Kinetics of guaiacol deoxygenation using methane over the Pt–Bi catalyst
Yang
Xiao
and
Arvind
Varma
*
Davidson School of Chemical Engineering, Purdue University, West Lafayette, IN 47907, USA. E-mail: avarma@purdue.edu; Fax: +1 765 494 0805; Tel: +1 765 494 8484
First published on 28th November 2016
Abstract
Using H2 as a reductant, catalytic hydrodeoxygenation (HDO) is typically used for upgrading bio-oils, generally produced from thermal degradation of lignin. In our recent prior work, methane was used to deoxygenate guaiacol over the Pt–Bi catalyst and was found to exhibit as good deoxygenation performance as hydrogen. In the present work, using methane as a reductant, detailed reaction pathways and kinetics of guaiacol deoxygenation are studied using differential and integral operating conditions. Kinetic parameters including rate constants and activation energies are determined for each individual reaction step. The model predicted values match experimental data well. Results from the present work are discussed and compared with the literature values. The present work provides a practical novel approach for bio-oil upgrading using methane as a reductant instead of hydrogen.
Introduction
Energy security and environmental concerns are drivers for utilization of biomass to produce bio-fuels. The vast quantity of biomass available in the United States has the potential to replace significant amounts of fuels that are currently derived from petroleum sources. Bio-oil, generally derived from fast pyrolysis of biomass, is one such candidate fuel.1 Its production process, however, remains under investigation, owing primarily to its high oxygen content, which may cause instability, poor combustion performance and low heating value.2–4 Too high levels of oxygen make pyrolysis oils distinctly different from petroleum fuels. Oxygen removal from pyrolyzed bio-oils, thus, is typically followed as an upgrading process to utilize these bio-oils as transportation fuels.5,6Since the pyrolysis bio-oil is always a mixture of more than 400 chemical species and different biomass sources result in different bio-oil compositions, model compounds such as phenol, guaiacol (2-methoxyphenol) and benzylphenyl ether7 are typically selected to represent bio-oils for more fundamental studies.8 Among these model compounds, guaiacol is attractive because it contains two common oxygenated groups in bio-oils: hydroxyl and methoxyl. Catalytic hydrodeoxygenation (HDO), as a common bio-oil upgrading approach, refers to oxygen removal by the use of H2 molecules over heterogeneous catalysts. Using Pt, Pd, Co, etc. as primary metals,9 Mo, S, etc. as promoters,10 and activated carbon (AC), Al2O3, ZrO2, SiO2, etc. as catalyst supports,11–13 various experimental and theoretical studies have been reported for guaiacol catalytic HDO.14–20
Although H2 is an ideal reductant for oxygen removal from pyrolysis oils, its use in HDO, however, leads to possible economic concerns owing to the high cost of H2 production and transportation. In some cases, instead of H2, methane has been used as a reductant. For example, for NOx to N2 conversion in the presence of O2,21,22 CH4 was considered as the hydrogen donor and was activated by surface oxygen species.23
For these reasons, we have recently reported a novel bio-oil upgrading approach,24 using CH4 instead of H2 as a reductant and Pt–Bi/AC as a catalyst, to deoxygenate guaiacol efficiently. It was found that using the mono-metallic Pt/AC catalyst, CH4 exhibited good deoxygenation performance as H2 in terms of guaiacol conversion and product distribution, but the catalyst deactivated rapidly. With addition of bismuth as a promoter, the lifetime of the bi-metallic Pt–Bi/AC was extended significantly without loss of activity, as compared to the Pt/AC catalyst.
In our other prior works,25,26 using Pt/AC and H2, reaction pathways and detailed kinetics of the guaiacol deoxygenation process were reported. On the basis of these prior studies,24–26 in the present work, using methane as the reductant both experimental and modeling investigations were conducted, to reveal the reaction pathways and kinetics of guaiacol deoxygenation, where partially deoxygenated compounds, including phenol and cyclopentanone, are target products. Kinetic measurements in both differential and integral reactors were carried out, and rate constants and activation energy values were determined for the individual reaction steps. These results are discussed and compared with our prior studies as well as other related works.
Experimental
Materials
The 5% Pt–1% Bi/C catalysts were prepared following the procedure described in our prior works.24,27,28 The metallic precursors were chloroplatinic acid hydrate (99.9% metal basis) and bismuth chloride (99.999%), both from Sigma Aldrich. The 20–120 mesh AC supports were from Norit Americas Inc. Briefly, Pt and Bi were loaded sequentially using the wet impregnation method. First, the Pt and Bi precursors were dissolved in 1.2 mol L−1 HCl and then added dropwise to the well-stirred AC slurry, with stirring continued for at least 8 h at room temperature. The mixture was then rinsed and dried in air at 100 °C before use. Guaiacol (>98.0%) and all other calibration compounds (catechol, phenol and cyclopentanone) were purchased from Sigma-Aldrich. GUA, CAT, PHE and CYC are used as abbreviations for guaiacol, catechol, phenol and cyclopentanone, respectively, in the later sections. Ultra high purity grade gases (99.98% oxygen, 99.999% argon, 99.98% helium and 99.999% hydrogen) were purchased from Indiana Oxygen.The 0.5% Pt/Al2O3 (metal dispersion = 31 ± 0.5%) standard (for chemisorption calibration) was from Micromeritics.
Kinetic measurements
The kinetic measurements were conducted in a fixed-bed reactor (316 L stainless steel, inner diameter = 12 mm) setup, described in our prior work.25,26 Prior to the reaction, the packed catalyst was activated at 400 °C and 1 atm for 4 hours under a gas mixture flow (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The reactor was then purged using N2 for 30 min. The standard reactor operating conditions were 400 °C, 1 atm, 0.02–0.05 g of catalyst for differential reactions (conversions typically <6–8%) and 0.3–0.5 g of catalyst for integral reactions (conversions typically >20%), total gas (CH4
2). The reactor was then purged using N2 for 30 min. The standard reactor operating conditions were 400 °C, 1 atm, 0.02–0.05 g of catalyst for differential reactions (conversions typically <6–8%) and 0.3–0.5 g of catalyst for integral reactions (conversions typically >20%), total gas (CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) flow rate of 100 mL min−1, and guaiacol feed rate of 0.025 mL min−1 (liquid, at room temperature).
1) flow rate of 100 mL min−1, and guaiacol feed rate of 0.025 mL min−1 (liquid, at room temperature).
The feed flow rates correspond to a molar ratio of 10 between CH4 and guaiacol. Blank tests with co-feeding guaiacol and CH4/N2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) over an AC support with no metal loading were conducted under the standard reaction conditions, and guaiacol conversion was less than 1%, while methane conversion was less than 3%, generating H2 and trace C2H6. This indicates that deoxygenation of guaiacol and methane decomposition to hydrogen/carbon are limited over the inert AC support. All experiments have carbon mass balances of 92 ± 2%, similar to those reported in the literature.29,30 Possible factors affecting mass balance include liquid hold-up in various locations in the system, particularly the condenser, and coke deposit on the catalyst.
1) over an AC support with no metal loading were conducted under the standard reaction conditions, and guaiacol conversion was less than 1%, while methane conversion was less than 3%, generating H2 and trace C2H6. This indicates that deoxygenation of guaiacol and methane decomposition to hydrogen/carbon are limited over the inert AC support. All experiments have carbon mass balances of 92 ± 2%, similar to those reported in the literature.29,30 Possible factors affecting mass balance include liquid hold-up in various locations in the system, particularly the condenser, and coke deposit on the catalyst.
Product analysis
As in our prior works, a GC (Agilent GC6890) with a flame ionization detector (FID), equipped with a DB-1701 column (30 m × 0.25 mm), was used for quantitative analysis of the liquid products.24–26 The gaseous effluent was analyzed using a Micro GC (Agilent 3000A Micro GC) equipped with two columns (column A, MolSieve 5 A, 10 m × 0.32 mm; column B: Plot U, 8 m × 0.32 mm) and two thermal conductivity detectors (TCD). For the reaction experiments, good repeatability generally within less than 2% deviation was achieved for all quantitative analyses.Catalyst characterization
Since the same catalyst, as in our prior related work,24 was used in the present work, catalyst characterization results, including BET surface area, pore diameter, chemisorption (Pt metal dispersion) and transmission electron microscopy, can be found in that article and its Supplementary Information. Briefly, the BET surface areas of Pt–Bi/C are high (>500 m2 g−1), which imply good capacity to adsorb reactants. The moderate mean pore diameter (about 3 nm) makes the catalyst accessible to larger molecules such as guaiacol (reactant) and catechol. The mean diameters of metal clusters given by TEM (3.3 nm) and chemisorption (3.9 nm) techniques are consistent, providing Pt metal dispersion of Pt–Bi/C by chemisorption of 29%.Results and discussion
Proposed reaction pathways
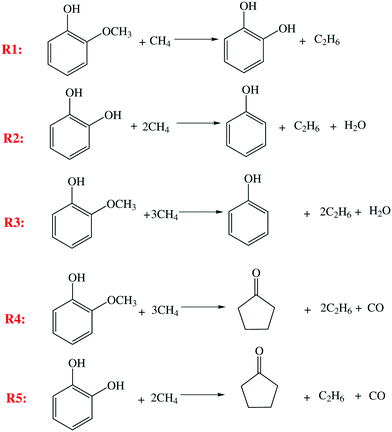
Since bismuth and the AC support do not show any activity for guaiacol deoxygenation, platinum is considered as the primary component that catalyzes guaiacol deoxygenation, while surface Pt atoms are the active sites. In our prior work, when Pt/AC as a catalyst and H2 as a reductant were used for guaiacol deoxygenation, reaction pathways, as shown in Fig. 1a, were proposed and confirmed by experimental observations,25 kinetic modeling and DFT calculations.26 Similar reaction pathways, as shown in Fig. 1b, apply in the present work since the same product species, including GUA, CAT, PHE and CYC, and similar distribution are found when Pt–Bi/AC as a catalyst and CH4 as a reductant are used. Both Fig. 1a and b describe reaction pathways containing five individual steps, denoted as R1–R5, producing catechol, phenol and cyclopentanone as liquid products, while different gaseous by-products arise from H2 and CH4, respectively. It is proposed that the same active site (surface Pt) is effective for all the five steps (R1–R5). Experimentally, no significant conversion was found when phenol and cyclopentanone were used as reactants in the feed, indicating no additional reactions beyond steps R1–R5.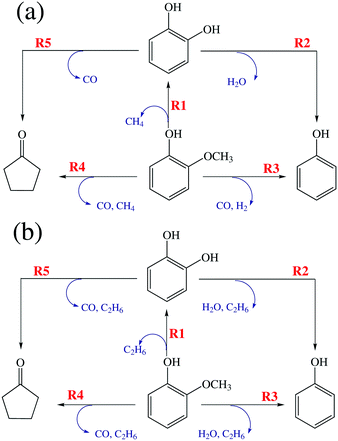 | ||
| Fig. 1 Proposed reaction pathways of guaiacol deoxygenation for the a) Pt/AC and H2 case and b) Pt–Bi/AC and CH4 case. | ||
Based on the above features, detailed reactions between guaiacol/intermediates and methane are proposed in Fig. 2.
For a deoxygenation reaction (such as R2 or R3), similar to H2, one molecule of CH4 provides only one H atom to oxygen-containing groups, forming H2O or other hydrogenated species, while the residual CH3 combines with another CH3 to form a C2H6 molecule.
Absence of mass transfer limitations
Before conducting kinetic measurements, mass transfer limitations were tested in a differential reactor, using the well-known procedure.31 The experimental results are shown in Fig. 3.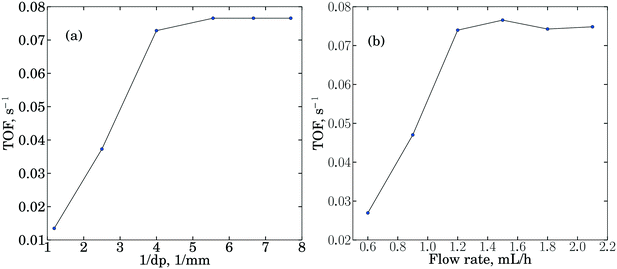 | ||
| Fig. 3 Mass transfer limitation tests for the a) internal diffusion effect and b) external diffusion effect. | ||
Fig. 3a is obtained by varying the packed catalyst particle diameter (dp) in the range 100 microns < dp < 1000 microns, while keeping other operating conditions (guaiacol flow rate = 1.8 mL h−1) unchanged. Fig. 3b is obtained by varying the guaiacol feed flow rates, while maintaining contact time (W/F) and other operating conditions constant (dp < 150 microns). Fig. 3a shows that when the catalyst particle diameter (dp) is larger than 250 microns, corresponding to 60 mesh, internal diffusion effects are discerned, while when (dp) < 150 microns, corresponding to 100 mesh, no significant internal effects are found. As shown in Fig. 3b, when the feed flow rate is smaller than 1.2 mL min−1, there is an obvious external mass transfer effect, while no such effect is observed when the feed flow rate is larger than 1.5 mL min−1. Thus, dp = 100 microns and a feed flow rate larger than 1.5 mL min−1 were used in the kinetic measurement experiments. Further, the criteria described by Weisz and Prater32 and Mears33 were both satisfied to verify the absence of mass and heat transfer effects in all experiments.
Kinetic model development
The reaction rates of step Ri in Fig. 1b and the corresponding turnover frequencies (TOF) are defined by eqn (1) and (2), respectively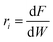 | (1) |
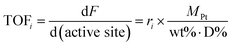 | (2) |
Since only one catalyst (Pt–Bi/AC), with a specific Pt metal loading amount (wt% = 5%) and a specific Pt metal dispersion (D% = 29%), was used in the kinetic experiments, the reaction rate and TOF are inter-converted as indicated in eqn (1) and (2).
The reaction pathways, including five individual steps (R1–R5) as shown in Fig. 1b, were established under integral operating conditions. When using guaiacol as the feed, under differential operating conditions (guaiacol conversion less than 6–8%), catechol is the only detectable product, i.e. only step R1 prevails while steps R3 and R4 are negligible. This is likely due to lower reaction rates, hence lower yields (smaller than 6–8%) of steps R3 and R4, as compared to step R1.
When catechol is used as feed, also under differential conditions, both phenol and cyclopentanone (corresponding to steps R2 and R5) exist in the liquid products. These experimental observations imply that when guaiacol is fed under differential operating conditions, R1 is faster than R3 and R4, and phenol and cyclopentanone are not produced owing to the low concentration of catechol. Therefore, kinetic parameters for steps R1, R2 and R5 could be obtained by running differential reactions. Using kinetic parameters of R1, R2 and R5, those for R3 and R4 could be further regressed by running integral reactions.
When differential operating conditions apply, reaction rates are obtained using eqn (3).
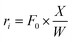 | (3) |
Based on the design equation for a fixed-bed reactor and the proposed pathways in Fig. 1b, consumption/formation rates of guaiacol, catechol, phenol and cyclopentanone are shown in eqn (4)–(7).
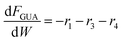 | (4) |
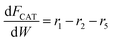 | (5) |
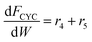 | (6) |
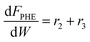 | (7) |
An overall material balance yields the following equations (eqn (8) and (9)):
| FGUA + FCAT + FCYC + FPHE = F0 | (8) |
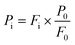 | (9) |
As described in the kinetic measurements section, excess methane (the molar ratio of methane to reactants is ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is used in all cases, thus partial pressure of methane may be considered as a constant contribution to the reaction rate expressions. The following data fitting and optimization are based on experimental measurements at various temperatures from 325 to 450 °C. Although only results at 400 °C are shown below because this standard operating temperature gives the optimum deoxygenation performance of guaiacol24 and results at 400 °C were repeated more than 5 times, fitting and optimization results for different temperatures give similar conclusions. All experiments at other temperatures were repeated at least twice and are discussed in the section on Activation energies and temperature effect.
1) is used in all cases, thus partial pressure of methane may be considered as a constant contribution to the reaction rate expressions. The following data fitting and optimization are based on experimental measurements at various temperatures from 325 to 450 °C. Although only results at 400 °C are shown below because this standard operating temperature gives the optimum deoxygenation performance of guaiacol24 and results at 400 °C were repeated more than 5 times, fitting and optimization results for different temperatures give similar conclusions. All experiments at other temperatures were repeated at least twice and are discussed in the section on Activation energies and temperature effect.
Differential reactions for steps R1, R2 and R5
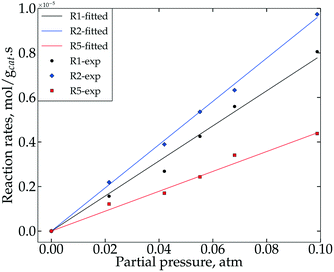
When differential operating conditions apply at 400 °C, for steps R1, R2 and R5, the corresponding reaction rates r1, r2 and r5 were obtained using eqn (3).Using different feed partial pressures of guaiacol or catechol, reaction rates were measured and plotted with respect to average bed reactant partial pressures (average pressures of the inlet and outlet), as shown in Fig. 4. All three data sets follow linear regressions, indicating first order kinetics for steps R1, R2 and R5. Thus, the corresponding reaction rates at 400 °C are described using eqn (10)–(12), where the regressed values of k1, k2 and k5 are listed in Table 1. The reaction rate regressions of R1, R2 and R5 at other temperatures are also essentially linear, as discussed later in the section on Activation energies and temperature effect.
| r1 = k1PGUA | (10) |
| r2 = k2PCAT | (11) |
| r5 = k5PCAT | (12) |
| Steps | k i × 10−5 |
|---|---|
| R1 | 8.29 ± 0.07 |
| R2 | 9.71 ± 0.03 |
| R5 | 4.47 ± 0.03 |
Using H2 as a reductant, Runnebaum et al.34 also reported 1st order kinetics for step R1 over the Pt/Al2O3 catalyst, while our prior work26 fitted a 2nd order for the same step. The difference between the present work and our prior work may be due to different reductants (H2 or CH4). For steps R2 and R5, both the present work and our prior work demonstrate 1st order kinetics. The reaction rate constant values, however, are smaller when CH4 is used as a reductant, indicating a higher activation energy for CH4.
Integral reactions of steps R3 and R4
As shown above, under differential operating conditions, step R1 is decomposed from the reaction network (Fig. 1) when guaiacol is fed over the catalyst. Similarly, steps R2 and R5 are decomposed under differential operating conditions when catechol is fed. To obtain insight into steps R3 and R4, integral operating conditions (guaiacol conversion >30%) were used while guaiacol is fed by packing more catalyst, producing distinct amounts of phenol and cyclopentanone. These, obviously, introduce not only steps R3 and R4 but also steps R1, R2 and R5. It should be clear that the integral operating conditions do not create new pathways (steps R3 and R4), as compared to the differential operating conditions (only step R1). Instead, under differential operating conditions, owing to low guaiacol conversion (6–8%), undetectability of products (PHE and CYC, estimated amount <2%) from steps R3 and R4 is due to the GC equipment detection limit and unavoidable loss during product collection and analysis. These limitations were overcome by higher guaiacol conversion under integral operating conditions.Since all five steps (R1 to R5) exist under integral operating conditions, eqn (4)–(7) can be re-written as eqn (13)–(16), where ki and ni are reaction rate constants and orders for steps Ri.
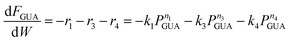 | (13) |
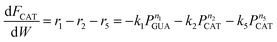 | (14) |
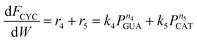 | (15) |
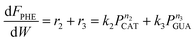 | (16) |
In this description, the reaction orders for steps 1, 2 and 5 are also kept flexible. With ki and ni as variables (total 10), predicted values of flow rates for GUA, CAT, CYC and PHE can be calculated by Runge–Kutta numerical integration using the following initial conditions:
| FGUA = F0, FCAT = 0, FPHE = 0 and FCYC = 0, when W = 0. | (17) |
In general, for parameter estimation involving exponents, the weighted least-squares method is recommended and was followed in the present work.35–37 This involves minimizing the error estimate given by eqn (18).
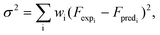 | (18) |
Since the differential reaction analysis yielded first-order kinetics for steps R1, R2 and R5, we used wi = Fi2 as the starting weighting functions. A sequential optimization strategy was then employed where only 2 (k3 and k4) or 4 (k3, k4, n3 and n4) variables out of 10 were kept flexible at the initial stage. Using these results as initial guesses, all 10 variables were allowed to vary in the final stage and the corresponding optimized parameters are shown in Table 2. Since the reaction orders n3 and n4 are 2, wi = Fi4 was also tested and converged to the same ni as for wi = Fi2 and essentially the same ki values as well. Thus, it appears that wi = Fi2 is an appropriate choice for the weighting function.
| n 1 | n 2 | n 3 | n 4 | n 5 | k 1 × 10−5 | k 2 × 10−5 | k 3 × 10−5 | k 4 × 10−5 | k 5 × 10−5 |
|---|---|---|---|---|---|---|---|---|---|
| 1.00 | 1.00 | 2.00 | 2.00 | 1.00 | 8.38 | 9.68 | 23.8 | 1.76 | 4.46 |
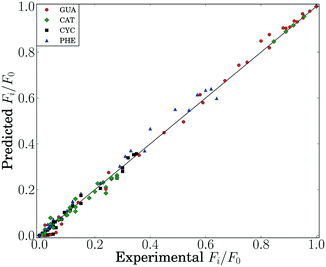
Using data from both integral and differential operating conditions, a comparison of the experimental and predicted flow rates is shown in Fig. 5. Fig. 5a shows that predicted flow rates are close to the experimental values for various catalyst amounts. Fig. 5b summarizes the goodness-of-fit in a parity plot. The values for all species are close to the diagonal line and relatively evenly distributed on both sides, indicating a good fit (normalized RMS error = 2.8%).
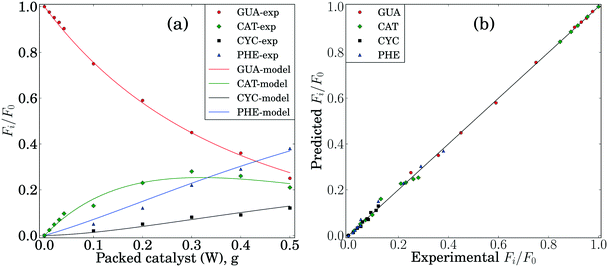 | ||
| Fig. 5 A comparison of experimental and predicted flow rate values for a) different catalyst amounts and b) parity plot. | ||
Because both experimental observations and kinetic modeling indicate that r1 is larger than both r3 and r4, it is worthwhile to test a reduced model with R3 and R4 steps excluded, which means that k3 = 0 and k4 = 0. The results, however, show that the reduced model (RMS error = 11.4%) does not fit the experimental data as well as the full model. Thus, steps R3 and R4 are important and cannot be neglected to predict the product distribution for guaiacol deoxygenation over the Pt–Bi/AC catalyst.
Activation energies and temperature effect
In the above sections, all reaction orders and rate constants at 400 °C were determined. Using the Arrhenius equation (eqn (19)), reaction activation energies (Ea) and exponential factors (A) were calculated.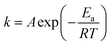 | (19) |
The Ea values are listed in Table 3 and also compared with our prior work,26 where hydrogen and Pt/AC were used.
| E a, kJ mol−1 | R 1 | R 2 | R 3 | R 4 | R 5 |
|---|---|---|---|---|---|
| CH4 case | 146 ± 11 | 110 ± 8 | 166 ± 13 | 184 ± 14 | 141 ± 9 |
| H2 case | 126 ± 6 | 100 ± 4 | 93 ± 3 | 149 ± 5 | 125 ± 2 |
The activation energy of step R1 was reported to be 71.2, 58.7 and 89.1 kJ mol−1 for Co–Mo, Ni–Mo and Ni–Cu catalysts, respectively,39,40 when H2 was used as a reductant. There are no data available for the CH4 case in the literature. Using Pt-based catalysts, our activation energies of step R1 are 146 and 126 kJ mol−1 for the CH4 and H2 cases, respectively, both higher than the literature data. The difference is likely due to the catalyst itself, including Pt metal nature, Pt particle size, support effect and Bi promoter addition.24,41Table 3 also shows that activation energies for the CH4 case are always 10–35 kJ mol−1 higher (except R3) than those for the H2 case. This is an explanation for the fact that, to achieve a similar guaiacol conversion at 300 °C for the H2 case, 400 °C is needed for the CH4 case as shown in our prior work.24 For step R3, activation energy of the H2 case is only 93 kJ mol−1, while it is 166 kJ mol−1 for the CH4 case. This is likely because H2 promotes step R3 by forming a more stable by-product, i.e. CO than C2H6, the by-product from the CH4 case.
With the kinetics fully known, it is now possible to see how well they fit the entire data set for all temperature, flow rate and pressure conditions investigated in this work (total of 140 data points for species concentrations). The entire data set is shown in the parity plot in Fig. 6, where the RMS error = 5.7%, indicating a good fit.
Concluding remarks
In the present work, kinetics of guaiacol deoxygenation using methane as a reductant over the Pt–Bi/AC catalyst is studied by both experimental and modeling approaches. It is shown that the liquid phase products remain the same as for the case using hydrogen as a reductant.26 The activation energies and other kinetic parameters are obtained based on data fit. For all the temperature, flow rate and pressure conditions investigated in this work, the model predictions match the experimental data well. Modeling results are discussed, analyzed and compared with literature reports. The present work provides a new practical approach for bio-oil upgrading using methane as a reductant instead of hydrogen.Nomenclature
| A i [mol s−1 gcat−1] | Pre-exponential factor of step i |
| D% | Metal dispersion |
| E ai [kJ mol−1] | Activation energy of step i |
| F 0 [mol h−1] | Feed flow rate |
| F i [mol h−1] | Flow rate of species i |
| k i [mol gcat−1 s−1 atm−n] | Reaction rate constant of step i when the reaction order is n |
| M Pt [kg kmol−1] | Molecular weight of Pt |
| N i [mol] | Amount of species i |
| n i [mol] | Reaction order of step i |
| P i [atm] | Partial pressure of species i |
| R [J K−1 mol−1] | Gas constant |
| r i [mol s−1 gcat−1] | Reaction rate of step i |
| t [s] | Contact time |
| TOFi [s−1] | Turnover frequency of step i |
| W [g] | Catalyst packing amount |
| w i [—] | Weighting function for species i |
| wt% | Metal loading percentage |
Acknowledgements
This work was supported by the R. Games Slayter Fund. The authors thank Ms. Marina Lourencone for her valuable assistance.References
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Valorization of Biomass: Deriving More Value from Waste, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- P. S. Nigam and A. Singh, Production of liquid biofuels from renewable resources, Prog. Energy Combust. Sci., 2011, 37, 52–68 CrossRef CAS.
- D. C. Elliott and T. R. Hart, Catalytic Hydroprocessing of Chemical Models for Bio-oil, Energy Fuels, 2009, 23, 631–637 CrossRef CAS.
- P. Mortensen, J.-D. Grunwaldt, P. Jensen, K. Knudsen and A. Jensen, A Review of Catalytic Upgrading of Bio-oil to Engine Fuels, Appl. Catal., A, 2011, 407, 1–19 CrossRef CAS.
- S. Xiu and A. Shahbazi, Bio-oil Production and Upgrading Research: A Review, Renewable Sustainable Energy Rev., 2012, 16, 4406–4414 CrossRef CAS.
- D. C. Elliott, H. Wang, M. Rover, L. Whitmer, R. Smith and R. Brown, Hydrocarbon Liquid Production via Catalytic Hydroprocessing of Phenolic Oils Fractionated from Fast Pyrolysis of Red Oak and Corn Stover, ACS Sustainable Chem. Eng., 2015, 3, 892–902 CrossRef CAS.
- B. Guvenatam, O. Kursun, E. H. Heeres, E. A. Pidko and E. J. Hensen, Hydrodeoxygenation of Mono- and Dimeric lignin Model Compounds on Noble Metal Catalysts, Catal. Today, 2014, 233, 83–91 CrossRef.
- A. Bridgwater, Review of Fast Pyrolysis of Biomass and Product Upgrading, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS.
- J. Wildschut, F. H. Mahfud, R. H. Venderbosch and H. J. Heeres, Hydrotreatment of Fast Pyrolysis Oil Using Heterogeneous Noble-metal Catalysts, Ind. Eng. Chem. Res., 2009, 48, 10324–10334 CrossRef CAS.
- W. Wang, Y. Yang, H. Luo, H. Peng and F. Wang, Effect of La on Ni–W–B Amorphous Catalysts in Hydrodeoxygenation of Phenol, Ind. Eng. Chem. Res., 2011, 50, 10936–10942 CrossRef CAS.
- A. Gutierrez, R. Kaila, M. Honkela, R. Slioor and A. Krause, Hydrodeoxygenation of Guaiacol on Noble Metal Catalysts, Catal. Today, 2009, 147, 239–246 CrossRef CAS.
- M. Saidi, F. Samimi, D. Karimipourfard, T. Nimmanwudipong, B. C. Gates and M. R. Rahimpour, Upgrading of Lignin-derived Bio-oils by Catalytic Hydrodeoxygenation, Energy Environ. Sci., 2014, 7, 103–129 CAS.
- S.-K. Wu, P.-C. Lai, Y.-C. Lin, H.-P. Wan, H.-T. Lee and Y.-H. Chang, Atmospheric Hydrodeoxygenation of Guaiacol over Alumina-, Zirconia-, and Silica-supported Nickel Phosphide Catalysts, ACS Sustainable Chem. Eng., 2013, 1, 349–358 CrossRef CAS.
- H. Zhao, D. Li, P. Bui and S. Oyama, Hydrodeoxygenation of Guaiacol as Model Compound for Pyrolysis Oil on Transition Metal Phosphide Hydroprocessing Catalysts, Appl. Catal., A, 2011, 391, 305–310 CrossRef CAS.
- V. N. Bui, D. Laurenti, P. Afanasiev and C. Geantet, Hydrodeoxygenation of Guaiacol with CoMo Catalysts. Part I: Promoting Effect of Cobalt on HDO Selectivity and Activity, Appl. Catal., B, 2011, 101, 239–245 CrossRef CAS.
- X. Zhang, T. Wang, L. Ma, Q. Zhang, Y. Yu and Q. Liu, Characterization and Catalytic Properties of Ni and NiCu Catalysts Supported on ZrO2–SiO2 for Guaiacol Hydrodeoxygenation, Catal. Commun., 2013, 33, 15–19 CrossRef CAS.
- T. Mochizuki, S.-Y. Chen, M. Toba and Y. Yoshimura, Deoxygenation of Guaiacol and Woody Tar over Reduced Catalysts, Appl. Catal., B, 2014, 146, 237–243 CrossRef CAS.
- R. Olcese, M. Bettahar, D. Petitjean, B. Malaman, F. Giovanella and A. Dufour, Gas-phase Hydrodeoxygenation of Guaiacol Over Fe/SiO2 Catalyst, Appl. Catal., B, 2012, 115-116, 63–73 CrossRef CAS.
- M. Badawi, J.-F. Paul, S. Cristol and E. Payen, Guaiacol Derivatives and Inhibiting Species Adsorption over MoS2 and CoMoS Catalysts under HDO Conditions: A DFT study, Catal. Commun., 2011, 12, 901–905 CrossRef CAS.
- K. Lee, G. H. Gu, C. A. Mullen, A. A. Boateng and D. G. Vlachos, Guaiacol Hydrodeoxygenation Mechanism on Pt(111): Insights from Density Functional Theory and Linear Free Energy Relations, ChemSusChem, 2015, 8, 315–322 CrossRef CAS PubMed.
- E. Kikuchi, M. Ogura, I. Terasaki and Y. Goto, Selective Reduction of Nitric Oxide with Methane on Gallium and Indium Containing H-ZSM-5 Catalysts: Formation of Active Sites by Solid-State Ion Exchange, J. Catal., 1996, 161, 465–470 CrossRef CAS.
- S. Burns, J. Hargreaves and S. Hunter, On the Enhancing Effect of Ce in Pd-MOR Catalysts for NOx CH4-SCR: A Structure-reactivity Study, Appl. Catal., B, 2016, 195, 121–131 CrossRef.
- H. Pan, Y. Jian, Y. Yu, N. Chen, C. He and C. He, Promotional Mechanism of Propane on Selective Catalytic Reduction of NOx by Methane over In/H-BEA at Low Temperature, Appl. Surf. Sci., 2016, 390, 608–616 CrossRef CAS.
- Y. Xiao and A. Varma, Catalytic Deoxygenation of Guaiacol Using Methane, ACS Sustainable Chem. Eng., 2015, 3, 2606–2610 CrossRef CAS.
- D. Gao, C. Schweitzer, H. T. Hwang and A. Varma, Conversion of Guaiacol on Noble Metal Catalysts: Reaction Performance and Deactivation Studies, Ind. Eng. Chem. Res., 2014, 53, 18658–18667 CrossRef CAS.
- D. Gao, Y. Xiao and A. Varma, Guaiacol Hydrodeoxygenation over Platinum Catalyst: Reaction Pathways and Kinetics, Ind. Eng. Chem. Res., 2015, 54, 10638–10644 CrossRef CAS.
- W. Hu, D. Knight, B. Lowry and A. Varma, Selective Oxidation of Glycerol to Dihydroxyacetone over Pt-Bi/C Catalyst: Optimization of Catalyst and Reaction Conditions, Ind. Eng. Chem. Res., 2010, 49, 10876–10882 CrossRef CAS.
- W. Hu, B. Lowry and A. Varma, Kinetic Study of Glycerol Oxidation Network over Pt-Bi/C Catalyst, Appl. Catal., B, 2011, 106, 123–132 CAS.
- J. Chang, T. Danuthai, S. Dewiyanti, C. Wang and A. Borgna, Hydrodeoxygenation of Guaiacol over Carbon-supported Metal Catalysts, ChemCatChem, 2013, 5, 3041–3049 CrossRef CAS.
- A. L. Jongerius, P. C. A. Bruijnincx and B. M. Weckhuysen, Liquid-phase Reforming and Hydrodeoxygenation as a Two-step Route to Aromatics from Lignin, Green Chem., 2013, 15, 3049–3056 RSC.
- C. Perego and S. Peratello, Experimental Methods in Catalytic Kinetics, Catal. Today, 1999, 52, 133–145 CrossRef CAS.
- P. Weisz and C. Prater, Interpretation of Measurements in Experimental Catalysis, Adv. Catal., 1954, 6, 143–196 CAS.
- D. E. Mears, Diagnostic Criteria for Heat Transport Limitations in Fixed Bed Reactors, J. Catal., 1971, 20, 127–131 CrossRef CAS.
- R. C. Runnebaum, T. Nimmanwudipong, D. E. Block and B. C. Gates, Catalytic Conversion of Compounds Representative of Lignin-derived Bio-oils: a Reaction Network for Guaiacol, Anisole, 4-Methylanisole, and Cyclohexanone Conversion Catalysed by Pt/γ-Al2O3, Catal. Sci. Technol., 2012, 113–118 CAS.
- A. C. Norris, Computational Chemistry: An Introduction to Numerical Methods, Wiley, New York, 1981 Search PubMed.
- H. S. Fogler, Elements of Chemical Reaction Engineering, 4th edn, Prentice Hall International, Inc, New Jersey, 2005 Search PubMed.
- G. E. P. Box, J. S. Hunter and W. G. Hunter, Statistics for Experimenters: Design, Innovation, and Discovery, 2nd edn, Wiley, New Jersey, 2005 Search PubMed.
- R. de Levie, When, Why, and How to Use Weighted Least Squares, J. Chem. Educ., 1986, 63, 10 CrossRef CAS.
- E. Laurent and B. Delmon, Study of the Hydrodeoxygenation of Carbonyl, Carboxylic and Guaiacyl Groups over Sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 catalyst, Appl. Catal., A, 1994, 109, 97–115 CrossRef CAS.
- M. V. Bykova, S. G. Zavarukhin, L. I. Trusov and V. A. Yakovlev, Guaiacol Hydrodeoxygenation Kinetics with Catalyst Deactivation Taken into Consideration, Kinet. Catal., 2013, 54, 40–48 CrossRef CAS.
- A. K. Deepa and P. L. Dhepe, Function of Metals and Supports on the Hydrodeoxygenation of Phenolic Compounds, ChemPlusChem, 2014, 79, 1573–1583 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |