Investigating high-school chemical kinetics: the Greek chemistry textbook and students' difficulties
Theodoros
Gegios
*,
Katerina
Salta
* and
Spyros
Koinis
Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece. E-mail: tgegios@chem.uoa.gr
First published on 4th November 2016
Abstract
In this study we present an analysis of how the structure and content of the Greek school textbook approach the concepts of chemical kinetics, and an investigation of the difficulties that 11th grade Greek students face regarding these concepts. Based on the structure and content of the Greek textbook, a tool was developed and applied to students in the form of test questions in order to investigate the difficulties that they encounter with concepts and facts of chemical kinetics. Our results indicate that the textbook presentation at several points does not take into consideration basic findings of chemistry education research, which could improve the understanding of the content, and that a large proportion of Greek students show low comprehension levels of the concepts and facts of chemical kinetics. Given the important role of the school textbook in the learning process, it seems that the structure and content of the textbook do not facilitate the conceptual understanding of the subject matter, and together with other factors contribute to the difficulties that students face regarding concepts in chemical kinetics.
Introduction
Research in chemical education has established that students experience difficulties in understanding concepts and often develop different conceptions from those that they are expected to learn. These conceptions have been variously referred to as misconceptions (Novak, 1988), misunderstandings (Turányi and Tóth, 2013), alternative conceptions (Gilbert and Swift, 1985) etc. In this article we will use the term “alternative conceptions” to denote conceptions which differ significantly from those which are scientifically accepted. Most alternative conceptions in chemistry do not derive from the learner's unschooled experience of the world (Taber, 2001), but from curriculum decisions, various pedagogical practices, imprecise use of language, and the abstract and symbolic nature of much of the subject matter of chemistry (Garnett et al., 1995).Chemical kinetics is a theme included in chemistry curricula at both secondary school and university levels. Chemical kinetics deals with the rates of chemical reactions, the factors and the ways these factors affect the rate as well as the relationship between the reaction rate and the reaction mechanism, all crucial for the understanding of how chemical reactions occur. The wide range of applications of chemical kinetics, especially in areas related to everyday life, such as the control of the rates of physiological processes, drug action and reactions of industrial and environmental importance, underlines the importance of this concept and constitutes an incentive for students to study this theme.
The study of chemical kinetics includes many abstract and complicated concepts, which require comprehension of (a) the difficult-to-understand fundamental ideas about the particle nature of matter, the kinetic theory of gases, statistical notions concerning the speed and energy distribution, as well as the role of energy in chemical reactions and (b) concepts related to various aspects of the nature of science.
The present investigation of the difficulties students face regarding the concepts of chemical kinetics focuses on the analysis of the structure and content of the Greek high school textbook, the primary source from which students obtain knowledge.
Theoretical background
Science textbooks and learning difficulties
The presentation of science content in school settings is generally covered by the textbook and the teacher. It has been reported that teachers make extensive use of the textbook in their planning and teaching, even when other sources of information are easily available (Bungum, 2008). Science textbooks contain much of the scientific information students receive (Mayer, 1983), which influences how students perceive the scientific enterprise (Chiappetta et al., 1991).Chemistry textbooks, as well as other science textbooks, should help to make science interesting, relevant, and understandable to students without diluting the subject matter to the point where it lacks meaning (Bucat and Cole, 1988). Moreover, it is undeniable that in the larger majority of classrooms, textbooks become the curriculum and determine, to a much larger extent than desired by science educators, what is taught and learned about science in these classrooms (Abd-El-Khalick et al., 2008).
Chemistry textbooks present curricular models, which are suitably designed representations of current or historical scientific models. Designing curricular models aims at finding an optimum level of simplification where the model is simple enough for students to understand (in terms of their prior knowledge). At the same time, the model should reflect scientific understanding well enough to provide a suitable foundation for progression to more sophisticated understandings (Taber, 2008). Developing effective curricular models of scientific concepts draws upon knowledge from both the particular scientific disciplines as well as the pedagogic content knowledge deriving from research in science education (Gess-Newsome and Lederman, 1999).
Although the school textbook is the main source from which students obtain knowledge, critical analysis of the textbooks has highlighted features which do not facilitate the understanding of the content. Chemistry textbooks often:
• Contain abbreviated and inadequate descriptions of phenomena, theories and concepts
• Present concepts quantitatively using formulas without giving an adequate qualitative introduction to the concept
• Refer to ideas and concepts which are not sufficiently developed around the models that the scientists have devised for their study (Gabel, 1983; Ruis, 1988).
In many cases, the textbooks with the above mentioned features either do not present the prerequisite knowledge or do not stimulate the recall of prior learning to correlate the concepts, resulting into memorizing vocabulary rather than understanding the concepts.
Another potential source of learning difficulties is language. Vague and imprecise language used in textbooks encapsulates ambiguous underlying reasoning, often encompassing contradiction and confounding interpretations with observations (Pedrosa and Dias, 2000). Furthermore, research findings provide evidence for a direct relationship between language used in textbooks and some of the alternative conceptions students develop at various levels of school chemistry (Garnett et al., 1995).
Textbook presentation of aspects of the nature of science
Curriculum innovations focusing on the improvement of the understanding of science have drawn attention to reflection on science, that is, increasing students' awareness of how scientific knowledge is constructed and applied, rather than focusing exclusively on the content of scientific ideas (Van Der Valk et al., 2007).Nevertheless, the absence or inadequate presentation of various aspects of the nature of science is common in school textbooks. Abstract and complicated concepts such as “model”, “theory” and “law” are presented in textbooks, while the words and the characteristics of the concepts, whose understanding would facilitate content comprehension, are not explained thereby leading to the development of alternative conceptions (Abd-El-Khalick et al., 2008; Niaz and Maza, 2011). Thus, elaborated theoretical models are presented as established facts, when theory is presented to students before the facts that need explanation are given (Gabel, 1983).
Moreover, in subject areas where different historical models are presented e.g. atomic structure, oxidation–reduction, acids–bases, chemical kinetics, textbooks should
• clarify the background and limitations each of the expressed models (Justi and Gilbert, 1999a).
• make clear indications of when a new model is being introduced, of how this new model differs from previous models, and of why the new model works better (Carr, 1984).
Although these observations contribute into promoting the development of scientific knowledge, studies have shown that, in general, textbook authors do not take into consideration the reported learning difficulties which result when the criteria above are not applied (Drechsler and Schmidt, 2005; Österlund et al., 2010).
Furthermore, the model-based approach in teaching and learning of several subject areas including chemical kinetics has been studied extensively and its use has been demonstrated (Justi and Gilbert, 1999a,b; Justi, 2002). Analyses of how textbooks introduce models of ‘chemical kinetics’ have shown that textbooks introduce ‘hybrid models’, i.e. those formed by merging characteristics of several distinct historical models. This presentation ignores the existence, the role and the limitations of the historical models from which they originate, and thus do not facilitate comprehension, since recognizing a model's limitations rather than focusing on its generality may serve to advance our “understanding” (Schamp, 1990). In addition, aspects of nature of science, such as the tentative nature of scientific theories, are not emphasized.
Chemical representations in textbooks
Chemical representations are an integral component of chemistry textbooks, conveying information regarding the structure and the function of things. These representations are effective tools to support students' understanding as they serve to stimulate thought, facilitating memory, information processing, and communication (Peeck, 1993; Roth et al., 1999; Tversky, 2005).Chemical representations can be classified in three types, macro, submicro and symbolic representations, that are directly related to each other. Macro representations depict observable properties of matter and chemical phenomena. Submicro representations are based on the particulate theory of matter, and depict arrangement and motion of particles of matter (molecules, atoms, subatomic particles, etc.). Symbolic representations involve the use of symbols, formulas, equations, structures, diagrams, etc. Understanding of a chemical phenomenon involves the ability to move fluently between above mentioned three levels of representation (Johnstone, 1993; Gabel, 1999; Gilbert and Treagust, 2009).
It has been mentioned that many students encounter difficulties in visualizing, interpreting and constructing submicro and symbolic representations because these representations are invisible and abstract while students' thinking relies heavily on sensory information (Novick and Nussbaum, 1981; Ben-Zvi et al., 1986; Griffiths and Preston, 1992; Keig and Rubba, 1993; Garnett et al., 1995; Wu et al., 2001; Gilbert and Treagust, 2009).
In addition, students face difficulties in making the translation between different types of representations (Keig and Rubba, 1993; Garnett et al., 1995; Kozma and Russell, 1997; Treagust et al., 2003; Chittleborough and Treagust, 2008).
The reader's ability to grasp the intended meaning of a representation is affected by many factors, including the surface features of the representation, prior knowledge of the content of representation and expertise in various associated skills such as graphical literacy skills, explanatory and reasoning skills (Kozma and Russell, 1997; Glazer, 2011).
The analysis of the difficulties students face in conceptualizing the meaning conveyed in representations has led to the development of criteria for the evaluation of chemical representations used in textbooks. More specifically representations must be explicit, completely related to the associated text content, and accompanied by an explicit, brief and comprehensive caption. In the case of a multiple representation, subordinate representations should be sufficiently linked (Gkitzia et al., 2011).
Textbooks as multimedia tools
The textbook can be viewed as a multimedia teaching aid, as subject matter is presented in more than one mode, such as in pictures and words. According to the cognitive theory of multimedia learning developed by Mayer (1997), meaningful learning occurs when learners engage in appropriate cognitive processing during learning, including selecting relevant incoming words and pictures for further processing, organizing the selected words and pictures into coherent mental representations, and integrating the representations with each other and with relevant prior knowledge activated from long-term memory (Mayer, 2011). This theory suggests that presenting an explanation in words and pictures together rather than solely in words, contributes to sound understanding. Text and representations should be combined, with their adequate integration leading to an effective science textbook, otherwise the representations either have a purely decorative role or may even cause alternative conceptions (Devetak and Vogrinc, 2013).To a large degree, the curricular models presented in chemistry textbooks influence the teaching models, namely the representations given by teachers in the classroom or through the teaching materials implemented. Furthermore, they also influence the subsequent comprehension of the content by the students and their attitude regarding the chemistry course. Therefore teachers, as facilitators of learning, should be aware of the problems and limitations of the students' textbook (Haggarty and Pepin, 2002).
However, it is very rare that the teachers of the subject evaluate the textbooks themselves, although they would benefit most from the concrete results of textbook evaluations. As groups of students are different as far as their knowledge, skills and experiences are concerned, the study of textbooks will facilitate lesson planning and aid in finding and applying versatile work methods and facilitate individualization of instruction (Ahtineva, 2005).
Students' difficulties in chemical kinetics
Learning difficulties in chemical kinetics has been the subject of many studies, which have been recently reviewed (Bain and Towns, 2016 and references therein). The reported difficulties concern not only high school and university students, but prospective chemistry teachers as well.The major difficulties cited in the literature include:
• The difficulty in distinguishing between the concepts of thermodynamics and kinetics, resulting in alternative conceptions such as: “Rate of reaction means the same as extent of reaction” (Griffiths, 1994), “In addition to temperature, pressure and concentration, Le Chatelier's principle is also applied to reaction rates” (Kousathana and Tsaparlis, 2002), “An increase in the temperature decreases the rate of exothermic reactions” (Banerjee, 1991; Garnett et al., 1995; Cakmakci, 2010; Turányi and Tóth, 2013), “The larger the equilibrium constant, the faster a reaction occurs” (Banerjee, 1991; Sözbilir et al., 2010), “Exothermic reactions occur faster than endothermic reactions” or vice versa (Cakmakci, 2010; Sözbilir et al., 2010).
• The conception that reactant concentrations in the rate law of the reaction have exponents equal to the stoichiometric coefficients of the reactants in the balanced equation for the reaction (BouJaoude, 1993; Cakmakci et.al., 2006; Turányi and Tóth, 2013).
• The mode of action of the catalyst, for example: a catalyst can affect the rates of the forward and reverse reactions differently and hence lead to a different equilibrium yield. (Hackling and Garnett, 1985), a catalyst increases the yield of products, and a catalyst does not affect or change the mechanism of a reaction (Cakmakci, 2010).
• Comprehension of concepts such as reaction rate and activation energy (Cakmakci, 2010).
Purpose of the study and research questions
Student learning is highly complex, and therefore optimizing pedagogy is at best a long-term goal (Taber, 2012). The motivation for this study was the recognition that both teachers and students experience difficulties with the theme of chemical kinetics (Justi, 2002; Chairam et al., 2009), and that there is room for improvement in the teaching and learning of this theme. The study focuses on• The analysis of the structure and content of the Greek high school textbook in order to identify existing portions of the text as well as gaps, which may impede comprehension of the material.
• The development of a tool for the purpose of investigating and recording students' difficulties.
The results of the study may be useful for future curriculum changes, for teaching purposes, for the preparation of new textbooks and for the development of teachers' pedagogical content knowledge, specifically regarding the theme of chemical kinetics.
Based on the findings reported in the literature and on the framework defining curriculum in Greece, we hypothesize that 11th grade Greek students hold alternative conceptions regarding the theme of chemical kinetics similar to those published and that the structure and content of the chemistry textbook may create obstacles to students' conceptual learning and lead to alternative conceptions.
The research questions that have guided this work are:
(1) How do the textbook structure and content approach concepts of chemical kinetics?
(2) What are the difficulties that 11th grade Greek students face regarding the concepts of chemical kinetics?
(3) Are the student difficulties in comprehending the concepts of chemical kinetics related to the structure and content of the textbook?
Methodology
The research context: the high school chemistry curriculum in Greece
In Greece, the system of elementary and secondary education is highly centralised. All students follow a common science curriculum and study from the same school chemistry textbook (Liodakis et al., 2005) published by the Greek Ministry of Education. The content of this book is aligned with the national curriculum and teachers are required to adhere very closely to the book content.It should be noted that both students and teachers consider the school textbook as the main source of reliable information, since the book's content defines and limits the scope of the questions on the national exams, which are required for students' entrance into the higher education system.
By the end of 10th grade, all students have taken the same chemistry courses. Chemical kinetics is taught at the 11th grade level (student age 16–17 years old). The relevant chemistry course consists of introductory concepts including the following themes: intermolecular forces, thermochemistry, chemical kinetics, chemical equilibrium and oxidation–reduction. The textbook content in chemical kinetics comprises the following issues: reaction rate concept, factors affecting reaction rates, collision theory, activated complex theory and energy diagram, rate law, reaction mechanisms (elementary reactions and composite two-step reactions with a rate determining step) and catalysis. During the period when data for this article were compiled, this course was compulsory only for the students choosing to follow biological and medical studies and optional for the students choosing university studies in engineering, science and agro-science departments.
Criteria of textbook analysis
The basic criteria for analyzing the structure and content of the chemical kinetics theme of the textbook under consideration are the following:• The content must be correct according to the actual knowledge of the specific scientific subject area and should take into account student developmental levels, as well as the degree of their understanding of and experience with scientific concepts from previous school years (Devetak and Vogrinc, 2013).
• Presentation of the concepts must be realized with adequate qualitative introductions (Gabel, 1983; Ruis, 1988).
• Carr's criteria with regard to the collision and transition state models (Carr, 1984).
• The content of the textbook should not contain vague or inaccurate wording, which encapsulates unclear underlying reasoning, does not facilitate content comprehension and which can lead to the development of alternative conceptions (Pedrosa and Dias, 2000; Cakmakci, 2009).
• Integration of textual and visual elements (Gkitzia et al., 2011; Devetak and Vogrinc, 2013).
Investigation of students' difficulties
The participating students received a test from their teachers, which was used both as an evaluation tool as well as to provide the data for this study. The school administration was informed and approved the use of the data for our study. In addition, the students and their parents were informed about the goals of the present study and that their responses to the test questions would be used in the study. The test was announced to the students in advance and administered to them after the completion of instruction of the chemical kinetics theme. The tests completed by those students who agreed to participate were given to the coordinating researcher by their teachers. It should be noted that students' names were not provided, and only their gender was reported (Taber, 2014).
(1) Analysis of the chemistry school textbook used in eleventh grade in Greece in order to precisely identify the content boundaries of the test and to ascertain whether its structure and content could potentially create obstacles to students' conceptual learning.
(2) Review of the literature on difficulties and alternative conceptions regarding the concepts of chemical kinetics.
(3) Division of the content into six curriculum topics and recording learning objectives (Table 1) that arise mainly from the general objectives of the Greek curriculum. Both curriculum topics and learning objectives were the basis on which test items were developed (Appendix 1).
| Curriculum topics | Learning objectives | Test questions | |
|---|---|---|---|
| 1 | Defining reaction rate (different ways of expressing reaction rates and how they relate to each other) | 1, 2 | 9, 13, 14, 17 |
| 2 | Rate law–reaction order | 3, 4 | 1, 8, 10, 11, 12, 15, 16 |
| 3 | Effect of temperature change on reaction rate | 5 | 2,3 |
| 4 | Energy reaction diagram | 6 | 18, 19, 20 |
| 5 | Reaction mechanism | 7, 9 | 4, 7 |
| 6 | Catalysis | 8 | 5, 6 |
(4) Development of the initial version of the test, including 10 multiple choice questions with four response choices each, and 10 objective short answer questions. Multiple choice questions were developed using either known alternative conceptions or students' answers to open-ended questions as distractors (Tamir, 1991). Content validity of the initial version of the test was confirmed by three experienced high-school chemistry teachers.
(5) Piloting the initial version of the test, on 246 students from 14 high schools during the 2011–2012 school year.
(6) Statistical analysis of students' responses, followed by revision of the test.
(7) Validation of the revised test by two groups of experts. One group included a full professor, an associate professor and two assistant professors, all from the Department of Chemistry, University of Athens, while the second group included three high school science teachers who had been teaching for more than 15 years. The experts were asked to:
• Confirm the assignment of test items with instructional objectives
• Verify the correct answer for each item
• Detect construction errors and
• Verify the ability of students to answer test items based on the chemistry school textbook.
The validating process resulted in slight amendments of the revised version to produce the final version of the test (Appendix 2). The final version consisted of 10 multiple choice questions and 10 objective short answer questions and was administered to the student sample during the 2012–2013 school year.
Results and discussion
Textbook analysis
A laconic description of the transition state theory follows, according to which in order for a reaction to take place, an intermediate product is formed during the collision of the reactants, which absorbs the activation energy and is termed as the “activated complex”.
In the presentation of the collision and transition state models, the following issues are not adequately discussed in the textbook:
• The limitations of each model, i.e., that the two models are limited to elementary reactions, where two reactants collide and form products: A + B → products, whose behavior is interpreted and therefore applies only to elementary reactions (Laidler, 1996; Silberberg, 2012).
• The working assumptions of each model. Specifically, the collision model is based on the kinetic theory of gases, and in its original form has the following basic premises: (a) the reactants behave as hard spheres, without an internal structure, (b) between collisions, the reactants do not influence each other by attractive or repulsive forces and (c) their collisions are elastic (Laidler, 1987; Justi and Gilbert, 1999b; Silberberg, 2012). Moreover, in this model we suppose that reactants bounce apart if they collide with less than a certain kinetic energy, but if they collide with a kinetic energy higher than that threshold, bonds can break and new bonds can form (Atkins and Jones, 2010). The transition state model approaches reality to a higher degree, since it acknowledges that the reactant particles have an internal structure and since it focuses on the variation in the potential energy of the system as well as the simultaneous changes in the internal geometrical configuration of the reactants as they approach each other (Silberberg, 2012). Understanding the background of each model, correlating and contrasting each model with the real system it represents, and juxtaposing the models help to reveal the weaknesses of each model as well as to differentiate each model from the system it represents. In this way, a better comprehension of the real system represented is achieved.
• The historical context of the two models in order to show the development of scientific knowledge.
• The purpose served in devising each model, i.e. which key observations and questions that arose from the scientists' experimental measurements offered interpretations? Specifically, the collision model provided an interpretation of why the reaction rate is proportional to the product of the reactant concentrations as well as of the effect of temperature on the reaction rate while the transition state model explained the role of the activation energy (Silberberg, 2012).
The above mentioned shortcomings render the text difficult to understand (Carr, 1984), thereby favoring rote learning over meaningful learning and further contribute to the emergence of alternative conceptions. Furthermore, it should be noted that the textbook terms the activated complex as an intermediate product, even though it's neither a reactant nor a product, but rather an unstable typification of the reactant system, which only exists at the point where the system has the highest potential energy (Silberberg, 2012).
• Information interpreting the shape of the curve or at least providing some reference to the fact that the diagram depicts the variation in the potential energy of the system throughout a reaction's progress from reactants through transition state to products, is absent from the main text.
• In the diagram, the axes are designated as “energy” and “reaction pathway”, without any additional explanation, and the magnitude of the reaction enthalpy is not included, in spite of its integral importance to the diagram. To facilitate comprehension of the diagram, surface features (curve and lines – energy levels) should be clearly discussed. For example, the fact that the energy which corresponds to the activated complex does not represent the reaction activation energy, as students often confuse a point with an interval in diagrams (Glazer, 2011).
• There is no reference to the fact that the diagram depicts an exothermic reaction and that in the case of an endothermic reaction, the corresponding curve would be different. In the entire chapter on chemical kinetics all the energy diagrams presented, depict exothermic reactions. According to Bowen and Roth (2002) parallel presentation of energy diagrams concerning both endothermic and exothermic reactions is educationally necessary as their comparison would provide students with the possibility to compare and contrast both types of energy diagrams, noting similarities and differences, leading thus to a better understanding of the theme, arising from the students' active engagement in interpreting the diagrams.
• The diagram caption refers to a property which is common to both theories presented in the textbook, and thus does not contribute to the distinction between the two theories (Fig. 1).
The above omissions may also raise issues regarding how effectively the diagram and the accompanying text can be used to clarify the meaning which is conveyed by the representation, thereby facilitating learning.
The text does not clarify this issue, with the likely result that some students will be led to the arbitrary generalization that the following statements are always true:
• An increase in concentration of a reactant causes an increase in the reaction rate, and
• As a reaction progresses, its rate decreases.
In the next paragraph where the rate law concept is presented it is stated that the concentration exponents (in the rate law) can be, among other values, zero and/or negative numbers.
This statement, together with the above mentioned statement, can confuse students as to what applies for a zero-order reaction.
The development and description of chemical kinetic events take place on two distinct levels: the “theoretical” and the “empirical”, or in other words in terms of the model or in terms of reality. The distinction, however, between the two levels is not at all obvious to students, who often confound the two levels because their difference is not clearly discussed and also because the two approaches are not treated as complementary to each other (Logan, 1984).
The reality and model distinction is important since it contributes to:
• Avoiding the development of alternative conceptions by students,
• An understanding of how science works,
• Highlighting the complexity of reality.
The distinction between the theoretical and practical levels could be indicated as follows:
From the results of a great number of kinetic studies it has been ascertained that an increase in the concentration of a reactant causes, in general, a rate increase, but in certain reactions the increase in concentration either has no effect on the rate e.g. zero-order reactions or even causes it to decrease. The collision model is limited to interpreting only elementary reactions for which reaction rate is proportional to the concentration of reactants.
A complete description should state that in the bimolecular elementary reaction, A(g) + B(g) → products, the change in temperature influences the reaction rate since it effects the average rate of the reactants and thus the rate of collisions between the reactants A and B, and more importantly the average kinetic energy of the reactants and thus the rate of effective collisions between the reactants A and B (Silberberg, 2012).
According to the text, “the rate of a reaction generally increases with an increase in temperature. In many cases, a temperature increase of 10 °C causes the doubling of the reaction rate. This occurs because the temperature increase causes the average kinetic energy of the reactants to increase, resulting in an increase in the number of effective collisions”. In this excerpt, the first two statements refer to what is actually happening generally in reactions, based on experimental observation while the last statement refers to the “theoretical” level and is the interpretation of the phenomenon according to the collision theory, concerning elementary reactions. Failure to clearly explain the points above causes students to confuse the different levels.
Moreover, this inadequate explanation could possibly cause alternative conceptions in students' minds, such as that an increase in temperature does not alter the frequency of the collisions between the reactants, because students tend to perceive scientific phenomena as monocausal, and fail to identify causes in particular when they are implicit (Grotzer and Bell Baska, 2003; Perkins and Grotzer, 2005).
Furthermore, according to Turányi and Tóth (2013), examples of enzymatic reactions in which the reaction rate decreases as a result of an increase in temperature should be given to high school students. These examples would not only help students to distinguish reality from the collision model, which states that an increase in temperature always causes an increase in the rate of all elementary reactions, but would also encourage student to recognize the limitations of models and the complexity of what actually occurs.
A graph of the distribution of energy amongst the particles in a gas at two different temperatures (Maxwell–Boltzmann distribution) follows. The graph is used in the text to contribute to the students' understanding of the influence of temperature on the reaction rate, as well as the difference between effective and ineffective collisions (Fig. 3).
The text accompanying the graph states that the graph illustrates the distribution of the gas molecules with regard to their kinetic energy. The shaded area under each curve represents the number of molecules whose energy is larger than the activation energy. From the comparison of the sizes of the two areas, it can be observed that at higher temperatures, the number of reacting molecules increases. This brief presentation together with a lack in prerequisite knowledge of the content is likely to create difficulty for students in clearly understanding the graph's meaning.
It should be noted that in this conceptually demanding graph, abstract complicated ideas are represented which students have not met in the past. Simply stating the name of the concept (energy distribution of the molecules, for example) is not adequate for students to comprehend. Rather, a complete description and commentary that would induce students to recall the knowledge they have already gained regarding the kinetic theory of gases, in order to provide the appropriate connection leading to conceptual understanding, is necessary. Furthermore, a clear interpretation of the graph's surface features is also required.
 | ||
| Fig. 4 An energy diagram of a reaction both with and without catalyst similar to that presented in school textbook. | ||
The diagram is abstract and should be accompanied by text which would interpret both the surface and more subtle aspects of catalysis, which are not overtly illustrated. This additional explanation would contribute to students' understanding of the abstraction associated and would help to avoid the development of incorrect alternative ideas, such as that reactions in both the presence or absence of a catalyst are elementary or that the catalyst's role is simply to lower the energy of the activated complexes, which is the same in both cases (Haim, 1989).
aA + bB → cC + dD, it is experimentally determined that:
rate = k[A]x[B]y (the rate law), where x and y are experimentally determined values. The text then clarifies that “In the case where the exponents x and y have the same value as the coefficients in the chemical equation, i.e. x = a and y = b, then the reaction is elementary” and is enhanced with the following example:
Given the reaction, N2O4(g) → 2NO2(g) with the rate law, rate = k·[N2O4]. What is the (overall) order of the reaction? Propose a mechanism.
Answer
The reaction is first order and is elementary.
This statement could be considered correct in the case that a + b ≤ 3 and when experimental data, e.g. a reaction intermediate is detected proving the opposite are absent, however its universal application as presented in the textbook is unclear and could lead to comprehension difficulties.
It is noted that a reaction mechanism is no more than a temporary and hypothetical model based on currently available kinetic data (Mortimer and Taylor, 2002). If new kinetic data, which do not support the mechanism, are discovered in the future, then a new mechanism will have to be proposed. We can never prove that a mechanism represents the actual chemical change, only that it is consistent with the data and an alternative mechanism may explain the experimental data equally well (Laidler, 1987).
A noteworthy example is the reaction H2 + I2 ⇌ 2HI, which was studied by Bodenstein (1894–1898). Both the forward and the reverse reactions were found to follow second order kinetics with rate laws υ1 = k1·[H2]·[I2] and υ−1 = k−1·[HI]2 respectively. Despite the fact that Bodenstein proposed several alternative mechanisms, “it was natural to conclude that reactions were elementary” (Laidler, 1987) and for many years reactions were thought to be prime examples of elementary reactions. However, in 1967, a detailed study of the reaction showed that iodine atoms are involved in the reaction, and thus the reaction is not elementary (Sullivan, 1967).
The school text is limited to the discussion of elementary reactions and complex reactions with mechanisms consisting of two irreversible steps – a fast one and a slow one.
Initially, the text refers to the process of the kinetic study of a reaction from which “the rate law is determined experimentally, and from which we can conclude whether the reaction is elementary or complex… in the case that the reaction is complex, intermediate elementary reactions are proposed, i.e., the reaction mechanism which is compatible with the experimentally determined rate law”.
The reaction given below as an example describes the chemical equation A + 2B → C + 2D, for which the experimentally determined rate law is the following:
rate = k·[A]·[B]. The proposed reaction mechanism shown below is consistent with the experimentally determined rate law, since the rate law is determined by the slowest step (rate determining step).
| A + B → AB (slow step) |
| AB → C + 2D (fast step) |
According to the literature, the elementary steps that are proposed for the mechanism must obey three criteria:
• The elementary steps must add up to the overall equation,
• Each step should involve one, two or, rarely, three reactant particles, and
• The experimental rate law must be equivalent to the rate law for the rate-determining, or slowest, step (Silberberg, 2009).
It should be noted that the textbook only mentions the third point. While the first criterion is obvious to the teacher, the same may not be true for the students. Neither the second criterion nor the concept of the “molecularity of elementary reactions” are mentioned in the text, despite the fact that these concepts contribute to making the correlation between the reaction mechanism model with what really occurs as well as comprehending and consolidating the particulate nature of matter. According to Justi (2002), students should use the idea of discrete and dynamic particles in producing their models of specific aspects in chemical kinetics, for example reaction mechanism, as these models may help students to overcome the counter-intuitiveness of the particulate nature of matter.
The second criterion has also been completely ignored even regarding the preparation of the national examination questions, as can be seen in the following question:
In a 10 L container, 20 mol of gas A and 40 mol of gas B are introduced, thus giving the elementary chemical reaction
| A(g) + 3B(g) → 2C(g) |
The rate constant for the reaction, at temperature T, is
| k = 0.1 mol−3 L3 s−1. |
(a) Give the rate law for this reaction.
(b) Find the order of the reaction.
(c) Calculate the initial reaction rate.
(d) Calculate the reaction rate at which, after time t, 20 mol of gas C will have formed in the container.
(National university entrance examinations, May 30, 2001).
The reaction in this question, A(g) + 3B(g) → 2C(g), is described as elementary, even though according to the second criterion this is almost impossible, given that in order for the reaction to take place, 4 reactant particles (A + 3B) would have to be in the same place at the same time. The fact that the second criterion was not met deceived the experienced exam question writers, not to mention the students.
The above question illustrates, among other points, the importance of the quality of high school textbooks over the entire range of the educational process, as it is not easy to change later what was previously taught in secondary school (Turányi and Tóth, 2013). In addition, the question highlights the problem for those teachers who recognize the deficiencies and inaccuracies in the textbook and thus face the dilemma of whether or not to teach those points not covered in the text, since the evaluation of students' responses on the National Examinations is based directly on the material given in the textbook and not on what is actually correct.
An increase in temperature results in the increase of the reaction rate because:
(a) the frequency of molecular collisions is greater
(b) the molecular collisions are more violent
(c) *a greater proportion of molecules has the minimum energy to result in effective collisions
(d) the molecular bonds weaken
* the correct response, according to the textbook.
The question above is a “best answer” (Tamir, 1991) type of multiple choice question, with which Greek students are not familiar, since the multiple choice questions in the textbook as well as those on the National Examinations are “correct answer” multiple choice questions. The lack of directions on how to answer the question as well the fact that the students are not familiar with this question type is likely to lead students to incorrect conclusions, such as that only one choice is correct and therefore the choices (a) and (b) are wrong. Therefore, if for example the statement, “An increase in temperature increases the reaction rate because the frequency of molecular collisions increases” is considered an incorrect response, then it is possible to arrive at the conclusion that an increase in temperature does not increase the frequency of total collisions between the reactants.
Students' difficulties
In order to analyze the results quantitatively, the questions were scored as follows: for each multiple choice question 1 point was given for the correct answer and 0 points for the distractors, and for each short answer question a rating scheme from 0 to 1 points including partial rating was developed by the first researcher (example in Appendix 3).The maximum score was 20 points. The average score was 11.50 ± 4.53 points. The results show that “internal-consistency” reliability of the test is satisfactory (Cronbach α = 0.83, 20 items). Descriptive statistics for students' responses to test questions are presented in Appendix 4.
Discussion of quantitative analysis results for each curriculum topic
Curriculum topic: defining reaction rate: different ways of expressing reaction rates and how they relate to each other
Curriculum topic: rate law-reaction order
It is noteworthy that this response is chosen by similar a percentage of students in both the low performance group (20.7%) and the high performance group (26.1%). Some students select the response (c) stating that the reaction rate increases, probably because they arbitrarily associate this choice with the cue of the presence of a catalyst as mentioned in question. For those students who don't know the zero-order reaction concept, but do know that the presence of a catalyst causes an increase in the reaction rate, this particular selection may result from heuristic reasoning (Maeyer and Talanquer, 2010).
Curriculum topic: effect of temperature change on reaction rate
The correlation between the change in rate of a reaction with the sign of the reaction enthalpy is an alternative conception documented in the literature which likely comes from students' inability to distinguish between the concepts of “reaction yield” and “reaction rate”, and namely from the application of Le Chatelier's law to reaction rate. Specifically the statement found in the Greek high school chemistry textbook, “an increase in temperature favors endothermic reactions” contains the imprecise term “favors”, which is likely interpreted by students as “causes an increase in the rate” – instead of the correct interpretation that it “causes an increase in yield” (Kousathana and Tsaparlis, 2002).
Curriculum topic: “reaction energy diagram”
On the diagram, many students confuse the activation energy of the forward reaction either with the energy of the activated complex, that is they confuse an interval with a point (Glazer, 2011), or with the activation energy of the reverse reaction. Possible sources for the lack of understanding regarding the diagram are the deficiencies in the textbook previously mentioned above in textbook analysis, point b.
Curriculum topic: reaction mechanism
The alternative conception above could stem from the previously mentioned reference in the textbook to the transition state theory that “according to the transition state theory, in order for a reaction to take place, an intermediate product is formed upon collision of the reactions. This intermediate product absorbs the activation energy and is termed the activated complex.” From this inaccurate description of the activated complex as an intermediate product, the students may be led to the reasonable conclusion that according this model, reactions occur at two stages, the first, which is endothermic is where the reactant bonds break, while the second is exothermic and is where the product bonds are formed.
The second most frequently selected incorrect answer was (c), probably due the tendency for some students to assume that every reaction is elementary, i.e. that the conversion of reactants to products – and all the events involved (bond breaking and formation) – takes place in one step. A poor understanding of the transition state theory probably contributes to this assumption. Specifically, in the presentation of the theory, it is generally stated that in order for the reaction A + BC → AB + C to take place, the reactants A and BC approach each other, creating the activated complex A⋯B⋯C, an unstable species in which the bond between A and B has begun to form while the bond between B and C has begun to break, thus the formation and breaking of bonds happen “at the same time”. Some students will recognize the equation above in the wording of the question and choose response (c) without recognizing, that in the framework of the theory, the reaction A + BC → AB + C is elementary, which is not mentioned in the textbook.
In the textbook topic which refers to the concept of mechanism, only one example of a complex reaction is given, for which a two-step mechanism is suggested. One example is not enough for an in-depth understanding of the concept. In order for students to overcome the difficulty in understanding the variety of potential mechanisms a specific reaction could result from, it is suggested that the teachers as well as the textbook give students the possibility to “see” microscopic representations. This could be accomplished with a comparison of both symbolic and microscopic representations of 2–3 alternative mechanisms by which a specific reaction could take place, and by choosing a reaction which relates to a phenomenon known to the students, such as the decomposition of ozone to oxygen molecules in the upper atmosphere. Emphasis on the micro level would improve student performance at all levels in chemistry – microscopic, macroscopic and symbolic (Gabel, 1993).
The percentage of students who choose response (d) is higher than that of the other two incorrect responses, possibly since the molecule NO3, an intermediate in both mechanisms, is not familiar to students, who then exclude any option involving it.
Curriculum topic: catalysis
Since these students know that both an increase in temperature and the addition of a catalyst to the reaction mixture lead to an increase in reaction rate, they probably assume that the presence of the catalyst, like the increase in temperature, causes an increase in the energy of the reaction mixture, that is that the same outcome (an increase in the reaction rate) results from the same cause (an increase in energy). Thus the students assume that “the rate of a reaction increases exclusively when the reactants having increased energy collide”, which is intuitively compatible with the collision model, the model dominating on chemical kinetics.
According to the foundation of the “activation energy” concept, it would be didactically useful to highlight that the “activation energy” of a reaction is a characteristic parameter of the mechanism by which reactants are converted to products and not of the chemical phenomenon described by the chemical equation, providing appropriate examples so as to clarify the subtle but significant difference between the two statements, “A catalyst enables the reaction to take place by means of following a different mechanism with lower activation energy,” and “A catalyst lowers the activation energy of a reaction”.
Conclusions
The results of our quantitative research indicate that a large proportion of Greek students display a low level of conceptual understanding of the concepts and facts regarding chemical kinetics. The most important findings, which result from the analysis of student responses to the test questions administered, reveal that:(1) Many students, when given the stoichiometric equation of a reaction, cannot correctly associate parameters such as the reaction rate with the rate of change in concentration of a reactant (or product) or the rate of change in concentration of a reactant with the corresponding rate of change in concentration of a product.
(2) Many students are unable to distinguish between kinetic and thermodynamic parameters.
(3) Many students seem to have difficulties understanding:
(a) the concept of a “zero-order reaction”.
(b) that the stoichiometric equation of a reaction does not reveal its mechanism.
(c) how a catalyst works.
(d) that in a reaction with a mechanism consisting of two steps, one fast and one slow, the slow step is the rate determining step.
(e) the wealth of information provided in the potential energy diagram of a reaction.
The students in our sample, who are oriented toward medical, pharmaceutical and engineering schools, are among the top students in their schools and are very motivated to achieve high scores on the National university entrance exams. They are, therefore, also very motivated to learn chemistry. The difficulties encountered, even by these students, in understanding basic concepts of chemical kinetics can be partially attributed to the abstract nature of these concepts (e.g. statistical notions concerning the speed and energy distribution), but also to factors such as how the concepts are introduced and the extent and depth of their processing in the classroom. A detailed study of these factors with appropriately designed investigations and subsequent discussion are necessary in order to answer the question “Should the high school chemistry curriculum include such demanding aspects of chemical kinetics, and if so to what extent?” Similar questions can be found in the literature regarding other demanding topics in Chemistry, such as quantum-chemical concepts (Tsaparlis and Papaphotis, 2002; Stefani and Tsaparlis, 2009).
In this work, we investigated and highlighted aspects of the textbook as a factor in understanding concepts of chemical kinetics.
Analysis of the school textbook revealed that there are several points of the content where textual and pictorial presentations of the subject matter do not facilitate its conceptual understanding and may be contribute to (a) rote learning, in contrast to meaningful learning, (b) the formation of alternative conceptions and (c) discouraging students from appreciating, liking or spending time studying chemistry, due to a lack of understanding of its content.
Specifically it was found that:
(1) The abbreviated qualitative descriptions concerning collision and transition state models do not promote the appropriate correlation of presented material with student's knowledge base in order to advance content comprehension.
(2) The fragmentary presentation of “reaction mechanism” concept does not constitute a solid foundation for the interpretation and understanding of related chemical phenomena.
(3) The energy reaction diagrams and the Maxwell–Boltzmann energy distribution curves are not accompanied by text and caption with sufficient and appropriate information either to recall prior knowledge or to present prerequisite knowledge in order to promote understanding of the messages they convey.
(4) Ambiguous ideas presented such as: (a) “if the exponents in the rate law are the same as the corresponding stoichiometric coefficients in the chemical equation, then the reaction is elementary”, (b) “the activated complex is a product”, and (c) the instantaneous rate of a reaction of unknown stoichiometry, can be determined from the graph of a product's concentration as a function of time, may result in students' confusion.
Students' difficulties seem to be related to the way concepts are presented in the textbook, however, this relationship cannot be solely considered cause-and-effect since teacher input, student study practices and other parameters play an important role as well.
The textbook approach to concepts of chemical kinetics follows the tradition in chemistry education which involves simply presenting the concepts and principles to students without engaging them in the processes of chemical inquiry that would actually encourage the generation of these concepts and principles. Within this traditional framework of teaching science, the motivations, strategies and arguments underlying the development, evaluation and revision of chemical models are overlooked (Erduran, 2001).
The acknowledgment that school science is crucial in the efforts of raising scientifically literate generations equipped with an adequate knowledge and understanding of science indicates that all components of school science must be assessed and developed continuously to achieve the optimum outcome (Irez, 2009).
Improving the presentation of concepts in the textbook requires the adoption and implementation of the perspective that an understanding of the nature of science and of the historical and social framework of science contributes to a more substantial comprehension of scientific knowledge, encourages students to become actively interested in science and highlights the educational and cultural dimensions in science courses (Matthews, 1994).
In total, the findings of textbook analysis indicate the necessity to modify the planning, writing and evaluation frameworks of the chemistry textbooks with the determination and implementation of more specific quality criteria and indices towards the goal of a more qualitative presentation of the chemistry content.
Since every school textbook will have some weaknesses, it is important that before chemistry teachers actually begin teaching, they should always examine chemistry textbooks from the student's point of view, in order to identify the weaknesses of the curricular models so that the teaching models can be presented in class without the gaps and flaws of the curricular models presented in chemistry textbooks (Gabel, 1983). However, this is not an easy task. Studies have shown that teachers share many of the reported alternative conceptions held by learners (Taber, 2008), and that chemistry teachers have a background in chemistry and therefore perceive the content differently from students who have little or no background in this discipline (Chiappetta et al., 1991). Moreover, in Greece many chemistry teachers do not have adequate knowledge of chemistry education, which leads to empirical teaching practices.
As a result, the gaps and flaws of the textbook are not obvious to teachers or easily detectable in their full scope since thorough and time-consuming analysis is necessary to identify and record them.
This demonstrates the need for the training and instruction of chemistry teachers, at regular intervals, in the field of chemical education in order to improve their pedagogic content knowledge as well as their ability to effectively meet the challenges of their role.
Appendix 1
Learning objectives
Students are expected to:(1) Analyze kinetic data (tables, graphs) and
(a) Calculate the reactant consumption rate or product formation rate
(b) Calculate the reaction rate
(2) Correlate the following different ways of expressing reaction rates:
• reactant consumption rate
• product formation rate
• reaction rate
in all the three different forms presented, i.e. instantaneous–initial–average, provided that the balanced equation describing the reaction is given.
(3) Determine the rate law and rate constant for a reaction from experimental data regarding initial reactant concentrations and the respective initial reaction rates.
(4) Apply the rate equation of a reaction and draw conclusions about the factors included.
(5) Qualitatively explain how a change in temperature affects the rate of an elementary reaction based on the collision theory.
(6) Plot, describe and interpret the energy diagram for an elementary reaction.
(7) Recognize whether a proposed mechanism is compatible with the experimental rate equation, provided that the overall balanced equation describing the reaction is given.
(8) State the properties of a catalyst and explain its mode of action.
(9) Acknowledge that the balanced equation for a reaction doesn't reveal its mechanism.
Appendix 2
Test questions
Part 1In the following items 1–10 circle the letter that corresponds to the correct or the best answer
(1) The reaction between the gases A and B,
| 2A(g) + 2B(g) → products |
If the initial concentrations of both reactants are doubled, the initial reaction rate will
(a) double
(b) triple
(c) quadruple
(d) octuple
(2) The gases A and B react according to the elementary reaction A(g) + B(g) → AB(g)
What is the effect of increasing the reaction temperature on the following?
(a) only i increases
(b) only ii increases
(c) both i and ii increase
(d) not enough information is given to answer the question
(3) When the temperature of an elementary reaction increases
(a) the reaction rate increases if the reaction is exothermic
(b) the reaction rate increases if the reaction is endothermic
(c) the reaction rate increases for both exothermic or endothermic reactions
(d) we cannot conclude how the reaction rate will change
(4) Quantities of gases A and BC are introduced into an evacuated reaction vessel, and the following reaction takes place
| A(g) + BC(g) → AB(g) + C(g) |
What is true concerning the reaction mechanism?
(a) initially the bond between B and C is broken, and then the bond between A and B is formed
(b) initially the bond between A and B is formed, and then the bond between B and C is broken
(c) breakage of the bond between B and C and formation of the bond between A and B occur simultaneously
(d) there is not enough data to draw a conclusion
(5) A catalyst increases the rate of a reaction by:
(a) decreasing the change in enthalpy of the reaction
(b) providing an alternative reaction mechanism
(c) increasing the average kinetic energy of the reactants
(d) increasing the activation energy of the reaction
(6) If, for a reaction, we define
E a as the activation energy of the reaction in the absence of a catalyst and
E acat as the activation energy of reaction in the presence of a catalyst, then:
(a) Eacat = Ea
(b) Eacat > Ea
(c) Eacat < Ea
(d) from the given information we cannot determine the relationship between Eacat and Ea.
(7) The rate equation for the reaction
| NO2(g) + CO(g) → NO(g) + CO2(g) |
Which of the following reaction mechanisms is consistent with this rate equation?
(a) Mechanism 1
(b) Mechanism 2
(c) either Mechanism 1 or 2
(d) neither Mechanism 1 nor 2
(8) The decomposition of ammonia in the presence of catalyst (Pt),
(a) the rate of the reaction remains constant
(b) the rate of the reaction decreases
(c) the rate of the reaction increases
(d) we cannot conclude how the rate of the reaction changes
(9) During the course of the reaction
| 2N2O5(g) → 4NO2(g) + O2(g) |
The instantaneous rate of consumption of N2O5 at time t is equal to:
(a) 1.5 mol L−1 min−1
(b) 3 mol L−1 min−1
(c) 6 mol L−1 min−1
(d) 9 mol L−1 min−1
Part 2
The following data are needed for questions 10–12
During the experimental study of the reaction 2H2(g) + 2NO(g) → 2H2O(g) + N2(g), the following data were obtained:
| Experiment no. | Initial [H2(g)] (mol L−1) | Initial [NO(g)] (mol L−1) | Initial reaction rate (mol L−1 s−1) |
|---|---|---|---|
| 1 | 0.10 | 0.10 | 0.09 |
| 2 | 0.20 | 0.10 | 0.18 |
| 3 | 0.10 | 0.20 | 0.36 |
(10) Select the correct answer
The rate equation for the reaction is:
(a) rate = k·[H2]2·[NO]2
(b) rate = k·[H2]·[NO]
(c) rate = k·[H2]2·[NO]
(d) rate = k·[H2]·[NO]2
(11) Calculate the value of the rate constant (k).
(12) Give the units for the rate constant (k).
The following data are needed for questions 13–14
During a kinetic study of the reaction
| 2A(g) → B(g) + C(g) |
| Time (s) | 0 | 20 | 40 | 60 | 80 | 100 |
| [A] (mol L−1) | 5.6 | 4.6 | 3.7 | 3.0 | 2.4 | 2.0 |
(13) Calculate the average consumption rate of A during the time interval between 20 s and 60 s.
(14) Calculate the average reaction rate for the time interval between 20 s and 60 s.
The following data are needed for questions 15–16
The rate equation for a reaction between the gases X and Y at a certain temperature is:
| rate = k·[X]2·[Y] |
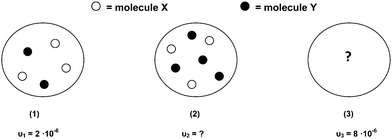
In the figure below, the circles represent three initial mixtures of X and Y in vessels of equal volume at the same temperature. Under each circle, the corresponding initial reaction rates are given in mol L−1 s−1
(15) Calculate the initial reaction rate in mixture (2).
(16) How many molecules each of X and Y are in mixture (3).
(17) Under appropriate conditions, the gas A is converted to gas B according to the reaction below:
| 2A(g) → B(g) |
The following graph represents the change in concentration of A during the course of the reaction.
Determine the average reaction rate during the first 5 minutes of the reaction.
The following data are needed for questions 18–20.
Consider the elementary reaction A + B → AB
Given that the reaction's activation energy (Ea), is 120 kJ mol−1 and the change in enthalpy (ΔH), is −85 kJ mol−1
(18) Draw the energy diagram of the reaction and label the axes.
(19) Indicate the activation energy on your diagram.
(20) Indicate the enthalpy of reaction on your diagram.
Appendix 3
An example of short answer question scoring (question 17)| Scoring description | Score |
|---|---|
| Response includes the equation that defines the average reaction rate as a function of the change in concentration of A over time, the correct extraction of data from the graph and correct arithmetic operations. | 1 point |
| Response includes the equation that defines the average reaction rate as a function of the change in concentration of A over time and the correct extraction of data from the graph. | 0.75 point |
| Response includes the equation that defines the average reaction as a function of the change in concentration of A over time. | 0.5 point |
| Response includes the equation that defines the average reaction rate as a function of the change in concentration of A over time but with the wrong sign. | 0.25 point |
| Response is incorrect, irrelevant, or inappropriate | 0 point |
| No response at all. | 0 point |
Appendix 4
Descriptive statistics for students' responses to test questions| Students | N = 619 |
| Questions | N = 20 |
| Cronbach's alpha | 0.832 |
| Mean | 11.4495 |
| Std. deviation | 4.52590 |
| Median | 11.5000 |
| Mode | 10.00 |
| Minimum | 0 |
| Maximum | 20 |
| Item difficulty indices | Average of 0.57, ranging from 0.24 to 0.76 |
| Item discrimination indices | Average 0.38, ranging from 0.22 to 0.54 |
References
- Abd-El-Khalick F., Waters M. and Le A., (2008), Representations of nature of science in high school chemistry textbooks over the past four decades, J. Res. Sci. Teach., 45, 835–855.
- Ahtineva A., (2005), Textbook analysis in the service of chemistry teaching, Univ. Sci., 10, 25–33.
- Atkins P. and Jones L., (2010), Chemical Principles, The quest for insight, 6th edn, New York: W. H. Freeman and Company.
- Bain K. and Towns M. H., (2016), A review of research on the teaching and learning of chemical kinetics, Chem. Educ. Res. Pract., 17, 246–262.
- Banerjee A., (1991), Misconceptions of students and teachers in chemical equilibrium, Int. J. Sci. Educ., 13, 355–362.
- Ben-Zvi R., Eylon B. and Silberstein J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63, 64–66.
- BouJaoude S., (1993), Students' systematic errors when solving kinetic and chemical equilibrium problems, Paper presented at the Annual Meeting of the the National Association for Research in Science Teaching Conference, Atlanta, Georgia, April 16–19.
- Bowen G. M. and Roth W.-M., (2002), Why Students May not Learn to Interpret Scientific Inscriptions, Res. Sci. Educ., 32, 303–327.
- Bucat R. B. and Cole A. R. H., (1988), The Australian Academy of Science School Chemistry Project, J. Chem. Educ., 65, 777–779.
- Bungum B., (2008), Images of physics: an explorative study of the changing character of visual images in Norwegian physics textbooks, NorDiNa, 4, 132–141.
- Cakmakci G., (2009), Emerging issues from textbook analysis in the area of chemical kinetics, Aust. J. Educ. Chem., 70, 31–38.
- Cakmakci G., (2010), Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students in Turkey, J. Chem. Educ., 87, 449–455.
- Cakmakci G., Leach J. and Donnelly J., (2006), Students' ideas about reaction rate and its relationship with concentration or pressure, Int. J. Sci. Educ., 28, 1795–1815.
- Carr M., (1984), Model Confusion in Chemistry, Res. Sci. Educ., 14, 97–103.
- Chairam S., Somsook E. and Coll R. K., (2009), Enhancing Thai students' learning of chemical kinetics, Res. Sci. Technol. Educ., 27, 95–115.
- Chiappetta E. L., Sethna G. H. and Fillman, D. A., (1991), A quantitative analysis of high school chemistry textbooks for scientific literacy themes and expository learning aids, J. Res. Sci. Teach., 28, 939–951.
- Chittleborough G. and Treagust D. F., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38, 463–482.
- Devetak I. and Vogrinc J., (2013), The Criteria for Evaluating the Quality of the Science Textbooks, in Khine M. S. (ed.), Critical Analysis of Science Textbooks: Evaluating Instructional Effectiveness, Dordrecht: Springer, pp. 3–15.
- Drechsler M. and Schmidt H.-J., (2005), Textbooks' and teachers' understanding of acid–base models used in chemistry teaching, Chem. Educ. Res. Pract., 6, 19–35.
- Ebbing D. D. and Gammon S. D., (2009), General Chemistry, 9th edn, Boston: Houghton Mifflin Company.
- Erduran S., (2001), Philosophy of Chemistry: An Emerging Field with Implications for Chemistry Education, Sci. Educ., 10, 581–593.
- Gabel D. L., (1983), What high school chemistry textbooks do well and what they do poorly, J. Chem. Educ., 60, 893–895.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70, 193–194.
- Gabel D. L., (1999), Improving teaching and learning through chemistry education research: a look at the future, J. Chem. Educ., 76, 548–554.
- Garnett P. J., Garnett P. J. and Hackling M. W., (1995), Students' alternative conceptions in chemistry: a review of research and implications for teaching and learning, Stud. Sci. Educ., 25, 69–95.
- Gess-Newsome J. and Lederman N. G., (1999), Examining Pedagogical Content Knowledge, New York: Kluwer, Dordrecht.
- Gilbert J. K. and Swift D. J., (1985), Towards a Lakatosian analysis of the Piagetian and alternative conceptions research programs, Sci. Educ., 69, 671–696.
- Gilbert J. K. and Treagust D., (2009), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, Dordrecht: Springer, pp. 1–8.
- Gkitzia V., Salta K. and Tzougraki C., (2011), Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12, 5–14.
- Glazer N., (2011), Challenges with graph interpretation: a review of the literature, Stud. Sci. Educ., 47, 183–210.
- Griffiths A. K. (1994), A critical analysis and synthesis of research on students' chemistry misconceptions, in Schmidt, H. J. (ed.), Proceedings of the 1994 international symposium problem solving and misconceptions in chemistry and physics, ICASE (International Council of Associations for Science Education), pp. 70–99.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Grotzer T. A. and Bell Baska B., (2003), How does grasping the underlying causal structures of ecosystems impact students' understanding? J. Biol. Educ., 38, 16–29.
- Hackling M. W. and Garnett P. J., (1985), Misconceptions of chemical equilibrium, Eur. J. Sci. Educ., 7, 205–214.
- Haggarty L. and Pepin B., (2002), An investigation of mathematics textbooks and their use in English, French and German classrooms: who gets an opportunity to learn about? Br. Educ. Res. J., 28, 567–590.
- Haim A., (1989), Catalysis: New Reaction Pathways, Not Just a Lowering of the Activation Energy, J. Chem. Educ., 66, 935–937.
- Irez, S., (2009), Nature of Science as Depicted in Turkish Biology Textbooks, Sci. Educ., 93, 422–447.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70, 701–705.
- Justi R., (2002), Teaching and Learning Chemical Kinetics, in Gilbert J., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer Academic, pp. 293–315.
- Justi R. and Gilbert J., (1999a), A cause of a historical science teaching: use of hybrid models, Sci. Educ., 83, 163–177.
- Justi R. and Gilbert J., (1999b), History and philosophy of science through models: the case of chemical kinetics, Sci. Educ., 8, 287–307.
- Keig P. and Rubba P., (1993), Translation of representations of the structure of matter and its relationship to reasoning, gender, spatial reasoning, and specific prior knowledge, J. Res. Sci. Teach., 30, 883–903.
- Kousathana M. and Tsaparlis G., (2002), Students' errors in solving numerical chemical-equilibrium problems, Chem. Educ.: Res. Pract. Eur., 3, 5–17.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Laidler K. J., (1987), Chemical Kinetics, 3rd edn, New York: Harper & Row, Publishers.
- Laidler K. J., (1996), A glossary of terms used in chemical kinetics, including reaction dynamics, IUPAC Recommendations 1996, Pure Appl. Chem., 68, 149–192.
- Liodakis S., Gakis D., Theodoropoulos D., Theodoropoulos P. and Kallis A., (2005), Chemistry B' Lyceum-positive stream (11th-grade), in Greek, Athens: OEDB.
- Logan S. R., (1984), Introductory reaction kinetics – an unacknowledged difficulty, Educ. Chem., 21, 20–22.
- Maeyer J. and Talanquer V., (2010), The role of heuristics in students thinking: ranking of chemical substances, Sci. Educ., 94, 963–984.
- Matthews M. R., (1994), Science teaching: The Role of History and Philosophy of Science, Routledge, New York.
- Mayer R. E., (1983), What have we learned about increasing the meaningfulness of science prose? Sci. Educ., 67, 223–237.
- Mayer R. E., (1997), Multimedia learning: are we asking the right questions? Educ. Psychol., 32, 1–19.
- Mayer R. E., (2011), Instruction Based On Visualizations, in Mayer R. E. and Alexander P. A. (ed.), Handbook of Research on Learning and Instruction, New York: Routledge, pp. 427–445.
- Mortimer M. and Taylor P., (2002), The Molecular World, Chemical Kinetics and Mechanism, The Open University, Royal Society of Chemistry.
- Niaz M. and Maza A., (2011), Nature of Science in General Chemistry Textbooks, Dordrecht: Springer.
- Novak J., (1988), Learning science and the science of learning, Stud. Sci. Educ., 15, 77–101.
- Novick S. and Nussbaum J., (1981), Pupils' understanding of the particulate nature of matter: a cross-age study, Sci. Educ., 65, 187–196.
- Österlund L.-L., Berg A. and Ekborg M., (2010), Redox models in chemistry textbooks for the upper secondary school: friend or foe? Chem. Educ. Res. Pract., 11, 182–192.
- Pedrosa M. A. and Dias M. H., (2000), Chemistry textbook approaches to chemical equilibrium and student alternative conceptions, Chem. Educ.: Res. Pract. Eur., 1, 227–236.
- Peeck J., (1993), Increasing picture effects in learning from illustrated text, Learn. Instr., 3, 227–238.
- Perkins D. N. and Grotzer T. A., (2005), Dimensions of causal understanding: the role of complex causal models in students' understanding of science, Stud. Sci. Educ., 41, 117–166.
- Roth W. M., Bowen G. M. and McGinn M. K., (1999), Differences in graph-related practices between high school biology textbooks and scientific ecology journals, J. Res. Sci. Teach., 36, 977–1019.
- Ruis S. P., (1988), Something's wrong with chemistry textbooks, J. Chem. Educ., 65, 720–721.
- Rushton G. T., Criswell B. A., McAllister N. D., Polizzi S. J., Moor L. A. and Pierre M. S., (2014), Charting an alternate pathway to reaction orders and rate laws in introductory chemistry courses, J. Chem. Educ., 91, 66–73.
- Schamp H. W., (1990), Model misunderstandings: teach the limitations, Sci. Teach., 57, 16–19.
- Silberberg M. S., (2009), Chemistry: The Molecular Nature Of Matter And Change, 5th edn, New York: McGraw-Hill.
- Silberberg M. S., (2012), Chemistry: The Molecular Nature of Matter and Change, 6th edn, New York: McGraw-Hill.
- Sözbilir M., Pinarbasi T. and Canpolar N., (2010), Prospective chemistry teachers' conceptions of chemical thermodynamics and kinetics, Eurasian J. Math. Sci. Technol. Educ., 6, 111–120.
- Stefani C. and Tsaparlis G., (2009), Students' Levels of Explanations, Models, and Misconceptions in Basic Quantum Chemistry: A Phenomenographic Study, J. Res. Sci. Teach., 46, 520–536.
- Sullivan J. H., (1967), Mechanism of the “Bimolecular” Hydrogen–Iodine Reaction, J. Chem. Phys., 46, 73–78.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ.: Res. Pract. Eur., 2, 123–158.
- Taber K. S., (2008), Towards a curricular model of the nature of science, Sci. Educ., 17,179–218.
- Taber K. S., (2012), Vive la Différence? Comparing “Like with Like” in Studies of Learners' Ideas in Diverse Educational Contexts, Educ. Res. Int., 168741.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving “human subjects”, Chem. Educ. Res. Pract., 15, 109–113.
- Tamir P., (1991), Multiple choice items: How to gain the most out of them, Biochem. Educ., 19, 188–192.
- Treagust D. F., Chittleborough G. D. and Mamiala T. L., (2003), The role of sub-microscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
- Tsaparlis G. and Papaphotis G., (2002), Quantum-chemical concepts: are they suitable for secondary students? Chem. Educ.: Res. Pract. Eur., 3, 129–144.
- Turányi T. and Tóth Z., (2013), Hungarian University Students' Misunderstandings in Thermodynamics and Chemical Kinetics, Chem. Educ. Res. Pract., 14, 105–116.
- Tversky B., (2005), Prolegomenon to scientific visualizations, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht: Springer, pp. 29–42.
- Van Der Valk T., Van Driel J. H. and De Vos W., (2007), Common characteristics of models in present-day scientific practice, Res. Sci. Educ., 37, 469–488.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38, 821–842.
| This journal is © The Royal Society of Chemistry 2017 |