic ball-and-stick model, Fig. 1) or a common macro-entity, which is directly connected to a submicroscopic entity (e.g. a macroscopic view of a piece of diamond in connection to its corresponding microscopic view, Fig. 7). In addition, a number of chemical symbols could be used in a VR of microcosm for identification purposes (category symbolic). These could be symbols clarifying the depicted submicro-entities (e.g. symbols Na+ and Cl− on the depicted balls representing the corresponding particles in a lattice of NaCl, Fig. 6) or symbols with an iconic value, such as Lewis structures or structural formulae, representing in fact submicro-entities in a symbolic way (Talanquer, 2011). However, a VR can also describe the characteristics of one or more entities of the microcosm. These could be qualitative, such as composition or structure, and/or quantitative, such as size, and/or number of entities. For instance, the VR of Fig. 6 describes qualitative characteristics, including composition and structure of the NaCl lattice, and quantitative characteristics, showing the relevant size of Na+ and Cl− for a number of such entities. On the contrary, in the VR of Fig. 7, although it describes similarly qualitative and quantitative characteristics, there are no data concerning the size of the carbon atoms.
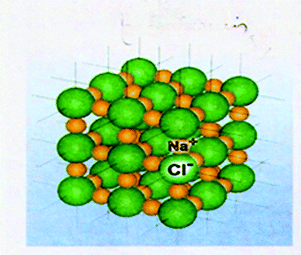 | ||
| Fig. 6 The ions of Na+ and Cl− in a lattice of NaCl. The figure is taken from the 8th grade textbook (Avramiotis et al., 2007). | ||
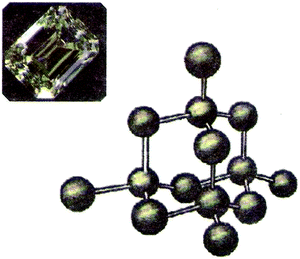 | ||
| Fig. 7 The diamond structure in connection to its macroscopic view. The figure is taken from the 8th grade textbook (Georgiadou et al., 1997). | ||
When a VR of the microcosm focuses on event(s), the aim could be the presentation of one or more events themselves. In this case, a specific identity could be attributed to an event. Thus, an event could be identified at the submicro-level as a specific chemical reaction (see Fig. 8, upper part), a formation of a chemical bond (see Fig. 9), an existence of a flow of charges in an electric circuit or in an electrolysis process, etc. For the same purpose, however at the macro-level, a VR of microcosm could also depict a macroscopic view of an experimental setup combined with submicroscopic elements, such as an electrolysis setup (Fig. 10), a heating device or an electric circuit. At the same level (macro-), an everyday analogous, such as that one of Fig. 11 (analogous to enzyme activity), could be depicted in order to help students to identify the event. In addition, a number of chemical symbols could be also depicted here (see Fig. 8, lower part) in order to determine through a symbolic way what is already depicted at the submicro-level (category symbolic).
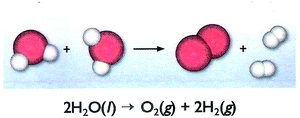 | ||
| Fig. 8 A VR concerning a chemical reaction. The figure is taken from the 8th grade textbook (Avramiotis et al., 2007). | ||
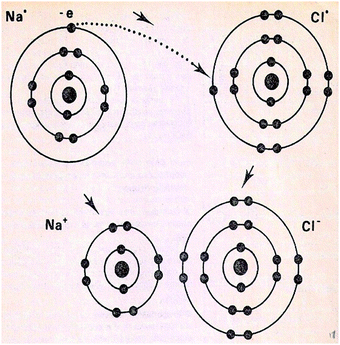 | ||
| Fig. 9 The formation of NaCl. The figure is taken from the 8th grade textbook (Frassari and Drouka-Liapati, 1993). | ||
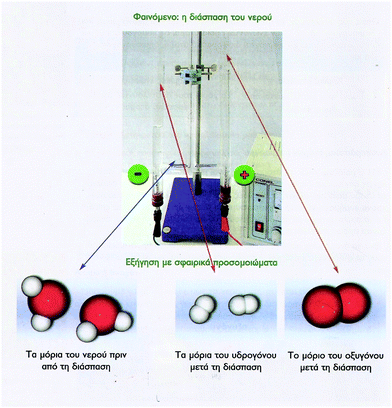 | ||
| Fig. 10 VR of a chemical reaction, where an experimental setup is also included. The figure is taken from the 8th grade textbook (Avramiotis et al., 2007). | ||
 | ||
| Fig. 11 An analogy for the activity of an enzyme. The figure is taken from the 9th grade textbook (Georgiadou et al., 1998). | ||
However in a VR, the process of an event can be also depicted. In this case, the categorization deals with how this event happens. More specifically, in an event either, entities could just be moving remaining unchanged, such as electrons around the nucleus, or entities could change through a chemical change or a transformation of atoms to ions (and vice versa). The VR of Fig. 11 for instance, additionally to its contribution in identifying an event (as reported above), could help students to understand analogically how during this event an enzyme acts in order to change the initial entities into others (chemical change). Similarly, Fig. 9, additionally to its contribution in identifying sub-atomic particles (entities) and the formation of a bond (event), could help students in understanding both, the structure of the two atoms that involve many sub-atomic entities (characteristics of entities) and how the movement of an electron can transform atoms to ions (processes of the event). On the contrary, the VR of Fig. 10, apart from the depiction of particular entities (as for their identity and characteristics), can help in understanding the event as an identity (a chemical reaction), but not as a process.
The focus of a VR could also be on the description of relations. These relations could be either, between submicro-entities as for particular characteristics (e.g. size of different atoms, freedom of movement between particles in liquid and gas states, etc.), or between characteristics of a submicro-entity and a macro-entity (e.g. structure of a lattice and durability of the corresponding substance). The VR of Fig. 12 for instance, apart from the depiction of particular entities, could help students to find relations between the atom and the ion of lithium as for their structure, composition and size.
 | ||
| Fig. 12 The atom and the ion of lithium: relations as for their structure, composition and size. The figure is taken from the 8th grade textbook (Avramiotis et al., 2007). | ||
Quantitative analysis
Table 2 shows the frequencies of categories of the systemic network found in each one of the three grades and in total. Since the number of VRs in each one of the three grades differs, the corresponding percentages (%) of Table 2 are calculated separately in each grade on the basis of the corresponding number of VRs, whereas the total percentage is calculated on the basis of the entire sample.| Categories | 8th grade (66) | 9th grade (92) | 10th grade (63) | Total (221) | ||||
|---|---|---|---|---|---|---|---|---|
| Entities | Identity | Submicro | Molecules | 42.4 (28) | 33.7 (31) | 55.6 (35) | 42.5 (94) | |
| Atoms | 75.7 (50) | 36.9 (34) | 69.8 (44) | 57.9 (128) | ||||
| Ions | 18.2 (12) | 14.1 (13) | 28.6 (18) | 19.5 (43) | ||||
| Sub-atoms | 16.7 (11) | 3.3 (3) | 7.9 (5) | 8.6 (19) | ||||
| Macro | Scientific models | 4.5 (3) | 10.7 (10) | 14.3 (9) | 10.0 (22) | |||
| Common macro-entities | 15.2 (10) | 19.6 (18) | 20.6 (13) | 18.6 (41) | ||||
| Symb. | Symbols clarifying entities | 36.4 (24) | 21.7 (20) | 23.8 (15) | 26.7 (59) | |||
| Symbols with iconic values | 4.5 (3) | 41.3 (38) | 7.9 (5) | 20.8 (46) | ||||
| Characteristics | Qualit. | Structure | 59.1 (39) | 70.7 (65) | 60.3 (38) | 64.3 (142) | ||
| Composition | 54.5 (36) | 56.5 (52) | 49.2 (31) | 53.8 (119) | ||||
| Quantit. | Size | 45.5 (30) | 21.7 (20) | 49.2 (31) | 36.7 (81) | |||
| Number of entities | One entity | 18.2 (12) | 28.3 (26) | 4.8 (3) | 18.6 (41) | |||
| Many entities | 71.2 (47) | 64.1 (59) | 92.1 (58) | 74.2 (164) | ||||
| Events | Identity | Submicro | Chemical reaction | 21.2 (14) | 23.9 (22) | 7.9 (5) | 18.6 (41) | |
| Charges flow | 4.5 (3) | 0.0 (0) | 3.2 (2) | 2.3 (5) | ||||
| Formation of a bond | 3.0 (2) | 1.1 (1) | 6.3 (4) | 3.2 (7) | ||||
| Intermolecular forces | 0.0 (0) | 2.2 (2) | 4.8 (3) | 2.3 (5) | ||||
| Miscellaneous | 10.6 (7) | 3.3 (3) | 7.96 (5) | 6.8 (15) | ||||
| Macro | Experimental device | 7.6 (5) | 0.0 (0) | 1.6 (1) | 2.7 (6) | |||
| Everyday analogous | 1.5 (1) | 2.2 (2) | 0.0 (0) | 1.4 (3) | ||||
| Symbolic | 18.2 (12) | 12.0 (11) | 0.0 (0) | 10.4 (23) | ||||
| Process | Movement of charges | 3.0 (2) | 1.1 (1) | 4.8 (3) | 2.7 (6) | |||
| Movement of uncharged particles | 1.5 (1) | 0.0 (0) | 0.0 (0) | 0.5 (1) | ||||
| Chemical change | 21.2 (14) | 18.5 (17) | 7.9 (5) | 16.3 (36) | ||||
| Atom to ion transformation | 9.1 (6) | 2.2 (2) | 4.8 (3) | 5.0 (11) | ||||
| Relations | Between micro-entities as for particular characteristics | 22.7 (15) | 15.2 (14) | 12.7 (8) | 16.7 (37) | |||
| Between micro- and macro-entities as for particular characteristics | 13.6 (9) | 6.5 (6) | 14.3 (9) | 10.8 (24) | ||||
In order to better evaluate the distribution of VRs of microcosm across grades, it would be helpful to have also a view of their contextualization in the units included in the curriculum of each grade (Table 1). As it is shown, VRs of microcosm are present in units such as, the ‘structure of matter’, where a study of atoms, molecules, ions and sub-atomic particles is included, the ‘chemical bond’, ‘chemical changes’, ‘changes of states’, ‘elements and their properties’, ‘inorganic compounds and their properties’ including acid, bases and salts, ‘organic compounds and their properties’ and a study of ‘mixtures’ including solutions.
Comparing entities and events depicted in the VRs of microcosm (Table 2), when the purpose is to attribute an identity, entities are much more frequent than events. Among entities and in particular those at the submicro-level, atoms are more frequent than any other particle of the microcosm, whereas ions appear to be significantly less frequent than atoms or molecules. This holds true for all grades, even for the 9th grade, where in the curriculum, although there is not a focus on the ‘structure of matter’, there are relevant VRs in the context of the units related to particular substances and their properties (see Table 1). Although approaching the submicroscopic world through the atom is something reasonable since this is one of the most fundamental idea (Feynman, 1963), however, such an emphasis on atoms could possibly contribute to the idea that other particles of the microcosm are just alter forms of the atom (Taber, 1998, 2003). On the other hand, entities at the macro-level are less frequent comparing to those at the submicro-level and show an increasing rate of their percentages (%) from the 8th to the 9th and 10th grades. Such a manipulation of the VRs across grades could be justified by the findings of a number of studies (Seufert, 2003; Cook, 2006; Cook et al., 2008) according to which, learners with low prior knowledge cannot understand and combine simultaneously different kinds of representations, focusing rather on the more familiar one. Thus, students for instance, of the 8th grade, whose prior relevant knowledge is very limited, could not have benefits from a coexistence of macro- and submicro-representations, rather the opposite. However, this justification contradicts to the corresponding manipulation of the symbolic representations, which does not follow the same rationale across these three grades (Table 2). On the contrary, what is obvious for the symbolic representations is that, the category ‘symbols with iconic values’ is much more present in the 9th grade than in the other two grades. It seems that in this grade, the VRs designers found the focus of the curriculum on particular substances and their properties (see Table 1) as an opportunity to familiarize students with chemical symbols, to a certain degree. As Nyachwaya and Wood (2014) suggest, the content of a textbook and the corresponding curricula play a significant role in the presence of symbolic representations in the textbook. When they analyzed chemical representations in physical chemistry textbooks they found an overwhelming presence of symbolic representation over the macro- and submicro-representations; they attributed this presence to the character of physical chemistry curriculum.
Regarding the characteristics of entities, VRs of microcosm frequently include depictions of their structure, composition and size, where usually many entities participate. Although there are some differences among grades due to corresponding differences in the curriculum, e.g. the emphasis on structures and composition than on size in the 9th grade, probably due to the focus on the structure and the composition of particular substances in this grade, the percentages of entities characteristics are generally quite significant.
Among events, expectantly for VRs of chemistry textbooks, chemical reactions dominate as identities at the submicro-level, although their percentages are not generally high. Chemical reactions are more frequently identified in the 8th grade, where they are explicitly introduced at the submicro-level, and the 9th grade, where they are studied in the context of the properties of particular substances also at the submicro-level, whereas their frequencies decrease in the 10th grade. Similarly, symbolic representations of events show a decrease rate from the 8th to the 10th grade. This is possibly due to the effort of the VRs designers to offer more visual aids to the students of the lower grades in order to be familiar with chemical reactions at the submicro- and the symbolic levels. On the contrary, symbolic representations of chemical reactions are much more frequently identified inside the main text in the 9th and especially in the 10th grade (something that is not under consideration in the present study), justifying their absence from the corresponding VRs. In accordance with Kapici and Savasi-Acikalin (2015), who found an increasing presence of symbolic representations in Turkish middle school science textbooks from sixth grade to eighth grade, the total presence of symbolic representations in the school textbooks becomes more intense along with the grade. However in our case, it seems that the interest of textbook designers conveys from the VRs of microcosm to the symbolic representations inside the text as the grade increases. In other words, designers possibly pay more attention to familiarize the students of higher grades with the chemical language, something that is expressed via the frequent use of chemical equations (at the symbolic level) inside the main text rather, than to use VRs of microcosm with coexisted submicro- and symbolic levels as a result of the consideration that the students of higher grades have the prior knowledge to benefit from such a coexistence. In addition, designers of VRs in all the grades do not pay significant attention to connect submicro- and macro-levels also here; percentages in identifying events at the macro-level are very low. As Treagust et al. (2003) suggest, it seems that students’ ability in connecting the submicroscopic events to the real world is overestimated.
As for the processes of the events, it seems that the focus is rather on the category of chemical change. However, there is also a decrease of the percentages (%) concerning the processes of chemical changes from the 8th to the 10th grade, probably due to their increasing presence in the main text in the form of chemical equations (as reported above). The fact that the depiction of events is limited, together with the also limited description of their process, leads to the conclusion that information concerning a dynamic aspect of the VRs of the sample is missing here.
When focusing on relations, the emphasis is rather on those between submicro-entities as for an attribute. There are not equally corresponding relations between submicro- and macro-entities. However, an effort to familiarize students with these relations through VRs would be rather expected to a certain degree, due to their importance, since their understanding is a precondition for the understanding of the real world (e.g.Johnstone, 1991, 1993; Talanquer, 2011).
Conclusions and implications for science education
The analysis of the VRs of the sample showed that their main conceptual framework includes entities and events of the microcosm as well as possible relations between them, giving emphasis to the entities rather than to the events, whereas relations are even less frequently found in the framework. However, the systemic network of Fig. 4 could be widely applied on the analysis of numerous VRs of the microcosm and could be a useful tool for both, science teachers and textbooks designers, in a more extended degree. Studying the network, one can have a more integrated view of the codes and aspects possibly involved in the main conceptual framework of a VR of the microcosm. This can help when the case is the way in which a reader (student) would possibly internalize the basic meaning of such a framework of a VR. A science teacher for instance, using the network, could locate categories in the main framework of a VR, which had not been initially so obvious by him/her. Thus, (s)he could help students trying to interpret the codes employed in the VR, taken into consideration these categories and the ways in which they could contribute to its interpretation. In addition, (s)he could also locate codes employed in the VR that are not included in the categories of the main framework of this particular VR, but they could be interpreted by a student (misleadingly) as being included.For example, in Fig. 13, the main conceptual framework includes the identification of sub-atomic particles (entities) and the formation of a bond (event), as well as both, the structure of the two atoms that involve many sub-atomic entities (characteristics of entities) and how the movement of an electron can transform atoms to ions (processes of the event). However, the relative size of protons and electrons is not included in the main conceptual framework of this VR (it is of secondary priority) and there is not an accurate depiction, respectively. However, it could be taken by a student as such, leading him/her to relevant misconceptions. As Gkitzia et al. (2011) suggest, such features of a representation constitute a basic criterion for its evaluation and they should be clear in order to ensure that students would understand the correct meaning of the representation. In addition, the systemic network can provide a teacher with a more clear view of the usage that a VRs of microcosm can have in the context of particular strategies. For instance, (s)he can better evaluate whether a VR is appropriate for the presentation of an entity, an event or a relation, whether it could be used in the context of an interpretation process, whether it is appropriate for questioning, etc.
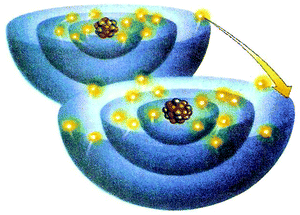 | ||
| Fig. 13 A VR showing the movement of an electron that transforms atoms to ions during the formation of NaCl. The figure is taken from the 10th grade textbook (Liodakis et al., 2011). | ||
Similarly, the systemic network could help designers of VRs and textbooks in general. Although, when focusing on certain attributes of a VR, it is possible to fail in attributing others (Onwu and Randall, 2006), the integrated view of the entire systemic network of Fig. 4 can help designers in anticipating such failures and avoiding the resulting students' alter interpretations. Thus, designers can better specify the categories of the main conceptual framework of a VR that they would like to focus on, and to realise the multiple ways in which any attributes of the VR could be interpreted by the students or the teachers. Also, they could stimulate students' attention targeting on these specific categories through an appropriate design or the use of clarifying captions/references. For instance, in the case of Fig. 13, a more accurate design of electrons and protons or a caption with an articulation clarifying the indicative relevant size of them could avoid possible students' misconceptions.
As for the distribution of the corresponding categories of the present VRs sample across grades, the conclusion drawn is that, the design of the curriculum/textbooks context in each one of the grades does have a relation to the corresponding main conceptual framework of the VRs of microcosm, to a certain degree. In particular, there are cases where, studying Tables 1 and 2, one can see that the frequencies in Table 2 are higher in the categories of VRs that are contextualised in relevant units of Table 1. For instance, the frequencies of the categories relating to the identity of entities at the submicro-level (atoms, molecules, ion, sub-atomic particles) are higher in the 8th and 10th grades, where the curriculum focuses especially on the ‘structure of matter’ and the ‘chemical bond’, comparing to the 9th grade, where this unit is not included. On the contrary, in the 9th grade, where the curriculum focuses on particular substances and their properties (see Table 1), the frequencies corresponding to the symbols with iconic values are higher (see Table 2). Nevertheless, it is worth noting that even in this grade (9th) the frequencies corresponding to the submicro-level, although lower, are noticeable. This could support the aspect that the systemic network is useful in any grade, since its categories can be found in VRs of microcosm contextualised in a plethora of topics. On the other hand, there are cases, where the curriculum is not probably the determinative factor for the main conceptual framework of the VRs of microcosm across grades. For instance, the decrease of frequencies in the category chemical change (in the process of the events) in the 10th grade seems to be due to a policy to familiarize students of that age with the chemical symbols using chemical equations inside the text, rather than due to the curriculum.
However, the question still remaining is how can the design of a VR of microcosm improve the teaching/learning procedure? For instance, a VR of the microcosm only at the submicro-level can help students to imagine and ‘see’ an unseen world, something that does not need any prerequisite in prior knowledge, but it fails to connect this world with the real one; this possibly fits to our findings concerning the relatively limited representations at the macro-level in the VRs of microcosm. On the other hand, a VR depicted in multiple levels (submicro, macro and symbolic) could help a student to better conceptualize its main framework, interplaying among the levels, but this prerequisites a significant prior knowledge basis (Cook, 2006; Cook et al., 2008; Seufert, 2003). However, something is probably missing here; that is the role of the science teacher. According to Seufert (2003) this role is quite significant for the students with a mediate prior knowledge. Students with significant prior knowledge do not need any help in combining different level of representations and these with no prior knowledge background have difficulties in multiple representations in spite of any help. On the contrary, students with a mediate knowledge background can significantly improve their ability to combine these levels when receiving instruction by a teacher. As a result, VRs comprising multiple representations could contribute to the teaching/learning procedure when textbooks designers introduce them after a basic relevant prior knowledge background has been established, even in lower grades. In such cases the role of the science teacher can significantly be strengthened by the use of the systemic network presented here, as explained above.
Appendix 1: the chemistry textbooks which were analyzed
8th Grade
(1) Avramiotis S., Aggelopoulos V., Kapelonis G., Sinigalias P., Spantidis D., Trikaliti A., and Filos G., (2007), Chemistry: B’ Gymnasium, Athens: OEDB.(2) Frassari T. and Drouka-Liapati P., (1993), Chemistry: B’ Gymnasium, Athens: OEDB.
(3) Georgiadou T., Kafetzopoulos K., Provis N., Spyrelis N. and Xiniadis D., (1997), Chemistry: B’ Gymnasium, Athens: OEDB.
9th Grade
(4) Frassari T. and Drouka-Liapati P., (1996), Chemistry: C’ Gymnasium, Athens: OEDB.(5) Georgiadou T., Kafetzopoulos K., Provis N., Spyrelis N. and Xiniadis D., (1998), Chemistry: C’ Gymnasium, Athens: OEDB.
(6) Theodoropoulos P., Papatheofanous P. and Sideri F., (2010), Chemistry: C’ Gymnasium, Athens: OEDB.
10th Grade
(7) Liodakis S., Gakis D., Theodoropoulos D., Theodoropoulos P. and Kallis A., (2011), Chemistry: A’ Lyceum, Athens: OEDB.(8) Mavropoulos M. and Kapetanou E., (1998), Chemistry: A’ Lyceum, Athens: OEDB.
(9) Sakellaridis O. P., (1990), Chemistry: A’ Lyceum, Athens: Idrima Evgenidou.
References
- Abd-El-Khalick F., Waters M. and Le A.-P., (2008), Representations of Nature of Science in High School Chemistry Textbooks over the Past Four Decades, J. Res. Sci. Teach., 45(7), 835–855.
- Adadan E., Irving E. K. and Trundle C. K., (2009), Impacts of Multi-representational Instruction on High School Students' Conceptual Understandings of the Particulate Nature of Matter, Int. J. Sci. Educ., 31(13), 1743–1775.
- Ainsworth S., (2006), DeFT: a conceptual framework for considering learning with multiple representations, Learn. Instr., 16(3), 183–198.
- Akaygun S. and Jones L. L., (2014), Words or pictures: a comparison of written and pictorial explanations of physical and chemical equilibria, Int. J. Sci. Educ., 36(5), 783–807.
- Albertazzi L., (2013), Handbook of experimental phenomenology: visual perception of shape, space and appearance, New York: Wiley.
- Arcavi A., (2003), The role of visual representations in the teaching and learning of mathematics, Educ. Stud. Math., 52(3), 215–241.
- Ardac D. and Akaygun S., (2004), Effectiveness of Multimedia-Based Instuction That Emphasizes Molecular Representations on Students' Understanding of Chemical Change, J. Res. Sci. Teach., 4(4), 317–337.
- Ardac D. and Akaygun S., (2005), Using Static and Dynamic Visuals to Represent Chemical Change at Molecular Level, Int. J. Sci. Educ., 27(11), 1269–1298.
- Avramiotis S., Aggelopoulos V., Kapelonis G., Sinigalias P., Spantidis D., Trikaliti A. and Filos G., (2007), Chemistry: B’ Gymnasium, Athens: OEDB.
- Bliss J. and Ogborn J., (1979), The Analysis of Qualitative Data, Eur. J. Sci. Educ., 1(4), 427–440.
- Bliss J., Monk M. and Ogborn J., (1983), Qualitative Data Analysis for Educational Research: a guide to uses of systemic networks, 1st edn, London: Croom Helm.
- Braga J., Phillips M. L. and Norris S., (2012), Visualizations and Visualization in Science Education, in Norris S. P. (ed.), Reading for Evidence and Interpreting Visualizations in Mathematics and Science Education, Sense Publishers, pp. 123–145.
- Carney R. N. and Levin R. J., (2002), Pictorial Illustrations Still Improve Students' Learning From Text, Educ. Psychol. Rev., 14(1), 5–26.
- Close F., Marten M. and Sutton C., (2002), The Particle Odyssey: A Journey to the Heart of Matter, New York: Oxford University Press.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40, 464–486.
- Cook M. P., (2006), Visual representations in science education: the influence of prior knowledge and cognitive load theory on instructional design principles, Sci. Educ., 90, 1073–1091.
- Cook M., Wiebe E. N. and Carter G., (2008), The Influence of Prior Knowledge on Viewing and Interpreting Graphics with Macroscopic and Molecular Representations, Sci. Educ., 92(5), 848–867.
- Dimopoulos K., Koulaidis V. and Sklaveniti S., (2003), Towards an analysis of images in school science textbooks and press articles about science and technology, Res. Sci. Educ., 33, 189–216.
- Eilam B. and Gilbert J. K., (2014), The Significance of Visual Representations in the Teaching of Science, in Eilam B. and Gilbert J. K. (ed.), Science Teachers' Use of Visual Representations: Models and Modeling in Science Education, Switzerland: Springer, pp. 3–28.
- Eliam B. and Poyas Y., (2010), External Visual Representations in Science Learning: The case of relations among system components, Int. J. Sci. Educ., 32(17), 2335–2366.
- Feynman R. P., (1963), Six easy pieces, Cambridge MA: Perseus Books.
- Frassari T. and Drouka-Liapati P., (1993), Chemistry: B’ Gymnasium, Athens: OEDB.
- Georgiadou T., Kafetzopoulos K., Provis N., Spyrelis N. and Xiniadis D., (1997), Chemistry: B’ Gymnasium, Athens: OEDB.
- Georgiadou T., Kafetzopoulos K., Provis N., Spyrelis N. and Xiniadis D., (1998), Chemistry: C’ Gymnasium, Athens: OEDB.
- Gilbert J. K., (2008), Visualization: an emergent field of practice and enquiry in science education, in Gilbert J. K., Reiner M. and Nakhleh M. (ed.), Visualization in science education, Dordrecht, The Netherlands: Springer, ch. 1, pp. 3–24.
- Gilbert J. K., (2010), The role of visual representations in the learning and teaching of science: an introduction, Asia-Pasific forum Sci. Learn. Teach., 11(1), 1–19.
- Gilbert J. K. and Treagust D., (2009), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, The Netherlands: Springer, pp. 1–8.
- Gkitzia V., Salta K. and Tzougraki C., (2011), Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12(1), 5–14.
- Griffiths K. A. and Preston R. K., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Grosslight L., Unger C. and Jay E., (1991), Understanding models and their use in science: conceptions of middle and high school students and experts, J. Res. Sci. Teach., 28(9), 799–822.
- Han J. and Roth W.-M., (2005), Chemical Inscriptions in Korean Textbooks: Semiotics of Macro- and Microworld, Sci. Educ., 90(2), 173–201.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Johnson P. and Papageorgiou G., (2010), Rethinking the introduction of particle theory: a substance-based framework, J. Res. Sci. Teach., 47(2), 130–150.
- Johnstone A. H., (1991), Why is Science Difficult to Learn? Things are Seldom What They Seem, J. Comp. Ass. Learn., 7, 75–83.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–705.
- Johnstone A. H., (2007), Science education: we know the answers, let's look at the problems, in Proceedings of the 5th Greek Conference “Science education and new technologies in education”, vol. 1, pp. 1–11, retrieved from http://kodipheet.chem.uoi.gr/fifth_conf/pdf_synedriou/teyxos_A/1_kentrikes_omilies/1_KO-4-Johnstone.pdf.
- Kapici H. O. and Savasi-Acikalin F., (2015), Examination of visuals about the particulate nature of matter in Turkish middle school science textbooks, Chem. Educ. Res. Pract., 16(3), 518–536.
- Kirschner P. A., (2002), Cognitive load theory: implications of cognitive load theory on the design of learning, Learn. Instr., 12(1), 1–10.
- Liodakis S., Gakis D., Theodoropoulos D., Theodoropoulos P. and Kallis A., (2011), Chemistry: A’ Lyceum, Athens: OEDB.
- Marton F., (1986), Phenomenography – a research approach investigating different understandings of reality, J. Thought, 21(2), 28–49.
- Marton F. and Booth S., (1997), Learning and Awareness, Mahwah, NJ: Lawrence Erlbaum Associates.
- Mason L., Pluchino P. and Tornatora M.-C., (2013), Effects of Picture Labeling on Science Text Processing and Learning: Evidence from Eye Movements, Read. Res. Quart., 48(2), 199–214.
- Mayer R. E., Steinhoff K., Bower G. and Mars R., (1993), A generative theory of textbook design: using annotated illustrations to foster meaningful learning of science text, Educ. Technol. Res. Devel., 43(1), 31–43.
- Mayring P., (2000), Qualitative Content Analysis, Forum Qual. Soc. Res., 1(2), 20.
- Mesa V., (2004), Characterizing practices associated with functions in middle school textbooks: an empirical approach, Educ. Stud. Math., 56, 255–286.
- Myers G. A., (1992), Textbooks and the sociology of scientific knowledge, English Spec. Purp., 11(1), 3–17.
- Nyachwaya J. M. and Gillaspie M., (2016), Features of representations in general chemistry textbooks: a peek through the lens of the cognitive load theory, Chem. Educ. Res. Pract., 17(1), 58–71.
- Nyachwaya J. M. and Wood N. B., (2014), Evaluation of chemical representations in physical chemistry textbooks, Chem. Educ. Res. Pract., 15(4), 720–728.
- Onwu G. O. and Randall E., (2006), Some aspects of students' understanding of a representational model of the particulate nature of matter in chemistry in three different countries, Chem. Educ. Res. Pract., 7(4), 226–239.
- Papageorgiou G., (2013), Can simple particle models support satisfying explanations of chemical changes for young students? in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, vol. 19, p. 524, in Series: Innovations in Science Education and Technology; Part IV – Chemical Reactions, Chemical Phenomena: Dordrecht, Springer, pp. 319-329.
- Pozzer L. L. and Roth W. M., (2003), Prevalence, function, and structure of photographs in high school biology textbooks, J. Res. Sci. Teach., 40(10), 1089–1114.
- Pozzer-Ardenghi L. and Roth W.-M., (2004), Making Sense of Photographs, Sci. Educ., 89(2), 219–241.
- Schnotz W., (2002), Toward an integrated view of learning from text and visual displays, Educ. Psychol. Rev., 14(1), 101–120.
- Seufert T., (2003), Supporting coherence formation in learning from multiple representations, Learn. Instr., 13(2), 227–237.
- Souza A. F. D. K. and Porto A., (2012), Chemistry and Chemical Education through Text and Image: Analysis of Twentieth Century Textbooks Used in Brazilian Context, Sci. and Educ., 21, 705–727.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Taber K. S., (2003), The atom in the chemistry curriculum: fundamental concept, teaching model or epistemological obstacle? Foundat. Chem., 5(1), 43–84.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Taber K. S. and García-Franco A., (2010), Learning processes in chemistry: drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Talanquer V., (2009), On cognitive constraints and learning progressions: the case of ‘structure of matter’, Int. J. Sci. Educ., 31(15), 2123–2136.
- Talanquer V., (2011), Macro, Submicro, and Symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Talanquer V., (2013), When atoms want, J. Chem. Educ., 90(11), 1419–1424.
- Treagust D., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Tulip D. and Cook A., (1993), Teacher and Student Usage of Science Textbooks, Res. Sci. Educ., 23, 302–307.
- Valverde G. A., Bianchi L. J., Wolfe R. G., Schmidt W. H. and Houang R. T., (2002), According to the book: using TIMSS to investigate the translation of policy into practice in the world of textbooks, Dordrecht, The Netherlands: Kluwer.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting Understanding of Chemical Representations: Students’ Use of a Visualization Tool in the Classroom, J. Res. Sci. Teach., 38(7), 821–842.
| This journal is © The Royal Society of Chemistry 2017 |