Life cycle assessment of novel supercritical methyl propionate process with carbon dioxide feedstock
S. C.
Stouten
,
A.
Anastasopoulou
,
V.
Hessel
and
Q.
Wang
*
Laboratory of Chemical Reactor Engineering/Micro Flow Chemistry and Process Technology, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, PO Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: q.wang0207@gmail.com
First published on 26th July 2017
Abstract
The alkoxycarbonylation reaction can be realized in continuous flow under supercritical conditions by utilizing CO2 as a feedstock instead of CO. Conventionally, the synthesis of the methyl propionate is achieved in the first step of the Lucite Alpha process through the hydroesterification of ethylene with methanol and carbon monoxide. In this paper, synthesis of the methyl propionate process by replacing the carbon monoxide feedstock with CO2 and using a more robust and less expensive catalyst is simulated and evaluated from the perspective of environmental influence. A life cycle assessment was done of the methyl propionate production via the supercritical process utilizing CO2 as feedstock. For all nine impact categories – AP, GWP, EP, FAETP, HTP, Land use, MAETP, ODP and CED –, the novel process was compared to the performance of the existing state-of-the-art carbon monoxide-based process, the Lucite Alpha process. An 80% impact reduction was found for both the Global Warming Potential and the Ozone Depletion Potential. The major contribution to the impact reduction stems from the change from CO to CO2 as a feedstock, since the impact from CO as feedstock is strongly negative while the impact from CO2 as feedstock is strongly positive. Yet, also the supercritical conditions themselves show a notable environmental benefit, besides providing the enabling function for the new chemistry. A remarkable effect on steam, electricity, and cooling energy is given. The higher pressure required for the supercritical CO2 process was found to have minimal effect on the electricity use for compression.
Introduction
Carbonylation is an important reaction for the chemical industry and is used to incorporate carbon monoxide into an olefin.1 This allows the synthesis of various types of products, such as carboxylic acids, aldehydes via hydroformylation,2–5 or esters via alkoxycarbonylation.6 What all these reactions have in common is the use of the toxic and flammable carbon monoxide as a feedstock. Due to its hazardous nature, the use of carbon monoxide in the high pressure carbonylation process requires considerable safety precautions. For the same reasons, also bulk transportation of carbon monoxide is a critical issue.7 Although various other sources of carbon monoxide have been investigated,8–12 these were found to be either too costly or too inefficient as a feasible alternative. Carbon dioxide (CO2) while potentially a very interesting alternative that is freely available, is also difficult to activate due to its high thermodynamic stability.13–16 Previously investigated methods that utilized CO2 as a feedstock could not compete with carbon monoxide-based processes.17–19Recently, the group of Beller20 successfully utilized CO2 as a feedstock for a ruthenium-catalyzed alkoxycarbonylation reaction, as shown in Scheme 1. This reaction uses CO2 gas, which is inexpensive compared to other, indirect, sources of CO2. In addition, the catalyst is based on the less expensive ruthenium, compared to more commonly used and more expensive palladium or rhodium based carbonylation catalysts. Finally, the catalyst system is also very robust, as it does not depend on the use of sophisticated ligands that are sensitive to deactivation by impurities in the feed.
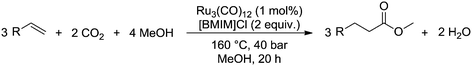 | ||
| Scheme 1 Methoxycarbonylation of alkenes with methanol and CO2 over a ruthenium catalyst, as performed by the group of Beller.20 | ||
The application of this novel alkoxycarbonylation reaction was further investigated at a laboratory scale by Stouten et al.,21 by performing the methoxycarbonylation of cyclohexene in continuous flow under supercritical conditions. The reaction rate was boosted more than five times by operating under flow conditions at a pressure of 120 bar and a temperature of 180 °C, obtaining a 77% yield with a 90 min residence time. Also investigated was the use of the catalyst in a heterogeneous manner, through immobilization on a solid support, as the robust nature of the catalyst makes it a promising candidate for such heterogenization. Although the immobilization was thus far unsuccessful, the advantages of a heterogeneous catalyst would be considerable.
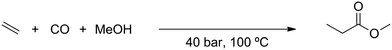
In short, the alkoxycarbonylation reaction discovered by the group of Beller is very interesting as a potential alternative for industrial carbonylation reactions that utilize carbon monoxide as a feedstock. In the Lucite Alpha process, the monomer for poly(methyl methacrylate) (PMMA) is produced via the intermediate methyl propionate.22 Synthesis of the methyl propionate is achieved in the first step of the process through the hydroesterification of ethylene with methanol and carbon monoxide. As it was shown by the group of Beller,20 synthesis of methyl propionate is also possible via the novel alkoxycarbonylation reaction, simply by replacing the carbon monoxide feedstock with CO2 and using a more robust and less expensive catalyst.
An issue to be considered for practical application is that the ionic liquid phase is continually diluted with water produced in the reaction. Indeed, literature findings have shown strong reliance of the performance of the supported aqueous-phase catalysts employed in the hydroformylation on the water loading.23 The change in the selectivity of the aqueous system has been attributed to the water-mediated hydrogen bonding to the catalyst.
Yet, measures are known to reduce and handle the aforementioned challenges. For example, adjusting the substrate surface so as to advert accumulation of traces of water in the SILP (supported ionic liquid phase), has proved to be an important means of ensuring continuous activation of the species. Moreover, the employment of a water scavenger or a perfluoroalkyl-functionalized silica substrate has demonstrated an improvement in the stability of the employed catalyst (140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TON).24 However, such engineering issues linked to product purification and catalyst recovery are likely to supersede the value of the final product.24 Continuous-flow systems could be a solution to that with both their steady-state homogeneous turnover in a stationary liquid phase and efficient integrated product separation. Also for this reason, this paper considers that kind of operation.
000 TON).24 However, such engineering issues linked to product purification and catalyst recovery are likely to supersede the value of the final product.24 Continuous-flow systems could be a solution to that with both their steady-state homogeneous turnover in a stationary liquid phase and efficient integrated product separation. Also for this reason, this paper considers that kind of operation.
The purpose of this continuous-flow based work is to study how the process based on the novel alkoxycarbonylation reaction compares with the industrial state-of-the-art, the Lucite Alpha process, from the perspective of environmental influence. To investigate this, a life cycle assessment (LCA) study was done to assess both processes. LCA studies assist the evaluation of processes and their ecological impact and, therefore, are often used as tools for decision making in the development of new processes.25–27 However, it is worth mentioning that there are two major approaches towards the sustainability evaluation of process intensified systems developed in R&D and project-funded work. In the case of industrial-lead explorations, such as H2020, lab-scale proof of concepts are transferred to pilot scale and these experimental data – at relevant industrial site – are used for sustainability analysis. The innovation degree of such process intensification (PI) is mediocre (TRL 3–5). On the contrary, in ground-breaking PI (TRL 1–2), where proof of concept at laboratory-scale is provided but no pilot plant demonstration is possible, sustainability assessment – at a preliminary level – is conducted based on the available, laboratory experimental data. In this study, the latter approach has been adopted given that the laboratory proof of concept for an analogous reaction using another substrate is demonstrated and an ex-ante environmental evaluation against the established industrial production route, the Lucite Alpha process, is being pursued.
Process modeling and simulation
To acquire the inventory data for the LCA study, the first step was to simulate the Lucite Alpha process, as well as the supercritical CO2 process using the Aspen Plus software. For the Lucite Alpha process, process information was acquired from relevant patents.28,29 For the supercritical CO2 process, the simulation was based on laboratory-scale experimental data.21Thermodynamic models and physical property methods
To determine the activity coefficients of the components in the liquid phase and the vapor–liquid equilibria for the Lucite Alpha process, the UNIFAC method30,31 with Redlich–Kwong equation of state and Henry's Law were applied. For the supercritical CO2 process however, the predictive Redlich–Kwong–Soave equation of state32,33 was used, to allow modeling of the supercritical conditions.Methyl propionate synthesis in the Lucite Alpha process
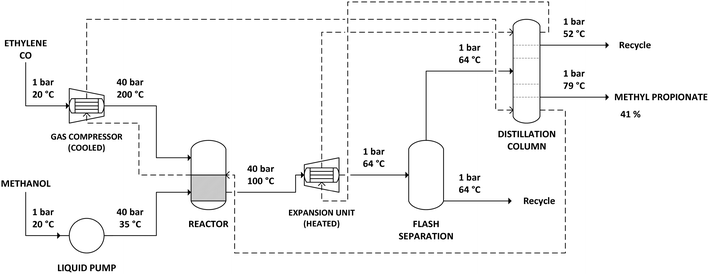
The simulation of the Lucite Alpha process was based on the process information available from the patents. The process flow sheet for the simulation is shown in Fig. 1. Ethylene and carbon monoxide gas feeds are compressed to a pressure of 40 bar. During compression, the gas feeds need to be cooled to prevent the temperature from exceeding 200 °C. Methanol is also pressurized to 40 bar, using a liquid pump. The reactor is a continuously stirred tank reactor (CSTR) operated at 100 °C and 40 bar, in which gas–liquid contact is maximized through agitation of the reaction mixture and gas re-circulation. The reaction is exothermic, with the stoichiometry shown in Scheme 2. The heat production by the exothermic reaction will require cooling of the reactor.The output of the reactor is a solution of methyl propionate and methanol in which the catalyst is dissolved. Subsequently, the solution is decompressed to atmospheric pressure and partially evaporated by a flash unit at 64 °C. The solution that remains is returned to the reactor to allow the catalyst to be recycled. The part of the solution that was evaporated, a mixture of methyl propionate and methanol, is then fed to a distillation column.
The distillation column was simulated in Aspen Plus using a RadFrac unit, with 80 equilibrium stages, a reflux ratio of 1.5 and operating at atmospheric pressure. The feed enters at stage 10. A 95% (m/m) solution of methyl propionate is obtained as bottom product, at 79 °C. As methyl propionate and methanol form an azeotrope at approximately 45% methanol and 55% methyl propionate, a mixture of both components is recovered as top product at 52 °C and recycled.
Methyl propionate synthesis in the supercritical CO2 process
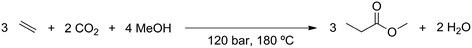
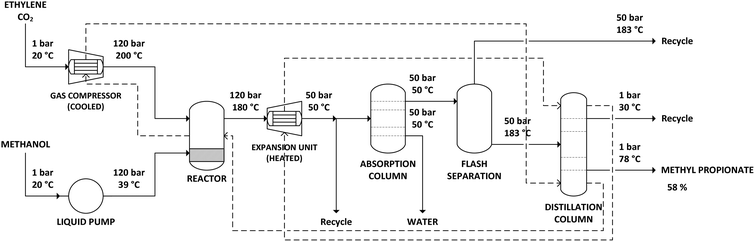
The supercritical CO2 process simulation was based on the literature in which the CO2-based methoxycarbonylation reaction was first performed under batch conditions,20 as well as on further study of the reaction under continuous supercritical conditions.21 Ethylene and CO2 gas feeds are compressed to a pressure of 120 bar. During compression, the gas feeds need to be cooled to prevent the temperature from exceeding 200 °C. Methanol is also pressurized to 120 bar, using a liquid pump. The reactor is a continuous flow reactor operated at 180 °C and 120 bar. Since the reactor operates at supercritical conditions, no gas–liquid phase separation exists. The reaction is exothermic, with the stoichiometry shown in Scheme 3. The heat production by the exothermic reaction will require cooling of the reactor.The output of the reactor is a supercritical mixture of methyl propionate, methanol and carbon dioxide in which the catalyst is dissolved, with most of the gaseous components remaining in the reactor. Subsequently, the solution is decompressed to 50 bar pressure at 50 °C. At these conditions, the solubility of the catalyst is poor, allowing it to be recovered and recycled. The product mixture is then passed through an absorption column, to remove water that was produced as part of the methoxycarbonylation reaction. The product mixture is then partially evaporated in a flash unit at 183 °C and 50 bar, to recover part of the CO2 before decompression to atmospheric pressure and purification in a distillation column.
The distillation column was simulated in Aspen Plus using a RadFrac unit, with 80 equilibrium stages, a reflux ratio of 1.3 and operating at atmospheric pressure. The feed enters at stage 10. Almost pure methyl propionate is obtained as bottom product, at 78 °C. As methyl propionate and methanol form an azeotrope at approximately 45% methanol and 55% methyl propionate, a mixture of both components and remaining gases is recovered as top product at 30 °C and recycled.
In addition to the supercritical CO2 process operating at 120 bar, the process was also simulated operating at 80 bar. In literature, experimental results show a decline in performance as going from 120 bar to 160 bar. Conversely, a further reduction in pressure from 120 bar to 80 bar may be feasible, possibly even improving performance. Reducing pressure below 80 bar was not considered beneficial, as the supercritical conditions, deemed essential for performance, would be lost. Compared to the process at 120 bar, only the initial pressurization of gas and liquid feed and the reactor pressure had to be lowered. After the reactor, the gas is depressurized to 50 bar in either case.
LCA methodology
The goal of the given LCA study is to compare the environmental footprint of the novel methyl propionate synthesis – at both low pressure (LP) and high pressure (HP) – against the conventional production pathway. For the purpose of the LCA modelling, the UMBERTO NXT LCA software (ifu Hamburg GmbH) has been utilized. As far as the system boundaries are concerned, a “cradle-to-gate” approach has been adopted, including only the material and energy flows linked to each sub-process of the conventional and novel production routes, for the conditions specified in the Fig. 1 and 2, respectively. For the latter process, the use of CO2 as raw material is associated with certain environmental benefits and in order to demonstrate this aspect in the LCA study, the concept of the “avoided burden” has been applied.34 Moreover, with respect to the catalyst involved in each examined chemical process, its complete recycling has been assumed, whereas the use of different substrates has been excluded. The transportation and storage of the final product, as well as, process maintenance have not been incorporated in the system boundaries of both processes.The functional unit has been defined as 1 ton of methyl propionate, and the inventory data required for the LCA modelling have been extracted from the Ecoinvent 3.0 embedded in the UMBERTO software. The values of the energy and material flows involved in the studied chemical processes have been acquired from the ASPEN simulations presented above. The LCIA method that has been selected is the CML2001 with the following impact categories being considered: acidification potential – average European (AP); global warming potential – 100 years (GWP); eutrophication potential – average European (EP); freshwater aquatic ecotoxicity potential – 100 years (FAETP); human toxicity potential – 100 years (HTP); land use; marine aquatic ecotoxicity potential – 100 years (MAETP); ozone depletion potential – 10 years (ODP); cumulative energy demand (CED).
Regarding the interpretation of the generated LCA results, the graphs presented in the discussion section below have been designed in such a way to reflect the contribution – in both absolute and normalized value representation – of the material and energy exchanged flows of each examined process to the aforementioned impact categories. In the case of the CO2 feedstock, the “credit” related to its use has been expressed with a negative value in those environmental impact categories which a relevant contribution has been observed.
Results and discussion
Global warming potential
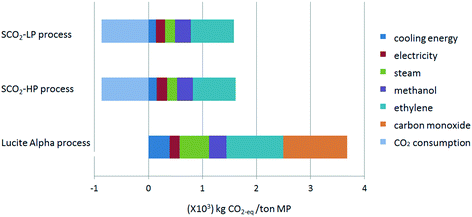
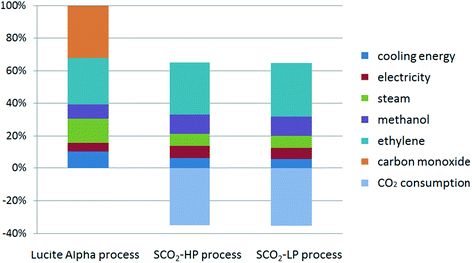
The global warming potential (GWP) is one of the most popular life cycle impact categories, reflecting the process' total emission in carbon dioxide equivalent (CO2-eq). The total CO2-eq emission, as well as the contribution factors for the three studied processes for MP synthesis are shown in Fig. 3. The negative CO2-eq emission in the case of the supercritical CO2 processes represents the consumption of CO2 for each functional unit of methyl propionate that is produced, taking into account the stoichiometry of 2 mol CO2 for 3 mol methyl propionate. The contribution from CO as the raw material in Lucite Alpha process is quite obvious and is responsible for almost 30% of the total CO2-eq emission (Fig. 4). Comparing the Lucite Alpha process with the supercritical CO2 process, the replacement of the CO feedstock with CO2 changes a large positive CO2-eq contribution to a large negative CO2-eq contribution. As such, it is immediately clear from the GWP results that a large reduction in CO2-eq emission is achieved simply from switching to the new methoxycarbonylation reaction with CO2 as a feedstock.Aside from the effect from switching to methoxycarbonylation with CO2, the absolute contribution from the energy (cooling energy), utility (steam), as well as the common raw materials (ethylene) for these two reaction systems are much lower for the supercritical CO2 process when compared to the Lucite Alpha process. As such, the impact from these three factors for the supercritical CO2 process is also lower (Fig. 4). Furthermore, since steam consumption reflects the heating energy (see the process simulation section), we can also conclude that the total energy consumption of the newly designed supercritical CO2 process is much lower than that of the existing process. The main reason for this reduction in energy consumption is the switch to supercritical conditions, creating a single phase system and, thus, eliminating mass transfer limitations inherent to a gas–liquid reaction. For the Lucite Alpha process, the biphasic system requires the reactor design to be aimed at maximizing the interfacial area between gas and liquid phases to boost mass transfer. However, this places considerable operational limits on the reactor, lowering the maximum conversion at which the process can be operated. Lower conversion is translated to less effective downstream recovery of the product. The low energy consumption of the supercritical CO2 process is the second obvious advantage compared to the existing Lucite Alpha process.
The absolute contribution from electricity and methanol consumption is quite similar in all processes. So the high pressure system of the supercritical CO2 process doesn't necessarily increase the demands of power consumption caused by pumps/compressors. According to the stoichiometry of these two reaction systems, the methanol consumption of the Lucite Alpha process is 75% of that occurring in the supercritical CO2 process. As such, the Lucite Alpha process consumes less methanol per functional unit of methyl propionate produced.
Surprisingly, the difference between high pressure and low pressure supercritical CO2 process is not that obvious, even though a higher energy consumption would be expected from operating at higher pressure. However, it can be calculated that very little additional work needs to be done by the compressor when increasing pressure from 80 to 120 bar. The required work can be found to scale roughly with ln(V2/V1), meaning it scales logarithmically with the compression ratio. And while the compression ratio from 1 to 80 bar is 80, the compression ratio from 80 to 120 bar is only 1.5.
Finally, the absolute CO2-eq emission for either supercritical CO2 process is only around 12% of that for the Lucite Alpha process.
Life cycle impact assessment for the Lucite Alpha process
Apart from the GWP, the other selected impact categories were also assessed for the Lucite Alpha and supercritical CO2 processes. The normalized quantitative results for the Lucite Alpha process are shown in Fig. 5. As it can be deduced, the consumption of CO has an obvious effect on all impact categories except for the land use. Among these eight impact categories, the contribution of CO feedstock use to the GWP is the lowest, which means the use of CO has higher influence on the other impact categories than the GWP. The normalized land use is dominated by methanol, accounting for almost 99%. Methanol contributes stronger to the other seven impact categories than to the GWP, except in the case of the CED. Ethylene plays the second most important role in the GWP, with a similar contribution to the AP and EP and a higher contribution to the CED. Conversely, ethylene contributes relatively little to the FAETP, HTP, land use, MAETP and ODP. The contribution from the cooling energy consumption to the HTP and ODP is more obvious than that to the GWP, while the contribution from the steam consumption only to the ODP is higher than that to the GWP. Finally, the electricity consumption has a higher contribution to the FAETP and MAETP than that to the GWP.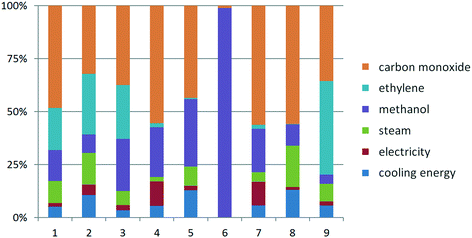 | ||
| Fig. 5 Normalized life cycle impact factors of Lucite Alpha process: 1. AP, 2. GWP, 3. EP, 4. FAETP, 5. HTP, 6. Land use, 7. MAETP, 8. ODP, 9. CED. | ||
Life cycle impact assessment for the supercritical CO2 process
For the supercritical CO2 process at high operating pressure, the normalized quantitative profile is strongly altered due to the change from CO to CO2 as a feedstock. As shown in Fig. 6, the most dominating factor in the AP, GWP, CED categories is ethylene, whereas in the remaining impact categories it is methanol. Yet steam, electricity and cooling energy follow roughly the same trend as for the Lucite Alpha process. For the supercritical CO2 process at low operating pressure the behavior is similar, so it is not shown separately.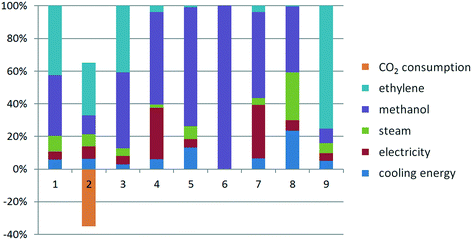 | ||
| Fig. 6 Normalized life cycle impact factors of the supercritical CO2 process at high pressure: 1. AP, 2. GWP, 3. EP, 4. FAETP, 5. HTP, 6. Land use, 7. MAETP, 8. ODP, 9. CED. | ||
Human toxicity potential and cumulative energy demand
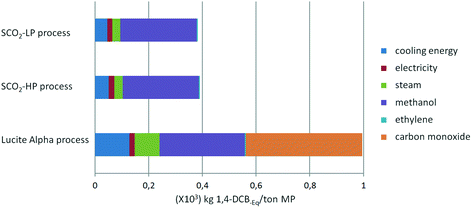
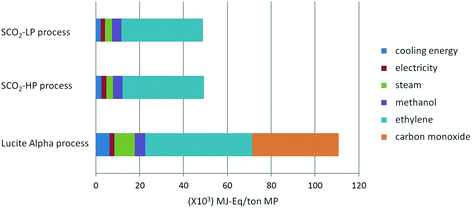
From Fig. 7 and 8, it is apparent that both the HTP and CED are reduced significantly by switching to the supercritical CO2 process, due to the high impact of CO as a feedstock in the Lucite Alpha process. On the other hand, methanol and ethylene contribute in a different manner to the HTP and CED. To elaborate, methanol contributes strongly to the HTP, even dominating in the case of the supercritical CO2 process. However, methanol's impact on the CED is only minimal. Ethylene, in contrast, is the dominating contribution for the CED, but barely contributes to the HTP at all. Cooling energy contributes considerably less, although more to the HTP than to the CED. Further reducing the HTP and CED is difficult, since the main contributors, methanol and ethylene, are essential feedstocks.Overall, changing from the Lucite Alpha process to the supercritical CO2 process results in a sharp decrease for all the life cycle impact categories studied, as depicted in Fig. 9. The GWP and ODP especially, decrease to 20% of that of the Lucite Alpha process. All other impact categories except for land use decrease to around 40%. The substitution of CO by CO2 accounts for most of these changes and is the main reason that the supercritical CO2 process performs such better. Because, not only is the CO feedstock a strong contributor to many impact categories, but also the use of CO2 can actually reduce the impact in some cases.
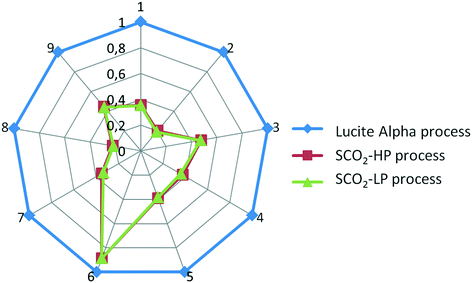 | ||
| Fig. 9 Comparison of all three processes. 1. AP, 2. GWP, 3. EP, 4. FAETP, 5. HTP, 6. Land use, 7. MAETP, 8. ODP, 9. CED. | ||
Conclusion
Methyl propionate production by the existing state-of-the-art process, the Lucite Alpha process, was compared to methyl propionate production by a novel supercritical CO2 process. Both processes were simulated using Aspen Plus software based on information from literature, and compared via a life cycle assessment involving nine different impact categories. On all impact categories except land use, the supercritical CO2 process was found to have a remarkably better environmental performance as compared to the Lucite Alpha process. For the GWP and ODP in particular, the impact was reduced to 20% of the corresponding value in the Lucite Alpha process. The strongest contributor to this impact reduction was found to be the use of CO2 feedstock over CO. Yet, it is important to note that the supercritical processing also notably reduces the overall environmental footprint – the other impact categories –. This is due to the supercritical conditions that eliminate mass transfer limitations, allowing operation under intensified conditions and removing the need for vigorous mixing using a continuously stirred tank reactor. Operating the supercritical CO2 process at 80 bar instead of 120 bar was found to have minimum effect on all impact categories.The major conclusions deduced from the given LCA study can be summarized as follows:
– Consumption of CO2 as feedstock results in a considerable reduction of the GWP – 80% as to the conventional one –.
– Operation under supercritical conditions shows a remarkable impact on steam, electricity, and cooling energy consumption.
– The process-related effect is also notable and stronger in other impact categories than GWP.
– Finally, the use of higher pressure in the supercritical process seems to have a minimal effect on the electricity use (for compression).
In short, the supercritical CO2 process is a very promising alternative to the existing Lucite Alpha process. However, with respect to the industrial application of the novel process, considerable effort needs still to be directed towards a reliable, stable operation and flawless working plant, the scale-up and economy of the supercritical operation, the life-time and regeneration/recycle of the smart, immobilized catalyst, and much more.
Acknowledgements
Funding by the Advanced European Research Council Grant “Novel Process Windows – Boosted Micro Process Technology” under grant agreement number 267443 is kindly acknowledged.References
- W. Bertleff, Ullmann's Encycl. Ind. Chem., 2012 Search PubMed.
- P. W. N. M. van Leeuwen and C. Claver, Rhodium Catalyzed Hydroformylation, Kluwer Academic Publishers, 2002 Search PubMed.
- O. Diebolt, C. Müller and D. Vogt, Catal. Sci. Technol., 2012, 2, 773 CAS.
- F. Hebrard and P. Kalck, Chem. Rev., 2009, 109, 4272–4282 CrossRef CAS PubMed.
- B. Breit, in Metal Catalyzed Reductive C–C Bond Formation, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, pp. 139–172 Search PubMed.
- G. Kiss, Chem. Rev., 2001, 101, 3435–3456 CrossRef CAS PubMed.
- T. Morimoto and K. Kakiuchi, Angew. Chem., Int. Ed., 2004, 43, 5580–5588 CrossRef CAS PubMed.
- T. Morimoto, K. Fuji, K. Tsutsumi and K. Kakiuchi, J. Am. Chem. Soc., 2002, 124, 3806–3807 CrossRef CAS PubMed.
- J. H. Park, Y. Cho and Y. K. Chung, Angew. Chem., Int. Ed., 2010, 49, 5138–5141 CrossRef CAS PubMed.
- J. J. Verendel, M. Nordlund and P. G. Andersson, ChemSusChem, 2013, 6, 426–429 CrossRef CAS PubMed.
- H. Konishi, T. Ueda, T. Muto and K. Manabe, Org. Lett., 2012, 14, 4722–4725 CrossRef CAS PubMed.
- A. Wiȩckowska, R. Fransson, L. R. Odell and M. Larhed, J. Org. Chem., 2011, 76, 978–981 CrossRef PubMed.
- T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- M. Aresta, C. F. Nobile, V. G. Albano, E. Forni and M. Manassero, J. Chem. Soc., Chem. Commun., 1975, 636–637 RSC.
- H. Mizuno, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2011, 133, 1251–1253 CrossRef CAS PubMed.
- L. Zhang, J. Cheng, T. Ohishi and Z. Hou, Angew. Chem., Int. Ed., 2010, 49, 8670–8673 CrossRef CAS PubMed.
- C. M. Williams, J. B. Johnson and T. Rovis, J. Am. Chem. Soc., 2008, 130, 14936–14937 CrossRef CAS PubMed.
- T. Fujihara, T. Xu, K. Semba, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2011, 50, 523–527 CrossRef CAS PubMed.
- S. Li, W. Yuan and S. Ma, Angew. Chem., Int. Ed., 2011, 50, 2578–2582 CrossRef CAS PubMed.
- L. Wu, Q. Liu, I. Fleischer, R. Jackstell and M. Beller, Nat. Commun., 2014, 5, 3091 Search PubMed.
- S. C. Stouten, T. Noël, Q. Wang, M. Beller and V. Hessel, Catal. Sci. Technol., 2016, 6, 4712–4717 CAS.
- C. Jimenez Rodriguez, D. F. Foster, G. R. Eastham and D. J. Cole-Hamilton, Chem. Commun., 2004, 1720–1721 RSC.
- U. Hintermair, T. Chinnusamy and W. Leitner, in New Strategies in Chemical Synthesis and Catalysis, ed. B. Pignataro, Wiley-VCH, 2012, pp. 273–297 Search PubMed.
- G. Franciò, U. Hintermair and W. Leitner, Philos. Trans. R. Soc., A, 2015, 373, 20150005 CrossRef PubMed.
- Q. Wang, I. Vural Gürsel, M. Shang and V. Hessel, Chem. Eng. J., 2013, 234, 300–311 CrossRef CAS.
- Q. Wang, B. Spasova, V. Hessel and G. Kolb, Chem. Eng. J., 2015, 262, 766–774 CrossRef CAS.
- S. Sundaram, D. Kralisch, Q. Wang and V. Hessel, Asia-Pac. J. Chem. Eng., 2015, 10, 483–500 CAS.
- S. Ziemian and I. A. York, WO2012063044 A1, Lucite International UK Limited, 2012.
- G. R. Eastham, D. W. Johnson, M. Waugh, J. A. Iggo and M. Beaumont, WO2016166525 A1, Lucite International UK Limited, 2016.
- S. Hung, I. Lai, H. Huang, M. Lee and C. Yu, Ind. Eng. Chem. Res., 2008, 47, 3076–3087 CrossRef CAS.
- S. B. Gadewar, G. Schembecker and M. F. Doherty, Chem. Eng. Prog., 2006, 102, 22–32 CAS.
- L. L. Williams, E. M. Mas and J. B. Rubin, J. Chem. Eng. Data, 2002, 47, 282–285 CrossRef CAS.
- S. Camy, J. S. Pic, E. Badens and J. S. Condoret, J. Supercrit. Fluids, 2003, 25, 19–32 CrossRef CAS.
- A. Azapagic and R. Clift, J. Cleaner Prod., 1999, 7, 101–119 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |