Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular simulations of MOF membranes for separation of ethane/ethene and ethane/methane mixtures†
Cigdem Altintas and
Seda Keskin *
*
Department of Chemical and Biological Engineering, Koc University, Rumelifeneri Yolu, Sariyer, 34450, Istanbul, Turkey. E-mail: skeskin@ku.edu.tr
First published on 10th November 2017
Abstract
Metal organic framework (MOF) membranes have been widely investigated for gas separation applications. Several MOFs have been recently examined for selective separation of C2H6. Considering the large number of available MOFs, it is not possible to fabricate and test the C2H6 separation performance of every single MOF membrane using purely experimental methods. In this study, we used molecular simulations to assess the membrane-based C2H6/C2H4 and C2H6/CH4 separation performances of 175 different MOF structures. This is the largest number of MOF membranes studied to date for C2H6 separation. We computed adsorption selectivity, diffusion selectivity, membrane selectivity and gas permeability of MOFs for C2H6/C2H4 and C2H6/CH4 mixtures. Our results show that a significant number of MOF membranes are C2H6 selective for C2H6/C2H4 separation in contrast to traditional nanoporous materials. Selectivity and permeability of MOF membranes were compared with other membrane materials, such as polymers, zeolites, and carbon molecular sieves. Several MOFs were identified to exceed the upper bound established for polymeric membranes and many MOF membranes exhibited higher gas permeabilities than zeolites and carbon molecular sieves. Examining the structure–performance relations of MOF membranes revealed that MOFs with cavity diameters between 6 and 9 Å, porosities lower than 0.50, and surface areas between 500–1000 m2 g−1 have high C2H6 selectivities. The results of this study will be useful to guide the experiments to the most promising MOF membranes for efficient separation of C2H6 and to accelerate the development of new MOFs with high C2H6 selectivities.
1. Introduction
The separation of different members of the C2 hydrocarbon family has industrial importance because these molecules are primary feedstocks for various chemicals.1 Ethane/ethene (C2H6/C2H4) separation is generally carried out using distillation. This is one of the most energy intensive single distillations practiced commercially. Separation of C2H6 from methane (CH4) similarly requires energy intensive distillation operations. The energy and equipment costs associated with these gas separations could be significantly reduced by the development of alternative separation methods.2 Adsorption-based and membrane-based gas separations provide very large reductions in the energy consumption and costs of these processes. The greatest limitation in the applications of adsorption and membrane-based gas separation technologies is the low selectivity of the materials used as adsorbents and membranes. Research on identification of new materials that can achieve C2H6 separations with high selectivity has gained significant attention in the last decade.Metal organic frameworks (MOFs) are considered as a new class of nanoporous materials that can be used as adsorbents and membranes in various gas separations. MOFs are composed of metal ions connected with organic ligands.3 They have well-defined pore structures,4 large surface areas (500–6500 m2 g−1),5 high porosities, good thermal and mechanical stabilities which make them strong candidates for gas separation applications.6,7 MOFs have been widely studied for adsorption of CO2, CH4, and H2 in addition to the separation of several gas mixtures including CO2/CH4, CH4/H2, CO2/N2, CO2/H2 and noble gases.8–12 Most of the studies related to the gas separation with MOFs in the literature focused on the CO2 capture whereas research on C2H6 separation using MOFs has recently started. Several experimental studies measured single-component adsorption isotherms of CH4, C2H4, and C2H6 in various MOFs and these initial studies showed that MOFs can be promising materials for C2H6 separations.13–23
Considering the large number of available MOFs, it is not practical to identify the most promising adsorbent materials using purely experimental manners. Most of the works used molecular simulations to study MOFs for adsorption-based C2H6 separations. Guo et al. used molecular simulations to study C2H6/CH4 separation in IRMOF-1 (isoreticular metal organic framework) and four different zeolitic imidazolate frameworks, ZIFs (ZIF-8, ZIF-71, ZIF-80, ZIF-90).24 They showed that ZIFs exhibit better C2H6/CH4 separation performance compared to MOR zeolite and IRMOF-1. Wu et al. studied four different ZIFs using molecular simulations and reported their C2H6/C2H4 selectivities.25 Pillai et al. carried out molecular simulations to explore C2H6/C2H4 separation in interpenetrated and non-interpenetrated IRMOF-8 and reported C2H6 selectivities.26 We recently performed the first large-scale molecular simulation study for C2H6/C2H4 and C2H6/CH4 separations using MOFs and reported adsorption selectivity of 278 different MOFs.27 Our results showed that there is a large number of MOFs that exhibit significantly higher adsorption selectivity than zeolites for separation of C2H6/C2H4 and C2H6/CH4 mixtures. All these studies suggest that MOFs have strong potential to be used in adsorption-based C2H6/C2H4 and C2H6/CH4 separations.
Membrane-based C2H6 separation is an alternative to the adsorption-based separation. Conventional polymeric membranes cannot achieve the desired performance, combination of high selectivity and high permeability, required for C2H6 separation.28–31 Polymers with high selectivity exhibit low permeability and polymers that have high gas permeability suffer from low selectivity. Due to this trade-off, recent studies have been directed towards developing more advanced membrane materials for C2H6 separations. Identification of MOF membranes that can achieve both high selectivity and high permeability will be very useful to replace polymeric membranes with MOFs. However, we have very limited information about the membrane-based C2H6 separation potential of MOFs. The number of studies focusing on C2H6/C2H4 and C2H6/CH4 separations with MOF membranes is scarce in the literature. Only two different types of MOFs were used as membranes for C2H6/C2H4 separation. Bux et al. predicted membrane selectivity of ZIF-8 as the product of adsorption and diffusion selectivities for an equimolar C2H6/C2H4 mixture.15 They reported that C2H6 adsorbs stronger than C2H4 but C2H4 diffuses faster and overcompensates the adsorption preference for C2H6, resulting in a MOF membrane that is weakly selective for C2H4. Pan and Lai reported single-component permeances of CH4, C2H4 and C2H6 through ZIF-8 membranes.32 Caro's group reported single-component permeances of CH4 and C2H6 for ZIF-90 membranes.33 MOFs were recently used as filler particles in polymers to fabricate mixed matrix membranes (MMMs) in order to improve C2H6 and C2H4 permeabilities of polymers.34–37
Predicting separation performances of MOF membranes requires diffusion coefficients of C2H6/C2H4 and C2H6/CH4 mixtures through the pores of MOFs. Stallmach et al. reported intra-crystalline self-diffusion of CH4 and C2H6 in MOF-5 (also known as IRMOF-1) using pulsed field gradient (PFG) NMR technique.38 Ford et al. reported self-diffusivity of CH4 and C2H6 in MOF-5 using experiments and molecular simulations.39 Chmelik et al. studied diffusion of C2H6/C2H4 mixtures in ZIF-8 using different NMR techniques and showed that C2H4 diffusion is 5 times faster than C2H6 diffusion.40 Molecular simulations were used to compute self-diffusivity of CH4 in MOF-5.9,41,42 Borah et al. recently conducted molecular dynamics simulations to predict diffusion behavior of pure CH4 and C2H6 in 6 different MOFs.43 Self-diffusivities of C2H6,44 C2H4,45 and transport diffusivities of CH4, C2H4, and C2H6 (ref. 46) in ZIF-8 were computed by molecular dynamics simulations. All these simulations were generally carried out for single-component gases since calculating diffusivities of gas mixtures is computationally demanding. We recently reported diffusion coefficients of C2H6/C2H4 and C2H6/CH4 binary mixtures in 5 different MOFs and using this diffusion data we predicted membrane selectivities and gas permeabilities of the 5 MOFs for C2H6 separations.27 Our results on a small number of MOFs demonstrated that MOFs are promising membranes for preferential separation of C2H6 from C2H4 due to their higher selectivities and higher gas permeabilities compared to zeolites and polymers.
The literature we summarized above shows that current studies on MOF membranes for C2H6 separations examine only a few types of structures. There is currently no large-scale computational study to identify the separation performances of different MOFs for C2H6/C2H4 and C2H6/CH4 mixtures. Considering the large variety and number of available MOFs, there may be many existing MOFs with better separation performances, high membrane selectivities and high gas permeabilities. In order to identify membrane-based C2H6 separation performances of a large number of MOFs, we used molecular simulations and computed adsorption equilibria and self-diffusivities of C2H6/C2H4 and C2H6/CH4 mixtures in 175 different MOFs. Using this data, we predicted adsorption selectivity, diffusion selectivity, membrane selectivity and gas permeability of 175 MOFs both for C2H6/C2H4 and C2H6/CH4 separations. Results were compared with well-known membrane materials, polymers, zeolites and carbon molecular sieves to evaluate the potential of MOFs. Relations between adsorption selectivity, diffusion selectivity and membrane selectivity of MOFs were discussed to understand the individual effects of adsorption and diffusion on the membranes' performances. We finally examined the relations between easily computable structural properties such as pore size, surface area and porosity of MOFs and their selectivities to provide structure–performance relationships that can serve as a map for experimental synthesis of new MOFs with better gas separation performances.
2. Computational details
2.1. MOFs
We used the same MOF database that we considered in our previous work.27 This database was originally prepared using the solvent-free MOF database constructed by Chung and coworkers47 and adding some well-known MOFs taken from our previous studies48 in order to cover widely studied subfamilies such as ZIFs, covalent organic frameworks (COFs), and bio-MOFs. This database does not have any MOF with open metal sites (OMS) in order to eliminate the necessity of performing detailed quantum-level calculations to accurately define the specific interactions between C2H4 and OMS of MOFs as discussed in detail before.27,49 We then refined our database to only include MOFs that have pore sizes (largest cavity diameters) larger than the kinetic diameters of the C2H6, C2H4 and CH4 molecules so that all these gases can enter into the MOFs' pores and diffuse. After this elimination, we ended up with 175 different MOF structures. All crystal structures of MOFs were taken from the Cambridge Crystallographic Data Centre (CCDC).50 The complete list of MOFs including the CCDC names and common names is given in Table S1 of ESI.† Structural properties of materials such as pore limiting diameter (PLD), largest cavity diameter (LCD), pore volume, porosity, and surface area were computed using Poreblazer algorithm51 in which the Dreiding force field52 was utilized. In this algorithm, He and N2 atoms were used as probe molecules for pore size and surface area calculations, respectively. The sigma parameters for He and N2 were used in their default values in the Poreblazer algorithm as 2.58 Å and 3.31 Å, respectively. The cut-off distance and cubelet size were used as 12.8 Å and 0.2 Å, respectively. The largest anticipated pore diameter was increased to 20 Å and the size of the bin was decreased to 0.25 Å in that algorithm.2.2. Molecular simulations
Grand Canonical Monte Carlo (GCMC)53 simulations were used to compute binary adsorption isotherms of C2H6/C2H4 and C2H6/CH4 mixtures in MOFs. In a GCMC simulation, adsorbed amounts of each gas component were calculated by specifying the bulk pressure, temperature and composition of the bulk gas mixture. The Dreiding force field52 was used for the MOFs. In cases where the potential parameters of atoms were not available from the Dreiding force field, these parameters were taken from the Universal Force Field (UFF).54 These force fields were selected based on the results of our initial simulation studies that give a good agreement with the available experimental uptake data of C2H6, C2H4 and CH4 in various MOFs as reported in our previous work.27 Single-site spherical Lennard-Jones (LJ) 12–6 potential was used to model CH4 (ref. 55) whereas two-site spherical LJ potentials were used for C2H6 and C2H4 molecules following the literature (given Table S2†).25 C2H6 and C2H4 molecules were described as uncharged united-atom models with one pseudo-atom representing –CH3 group and –CH2 group that was located at the position of carbon atom similar to the TraPPE united atom force field.55 Since the adsorbate molecules did not contain any dipole, the long-range electrostatic contribution was omitted following the previous studies in the literature.25 The cut-off distance for truncation of the intermolecular interactions was set to 12 Å for GCMC simulations. A simulation box of 2 × 2 × 2 crystallographic unit cells was used. Periodic boundary conditions were applied in all simulations. During the simulations, 1.5 × 107 steps were performed to guarantee the equilibration and 1.5 × 107 steps were performed to sample the desired properties.Computing membrane properties of MOFs requires diffusivities of gas molecules in the pores of materials. In order to obtain self-diffusivities of C2H6/C2H4 and C2H6/CH4 mixtures in MOFs, we performed Equilibrium Molecular Dynamics (EMD) simulations. The initial states of EMD simulations with the appropriate gas loadings were obtained from the GCMC simulations and each system was equilibrated for 20 ps prior to taking data. The Nosé–Hoover thermostat was applied to run EMD simulations at NVT (constant number of molecules, volume and temperature) ensemble.53 At least 10 independent EMD simulations with a length of 16 ns were performed to compute self-diffusivities of gases at the given loadings. The estimated uncertainties of the self-diffusivities were at least one order of magnitude smaller than the reported diffusion coefficients. More details of using GCMC and EMD simulations to obtain adsorption data and diffusion coefficients in various MOFs can be found in our previous studies.56,57
Molecular simulations should be performed for multiple materials on time scales shorter than the same materials can be assessed experimentally. Since we considered a large number of MOF membranes in this work, we used rigid framework assumption. Almost all molecular simulations for MOF membranes in the literature used this assumption because it saves a significant amount of computational time. Recent studies showed that including lattice flexibility does not make any significant change in the gas adsorption results of MOFs that have pore sizes larger than the guest molecules.58–60 Chmelik et al. could not find any evidence for gate-opening effect or another structural transitions of ZIF-8 upon adsorption of C2H6/C2H4 mixture.40 On the other hand, lattice flexibility can be important for the diffusion of large gas molecules in the MOFs having narrow windows.44,46 We recently carried out flexible EMD simulations to examine the effect of MOF's flexibility on the predicted membrane performance.61 Considering flexibility of the framework made a negligible effect on the gas permeability and selectivity of the MOFs having large pores whereas more pronounced changes were seen in gas permeabilities of the materials having narrow pores. Another recent study on Xe separations showed that flexibility should be considered in shape selective screening studies for the highest degree of accuracy and to achieve the best ranking of high-performance materials.62 Since MOFs considered in this work were specifically chosen to have larger pore diameters than the kinetic diameters of the three gas molecules we studied, flexibility effects were expected to be small and they were not taken into account for computational efficiency. The idea of our calculations is that once the potential value of a membrane material has been demonstrated by molecular simulations, further detailed studies such as flexible simulations can be performed to increase the precision of initial assessment.
2.3. Calculation of membrane properties
Adsorption selectivities (Sads) of MOFs for C2H6/C2H4 and C2H6/CH4 separations were calculated using the results of mixture GCMC simulations as we previously reported.27 Sads is defined as the ratio of compositions of the adsorbed gases (x) in the adsorbent material normalized by the ratio of bulk phase compositions (y) of component i to component j:| Sads(i/j) = (xi/xj)/(yi/yj) | (1) |
Adsorption selectivities of MOFs were computed at 10 bar, 298 K for equimolar C2H6/C2H4 and C2H6/CH4 mixtures. The ratio of self-diffusivities of gases obtained from the EMD simulations was used to define the diffusion selectivity of MOFs. Diffusion selectivity, (Sdiff) was calculated as the ratio of the self-diffusivities (Di,self) of each gas component in the binary mixture where ci represents the corresponding adsorbed loading of gas species i calculated from the GCMC simulations at 10 bar, 298 K:
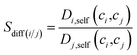 | (2) |
Once the adsorption and diffusion selectivities of MOFs were computed for a given gas mixture, membrane selectivity (Smem), also known as permeation selectivity, was calculated as the multiplication of adsorption selectivity and diffusion selectivity at a membrane feed pressure of 10 bar as described in the literature.56 The validity of this model was shown by comparing its predictions with the experimentally measured selectivity and permeability data of MOF membranes for various gas separations in previous studies.56
| Smem(i/j) = Sads(i/j) × Sdiff(i/j) | (3) |
Not only high selectivity but also high gas permeability is required for an efficient and economic membrane process. Therefore, we also computed gas permeabilities through MOFs using the following expression,63
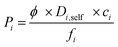 | (4) |
3. Results and discussion
3.1. Membrane properties of MOFs
We validated the accuracy of our GCMC simulations to predict the adsorption of C2H6, C2H4 and CH4 in various MOFs such as CuBTC, PCN-16, Co-MOF-74 and Mg-MOF-74 by comparing results of our molecular simulations with the available experimental data of different research groups in our previous work.27 In this work, we aimed to validate the accuracy of our EMD simulations for the diffusivity of C2H6 and CH4 in the MOFs' pores. There is limited information about the diffusivity of these gases in MOFs due to the difficulty of measurement of self-diffusivity using purely experimental techniques and high computational demands of molecular simulations. Table 1 compares C2H6 and CH4 diffusivities calculated from our molecular simulations with the available experimental and computational data taken from the literature. Our simulated data for C2H6 and CH4 diffusivities in MOFs agreed well with the previous simulation studies of different research groups. C2H6 diffusivities in MOF-5 predicted by our molecular simulations agreed well with the experimental measurements of Stallmach et al.38 and Ford et al.39 whereas simulations predicted an order of magnitude slower CH4 diffusivity in MOF-5 compared to the experiments. This discrepancy was attributed to the imperfections in the micropore structure which influenced the experimental studies but which were not taken into account in the EMD simulations.38,39 Overall, the good agreement between our simulations and reported values in the literature for diffusion of C2H6 and CH4 in MOFs suggests that simulated diffusion coefficients can be used to model gas transport through the MOF membranes.| MOF | This work | Other simulations | Ref. | Experiments | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| DCH4 (cm2 s−1) | DC2H6 (cm2 s−1) | DCH4 (cm2 s−1) | DC2H6 (cm2 s−1) | DCH4 (cm2 s−1) | DC2H6 (cm2 s−1) | ||||
| NU-125 | Single | 3.89 × 10−4 | 7.87 × 10−5 | 2–3 × 10−4 | 1–1.7 × 10−4 | 43 | |||
| Binary | 3.39 × 10−4 | 1.47 × 10−4 | 1.2 × 10−4 | 1.2 × 10−4 | 43 | ||||
| PCN-14 | Single | 2.10 × 10−4 | 7.62 × 10−5 | 1–1.5 × 10−4 | 0.3–1 × 10−4 | 43 | |||
| Binary | 1.92 × 10−4 | 8.02 × 10−5 | 1 × 10−4 | 6 × 10−5 | 43 | ||||
| COF-10 | Single | 9.88 × 10−4 | 7.7 × 10−4 | 66 | |||||
| MOF-5 | Single | 3.37 × 10−4 | 1.51 × 10−4 | 3 × 10−4 | 1.5 × 10−4 | 39 | 2 × 10−3 | 1.8 × 10−4 | 39 |
| Single | 3.1 × 10−4 | 41 | 1.7 × 10−3 | 2.1 × 10−4 | 38 | ||||
| Single | 3.08 × 10−4 | 42 | |||||||
| Single | 3.5 × 10−4 | 9 | |||||||
Combining adsorption data obtained from the GCMC simulations and diffusion data obtained from the EMD simulations, we computed selectivity and permeability of MOF membranes for C2H6/C2H4 and C2H6/CH4 mixture separations as shown in Fig. 1. In order to compare MOFs with traditional membrane materials, we collected selectivity and permeability data of zeolites, carbon molecular sieves (CMSs) and polymers for C2H6/C2H4 separations. At that point it is important to reiterate that membrane materials that preferentially select C2H6 over C2H4 are very scarce. Zeolites, CMSs and polymers are generally C2H4 selective. In order to be consistent with the literature data, we showed C2H4/C2H6 selectivity and C2H4 permeability of MOF membranes in Fig. 1(a). Traditional polymeric membranes, such as Matrimid, 4,4′-(hexafluoroisopropylidene)dipthalicanhydride-2,4,6-trimethyl-1,3-phenylene diamine (6FDA-DAM), 4,4′-(hexafluoroisopropylidene)dipthalicanhydride:3,3′,4,4′-biphenyltetracarboxylic dianhydride-2,4,6-trimethyl-1,3-phenylene diamine (6FDA:BPDA-DAM) selectively separate C2H4 from C2H6, generally due their sorption selectivities.67 The black solid line in Fig. 1(a) represents the experimental C2H4/C2H6 upper bound for polymers which was established by Rungta et al.67 based on the pure gas permeability data, similar to the Robeson's upper bound.68 Polymeric membranes are mostly located below this bound and it is highly desired to identify new membrane materials that can exceed this bound by exhibiting higher selectivity and/or higher permeability than polymers. Since MOFs are highly porous materials compared to polymers, C2H4 permeabilities of MOF membranes are significantly higher than those of polymers. According to the upper bound, the C2H4 permeabilities of polymeric membranes are in the range of 0.1–104 Barrer whereas MOF membranes we considered in this work exhibit C2H4 permeabilities in the range of 42–6.75 × 105 Barrer. Therefore, we extrapolated the Robeson's upper bound with a dashed line in Fig. 1(a) to show the high C2H4 permeabilities of MOFs. In fact, 4 MOFs, YUTYOC, IDIWOH, OWITAQ and OWITUQ were found to exceed the upper bound due to their high C2H4 permeabilities.
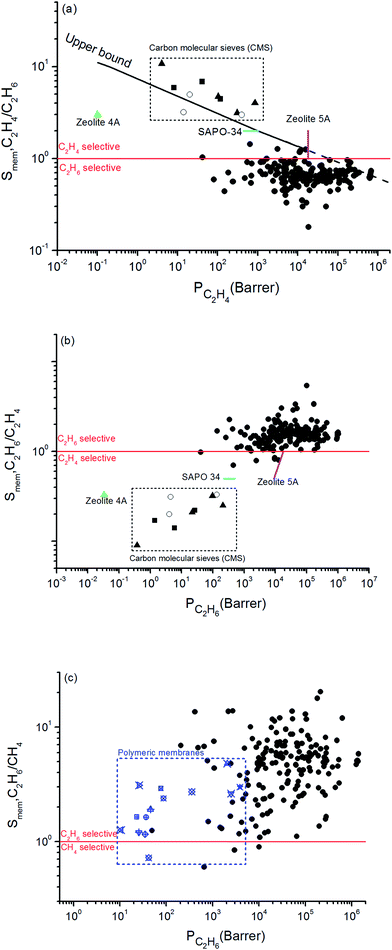 | ||
| Fig. 1 Selectivity and permeability of MOFs for (a) C2H4/C2H6, (b) C2H6/C2H4, (c) C2H6/CH4 separations. Data for CMSs shown within the box69–71 and data for zeolites shown with color symbols75–77 were taken from the literature in (a) and (b). Data for polymeric membranes shown with blue symbols in (c) were taken from the literature.34,73,74,78–81 | ||
The red solid line shows the selectivity preference of the membranes in Fig. 1(a). MOFs located above (below) this line are C2H4 (C2H6) selective. A significant number of MOF membranes was identified to show C2H6 selectivity over C2H4 and these MOFs were located below the red line. Well-known zeolites such as zeolite 4A, zeolite 5A, SAPO-34 and CMSs are C2H4 selective membranes in C2H6/C2H4 separations.67 For example, zeolite 4A membrane has C2H4 selectivity of 3 and C2H4 permeability of 0.1 Barrer whereas the MOFs we considered in this work are mostly C2H6 selective with significantly higher C2H4 permeabilities.67 The maximum C2H4 selectivity of CMS membranes was reported to be around 10 and their maximum C2H4 permeabilities were around 1000 Barrer.69–71 A recent study reported that a ZIF-8-filled 6FDA-DAM MMM exhibit C2H4 selectivity of 3.2 and permeability of 72.9 Barrer depending on the ZIF loading in the polymer.72 All these comparisons show that C2H4 permeability of MOF membranes are significantly higher than that of zeolite 4A, zeolite 5A, SAPO-34, and ZIF-8-filled MMM. Since majority of the MOFs we examined in this work are C2H6 selective, we additionally showed the C2H6 selectivity and C2H6 permeability of MOFs in Fig. 1(b). This figure shows that MOF membranes can selectively separate C2H6 from C2H4 with high selectivity. 169 out of 175 MOFs are C2H6 selective with selectivities in the range of 1.0–5.4. Among these MOFs, EYOPUE has the highest C2H6/C2H4 selectivity (5.4) and OWITIY has the highest C2H6 permeability (1.04 × 106 Barrer). Selectivity of 11 MOFs was found to be slightly larger than unity which means they do not have a strong preference for C2H6 or C2H4 and therefore they cannot be used as selective membranes for C2H6/C2H4 separations. A small number of MOF membranes (6 out of 175) was identified to show low/mediocre C2H4 selectivity over C2H6 and located below the red line.
Fig. 1(c) represents C2H6/CH4 selectivity and C2H6 permeability of MOF membranes. Although an upper bound is not established yet, several polymeric membranes were tested for C2H6/CH4 separation and we collected this data from the literature to compare MOF membranes with polymers.34,71–76 Most of the polymeric membranes exhibit C2H6 permeabilities between 10–100 Barrer and C2H6/CH4 selectivities between 0.7 and 3. Only polydimethylsiloxane (PDMS) and polympentanamer (PPM) membranes show higher C2H6 permeabilities reaching to 2070 Barrer (ref. 73) and 3900 Barrer,74 respectively. Most of the MOFs studied in this work exhibit higher C2H6 permeabilities and higher C2H6/CH4 selectivities than these polymers. Among 175 MOFs, all the MOFs except 3 of them (XENZUN, GITVAH and YARYEV) were identified to be C2H6 selective over CH4 and their C2H6 permeabilities were computed to be in the range of 49.5–1.39 × 106 Barrer. The most selective MOF for C2H6/CH4 separation was identified as NEXXIZ, with a selectivity of 20.5 and C2H6 permeability of 2.12 × 105 Barrer. OWITAQ was identified as the most permeable MOF with C2H6 permeability of 1.39 × 106 Barrer and C2H6/CH4 selectivity of 6. All these results indicate that MOFs are highly promising membrane materials for preferential separation of C2H6 from CH4.
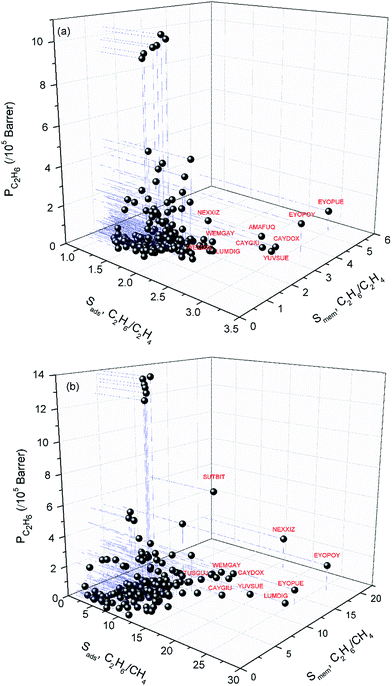
Combination of adsorption and diffusion selectivity determines the membrane selectivity of a MOF. In order to evaluate the individual effects of gas adsorption and diffusion on the membrane performance of MOFs, we examined the relation between adsorption, diffusion, and membrane selectivity in Fig. 2. All selectivities were computed for equimolar gas mixtures. The colored dots in Fig. 2(a) show the distribution of the diffusion selectivities of MOFs for C2H6/C2H4 mixture. The LJ energy parameter was higher for C2H6 (εC2H6/kB = 108 K) than C2H4 (εC2H4/kB = 92.8 K) to reflect stronger dispersion interactions. Since C2H6 is energetically preferred over C2H4, C2H6 (C2H4) is the strongly (weakly) adsorbed component in all MOFs. Therefore, adsorption selectivities favor C2H6 over C2H4 (C2H6/C2H4 selectivity > 1) for all MOFs. Diffusion selectivities favor C2H4 (C2H6/C2H4 selectivity < 1) in most of the MOFs since C2H4 molecules diffuse faster than C2H6 molecules. C2H4 molecules are lighter, smaller and weakly adsorbed into the pores of MOFs which leads to faster diffusion of C2H4 than C2H6. For 50 MOFs shown by red color, the diffusion selectivity for C2H6 over C2H4 is ranged from 0.45 to 0.83. Since the membrane selectivity was estimated as the multiplication of the adsorption and diffusion selectivities, the predicted membrane selectivities of these MOFs for C2H6 are lower than their adsorption selectivities as shown in Fig. 2(a). In other words, these MOFs are more useful in adsorption-based C2H6/C2H4 separations than in membrane-based separations in terms of C2H6 selectivity. 113 MOFs shown by green color have diffusion selectivities around unity (0.83–1.10), which means diffusion does not strongly favor one gas species over other in the mixture. Since diffusion selectivities are close to one, membrane selectivities of these MOFs are only slightly lower than their adsorption selectivities. On the other hand, 12 of the MOFs shown by blue color exhibit diffusion selectivities higher than unity (1.11–1.82). That means self-diffusion coefficient of C2H6 is slightly higher than C2H4. In this case, both adsorption and diffusion favors the same component, C2H6 over C2H4 in the mixture. As a result, membrane selectivities of these MOFs are higher than their adsorption-based selectivities. In fact, it is highly desired to find materials in which both adsorption and diffusion favor the same gas component and lead to high membrane selectivities. Table 2 summarizes the top ten C2H6 selective MOF membranes together with their adsorption, diffusion, membrane selectivities in addition to the self-diffusivities of each gas species in these materials. Both adsorption and diffusion selectivities of the MOFs listed in Table 2 are higher than 1, which means C2H6 is favored over C2H4 by both mechanisms. For example, EYOPUE has both high adsorption selectivity and high diffusion selectivity for C2H6 over C2H4. As a result it was identified as the most selective MOF membrane. Therefore, it is more useful to utilize the MOFs listed in Table 2 as membranes rather than as adsorbents for selective separation of C2H6 from C2H4.
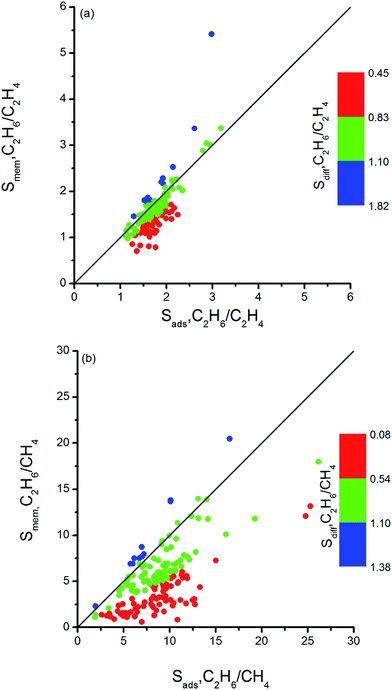 | ||
| Fig. 2 Adsorption, diffusion and membrane selectivities of MOFs for (a) C2H6/C2H4 and (b) C2H6/CH4 separations. | ||
| MOF | Sads, C2H6/C2H4 | DC2H6 (cm2 s−1) | DC2H4 (cm2 s−1) | Sdiff, C2H6/C2H4 | Smem, C2H6/C2H4 | PC2H6 (Barrer) | PC2H4 (Barrer) |
|---|---|---|---|---|---|---|---|
| EYOPUE | 2.98 | 1.45 × 10−4 | 7.97 × 10−5 | 1.82 | 5.41 | 1.01 × 105 | 1.86 × 104 |
| EYOPOY | 3.18 | 1.62 × 10−4 | 1.53 × 10−4 | 1.06 | 3.37 | 1.73 × 105 | 5.12 × 104 |
| AMAFUQ | 2.61 | 8.09 × 10−5 | 6.27 × 10−5 | 1.29 | 3.36 | 4.42 × 104 | 1.32 × 104 |
| YUVSUE | 2.87 | 1.56 × 10−5 | 1.47 × 10−5 | 1.06 | 3.04 | 1.60 × 104 | 5.25 × 103 |
| CAYDOX | 2.94 | 1.28 × 10−4 | 1.25 × 10−4 | 1.02 | 3.01 | 4.95 × 104 | 1.64 × 104 |
| CAYGIU | 2.79 | 9.65 × 10−5 | 9.34 × 10−5 | 1.03 | 2.88 | 3.71 × 104 | 1.29 × 104 |
| BUSNAF | 2.14 | 2.84 × 10−5 | 2.41 × 10−5 | 1.18 | 2.53 | 1.24 × 104 | 4.92 × 103 |
| TUSGUJ | 1.92 | 3.80 × 10−6 | 3.19 × 10−6 | 1.19 | 2.28 | 6.05 × 103 | 2.65 × 103 |
| UHAXUW | 2.22 | 6.58 × 10−7 | 6.45 × 10−7 | 1.02 | 2.26 | 3.40 × 102 | 1.50 × 102 |
| NEXXIZ | 2.18 | 1.27 × 10−4 | 1.23 × 10−4 | 1.03 | 2.25 | 1.45 × 105 | 6.44 × 104 |
It is interesting to discuss the MOFs for which the diffusion selectivity for C2H4 overcompensates the adsorption selectivity for C2H6 and makes the membrane C2H4 selective. We listed adsorption, diffusion, membrane selectivities and gas permeabilities of the C2H4 selective MOFs in Table 3. All these five MOFs are weakly selective for C2H4. For example, XENZUN was predicted to show the highest membrane selectivity. Adsorption weakly favors C2H6 over C2H4 in this MOF whereas diffusion favors C2H4 over C2H6 and dominates the adsorption selectivity. AVEROJ has a low C2H4/C2H6 adsorption selectivity but the diffusion selectivity strongly favors C2H4 over C2H6 and makes the membrane C2H4 selective. KEXFAU has the highest adsorption selectivity for C2H4/C2H6 separation as can be seen from Table 3, but its diffusion selectivity is close to unity making the membrane weakly selective for C2H4. These examples signify the importance of diffusion selectivity in governing membrane's separation performance. If the adsorption selectivity does not strongly favor one component in the mixture, then diffusion selectivity determines the membrane's gas separation performance.
| MOF | Sads, C2H4/C2H6 | DC2H4 (cm2 s−1) | DC2H6 (cm2 s−1) | Sdiff, C2H4/C2H6 | Smem, C2H4/C2H6 | PC2H4 (Barrer) | PC2H6 (Barrer) |
|---|---|---|---|---|---|---|---|
| XENZUN | 0.74 | 7.62 × 10−7 | 3.94 × 10−7 | 1.93 | 1.43 | 6.47 × 102 | 4.53 × 102 |
| AVEROJ | 0.57 | 5.47 × 10−6 | 2.44 × 10−6 | 2.24 | 1.27 | 3.12 × 103 | 2.45 × 103 |
| TUDJOS | 0.62 | 2.22 × 10−5 | 1.10 × 10−5 | 2.01 | 1.25 | 1.59 × 104 | 1.28 × 104 |
| YARYEV | 0.70 | 2.19 × 10−5 | 1.26 × 10−5 | 1.74 | 1.21 | 1.17 × 104 | 9.65 × 103 |
| KEXFAU | 0.80 | 2.15 × 10−5 | 1.46 × 10−5 | 1.47 | 1.17 | 1.12 × 104 | 9.60 × 103 |
Similar selectivity analysis was done for C2H6/CH4 separation in Fig. 2(b). Adsorption strongly favors C2H6 and strongly adsorbed C2H6 molecules move slower than weakly adsorbed, lighter CH4 molecules. As a result, diffusion selectivity favors CH4 over C2H6 and becomes less than 1 for almost all MOFs as shown by red and green points in Fig. 2(b). Since adsorption strongly favors C2H6 and diffusion weakly favors CH4, membrane-based C2H6 selectivities of MOFs are less than their adsorption-based selectivities. There are 11 MOFs shown by blue color in Fig. 2(b) in which diffusivity of C2H6 is slightly higher than the diffusivity of CH4. Our EMD simulations showed that gas molecules generally diffuse only in one direction in these MOFs and the high number of slowly diffusing C2H6 molecules hinders the fast diffusion of CH4 molecules in the pores. As a result, diffusion selectivities are around 1.1–1.3 and these MOFs are promising membrane materials since both adsorption and diffusion favors the same component C2H6 over CH4. Performances of the top ten promising MOF membranes for selective separation of C2H6 from CH4 were summarized in Table 4. For example, adsorption and diffusion favor C2H6 over CH4 in NEXXIZ, TIRQOB, ZUQPOQ, UHAXUW whereas high adsorption selectivity towards C2H6 dominates the diffusion selectivity towards CH4 in other MOFs as shown in Table 4.
| MOF | Sads, C2H6/CH4 | DC2H6 (cm2 s−1) | DCH4 (cm2 s−1) | Sdiff, C2H6/CH4 | Smem, C2H6/CH4 | PC2H6 (Barrer) | PCH4 (Barrer) |
|---|---|---|---|---|---|---|---|
| NEXXIZ | 16.49 | 1.41 × 10−4 | 1.14 × 10−4 | 1.24 | 20.46 | 2.12 × 105 | 1.04 × 104 |
| EYOPOY | 26.16 | 1.36 × 10−4 | 1.98 × 10−4 | 0.69 | 17.97 | 1.81 × 105 | 1.01 × 104 |
| TIRQOB | 13.07 | 1.81 × 10−6 | 1.70 × 10−6 | 1.07 | 13.98 | 2.72 × 103 | 1.95 × 102 |
| CAYDOX | 14.01 | 1.10 × 10−4 | 1.11 × 10−4 | 0.99 | 13.88 | 5.20 × 104 | 3.74 × 103 |
| ZUQPOQ | 10.10 | 3.71 × 10−6 | 2.72 × 10−6 | 1.37 | 13.81 | 2.18 × 103 | 1.58 × 102 |
| UHAXUW | 10.08 | 6.34 × 10−7 | 4.68 × 10−7 | 1.35 | 13.65 | 4.15 × 102 | 3.04 × 101 |
| EYOPUE | 25.29 | 1.14 × 10−4 | 2.20 × 10−4 | 0.52 | 13.10 | 1.01 × 105 | 7.68 × 103 |
| LUMDIG | 24.77 | 2.01 × 10−5 | 4.12 × 10−5 | 0.49 | 12.08 | 2.81 × 104 | 2.33 × 103 |
| SUTBIT | 12.32 | 2.13 × 10−4 | 2.17 × 10−4 | 0.98 | 12.07 | 6.31 × 105 | 5.23 × 104 |
| CAYGIU | 13.09 | 1.12 × 10−4 | 1.23 × 10−4 | 0.91 | 11.86 | 5.31 × 104 | 4.48 × 103 |
As we discussed above, some MOFs are promising for adsorption-based gas separations whereas some others are good candidates for membrane-based gas separations. We aimed to identify the MOFs that can be used both as effective adsorbents and membranes for the preferential separation of C2H6 from C2H4 and CH4. Selectivity is generally considered as the most critical factor to assess equilibrium and kinetic-based separation performances of materials. High adsorption selectivity is desired for adsorbents whereas both high selectivity and permeability are required for membranes. Therefore, we considered these three performance metrics, adsorption selectivity, membrane selectivity and permeability of the desired gas in order to identify the most promising MOFs that can be used both as adsorbents and membranes. For C2H6/C2H4 separation, adsorption and membrane selectivities of MOFs were computed to be 1.1–3.2 and 0.7–5.4, respectively and C2H6 permeabilities of MOFs were predicted to be 41–1.04 × 106 Barrer. For C2H6/CH4 separation, adsorption and membrane selectivities were calculated to be 1.9–26.2 and 0.60–20.5, respectively with C2H6 permeabilities of 49.5–1.39 × 106 Barrer. We set the minimum adsorption and membrane selectivity to 2 and showed the top ten MOFs with the highest C2H6 permeabilities for C2H6/C2H4 separation in Fig. 3(a). Similarly, for C2H6/CH4 separation, we considered the MOFs that have adsorption and membrane selectivities larger than 10. After these two constraints we identified the MOFs with the highest C2H6 permeabilities in Fig. 3(b). Results show that MOFs named as EYOPOY, NEXXIZ, EYOPUE, CAYDOX, WEMGAY, CAYGIU, LUMDIG and YUVSUE are common in the top ten promising material list of C2H6/C2H4 and C2H6/CH4 separations. For example, EYOPOY has high adsorption-based selectivity both for C2H6/C2H4 (3.2) and C2H6/CH4 (26.2) in addition to high membrane-based selectivity both for C2H6/C2H4 (3.4) and C2H6/CH4 (18). This result suggests that these 8 MOFs can be used as effective adsorbents and membranes for the selective separation of C2H6 from C2H4 and CH4.
3.2. Structure–performance relations for MOFs
We so far focused on the gas separation performance of MOFs as adsorbents and as membranes. Establishing relation between structures and separation performances of MOFs would be very useful to save computational time and to guide the experimental studies for the synthesis of materials with the desired topology. However, clear identification of this type of relations is challenging because separation performance of a material is determined by the interplay of various factors such as chemical topology, porosity, pore size and shape and it cannot be simply correlated to only a single or two structural properties.82 In order to simply structure–performance analysis, we examined the relation between selectivity and easily computable structural properties of MOFs such as pore size, porosity, and surface area. Fig. 4 shows that there is a correlation between adsorption selectivity and LCD as well as porosity of MOFs. MOFs with LCDs around 4.5–6 Å generally exhibit higher C2H6/C2H4 and C2H6/CH4 selectivities (>2 and >10, respectively) than MOFs with larger pore sizes. As the LCD increases, selectivity generally decreases. MOFs that have large LCDs (>6 Å) have lower C2H6/C2H4 and C2H6/CH4 selectivities (<2 and <10, respectively) since both gas molecules can easily pass through the pores. Fig. 4 also shows that increasing porosity decreases the adsorption selectivity and this is the common outcome for both gas separations. The porosity of MOFs we considered in this work ranges from 0.22 to 0.83. Although the color labeling is not distinct in Fig. 4, it is clear that MOFs with porosity lower than 0.50 exhibit higher selectivity.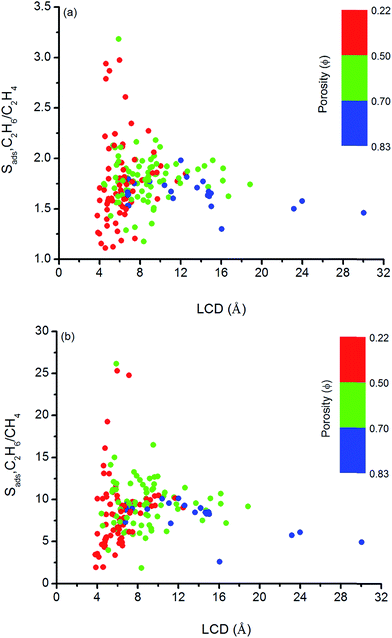 | ||
| Fig. 4 Relation between adsorption selectivities and LCDs of MOFs as a function of porosities for (a) C2H6/C2H4 and (b) C2H6/CH4 separations. | ||
Similarly, Fig. 5 shows that MOFs having lower surface areas exhibit higher adsorption selectivity. For example, MOFs with surface areas in the range of 500–1000 m2 g−1 and 750–1000 m2 g−1 tend to show adsorption selectivities higher than 2 and 10 for C2H6/C2H4 and C2H6/CH4 separations, respectively. Overall, results of our structure–performance analysis suggest that MOFs with LCDs around 4.5–6 Å, porosities less than 0.50, and surface areas in the range of 500–1000 m2 g−1 can be potentially promising adsorbents for efficient C2H6 separations.
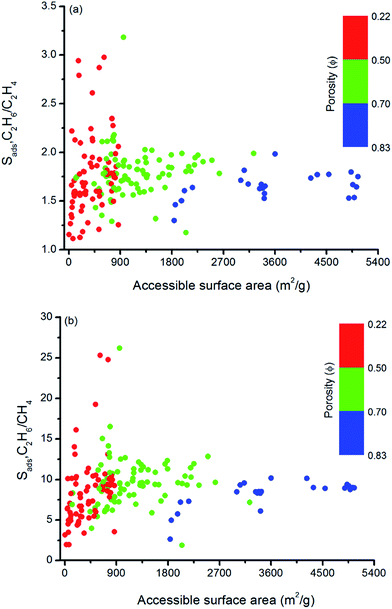 | ||
| Fig. 5 Relation between adsorption selectivities and surface areas of MOFs as a function of porosities for (a) C2H6/C2H4 and (b) C2H6/CH4 separations. | ||
Similar structure–performance analysis was carried out to unlock the relation between membrane selectivity of MOFs, pore sizes, porosities and surface areas. Fig. 6 shows that membranes with LCDs in the range of 6–7 Å and 6–9 Å are more selective for separation of C2H6 from C2H4 and CH4, respectively. It is also observed that among the two MOFs with close LCDs but different porosities, the MOF with lower porosity generally have higher membrane selectivity. For example, EYOPUE and SUBDOI have close LCDs (5.97 Å and 6.29 Å) but different porosities (0.46 and 0.56). The one with the lower porosity exhibits high membrane selectivity (5.41) whereas the other one has low membrane selectivity (1.14). This example underlines the importance of the material's porosity on the membrane selectivity. Similar to the adsorption selectivity, as the surface area and porosity decrease, membrane selectivity increases as shown in Fig. 7. For C2H6/C2H4 and C2H6/CH4 separation, we obtained the highest membrane selectivities (5.4 and 20.5, respectively) when the surface areas of the MOFs are between 500–1000 m2 g−1 and 750–1000 m2 g−1, respectively.
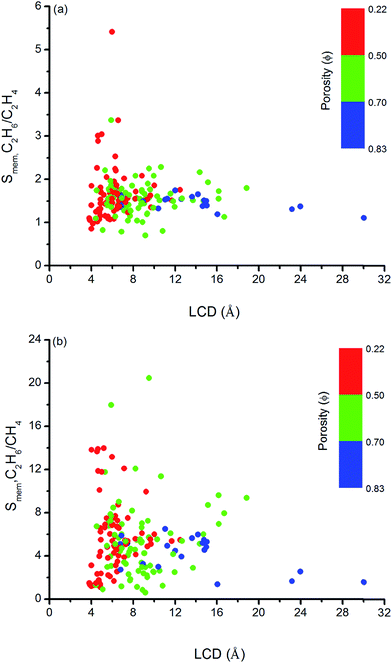 | ||
| Fig. 6 Relation between membrane selectivities and LCDs of MOFs as a function of porosities for (a) C2H6/C2H4 and (b) C2H6/CH4 separations. | ||
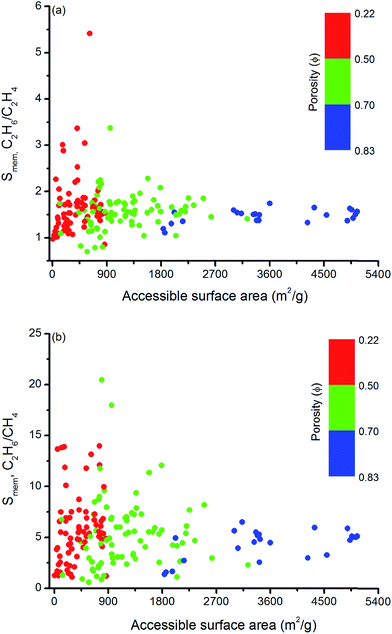 | ||
| Fig. 7 Relation between membrane selectivities and accessible surface areas of MOFs as a function of porosities for (a) C2H6/C2H4 and (b) C2H6/CH4 separation. | ||
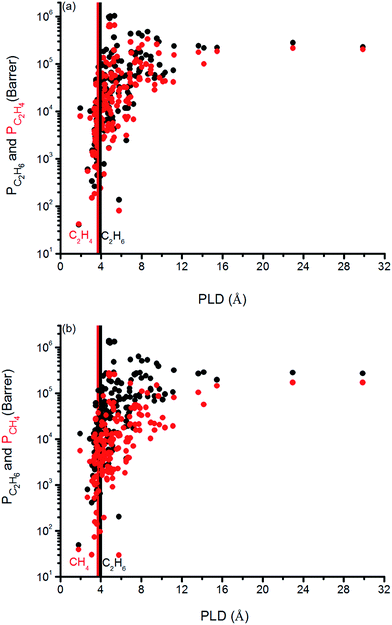
We finally investigated the effect of pore size on the gas permeabilities of MOFs in Fig. 8. Vertical solid lines in this figure represent the kinetic diameter of the gas molecules present in the mixture. We specifically focused on the PLD, the smallest pore diameter in the structure, rather than LCD since we refined our MOF database to have the LCDs greater than the kinetic diameters of the gas molecules as we discussed before. It can be seen that once the PLD is slightly larger than the kinetic diameter of a gas molecule, permeability of that gas increases. In Fig. 8(a), C2H6 (C2H4) permeability increases from 105 to 106 (104 to 105) Barrer when PLD is larger than 3.76 (3.68) Å, which is the kinetic diameter of C2H6 (C2H4) molecule. In Fig. 8(b), C2H6 and CH4 permeabilities increase from 105 to 106 Barrer and from 104 to 105 Barrer for the MOFs that have PLD values slightly larger than 3.76 Å and 3.73 Å, the kinetic diameters of C2H6 and CH4, respectively. These increases can be attributed to the easier diffusion of gas molecules in the larger pores of MOFs. These results suggest that it is reasonable to choose MOFs with PLD values slightly larger than the kinetic diameters of the gas molecules that are desired to be separated in order to obtain high gas permeabilities.
4. Conclusions
In this study, we used GCMC and EMD simulations to compute adsorption and diffusion data of C2H6/C2H4 and C2H6/CH4 mixtures in 175 different MOFs. Using this data, membrane performances of MOFs were assessed for these two important gas separations. Majority of the MOFs we considered was identified as C2H6 selective membranes and a small number of MOFs was identified as C2H4 selective. This result is important since membranes that are selective for C2H6 over C2H4 are scarce in the literature and almost all traditional membrane materials such as polymers, zeolites and CMSs are C2H4 selective. MOF membranes that we considered in this work were found to exhibit higher gas permeabilities than these well-known membrane materials due to their highly porous structures. Examining the structure–performance relations of MOF membranes revealed that MOFs with porosities lower than 0.50, LCD values between 6–9 Å, and surface areas between 500–1000 m2 g−1 have the highest selectivities for C2H6/C2H4 and C2H6/CH4 separations.The idea of our work was to identify promising MOF membranes for C2H6 separations using molecular simulations in order to direct experimental efforts, time and resources to those promising materials for experimental fabrication and testing of membranes under real operating conditions. It is very important to discuss the assumptions made in computational studies in order to evaluate the potential of new membrane materials in real applications. We assumed perfect MOF crystals in our GCMC and EMD simulations and predicted gas separation performances of MOFs as defect-free membranes. In reality, defects may be formed during membrane fabrication and they may reduce the membrane's expected selectivity. The idea of our calculations is that once the potential value of a membrane material has been demonstrated by molecular simulations, further experimental studies can be used to increase the precision of initial assessment. Our molecular simulations do not provide any information about the stability of MOFs, which is very important for real applications. An efficient membrane material must keep its structural stability under industrial operation conditions. This issue is more likely to be addressed experimentally. We searched for the stability information of the two MOFs, EYOPUE and NEXXIZ, which were identified as the top promising membrane material for selective separation of C2H6 from C2H4 and CH4, respectively. Experiments reported that they can retain their crystalline integrity at ambient conditions.83,84 The value of our computational work is that it can provide a motivation to perform detailed experimental studies for the thermal and structural stability of promising membrane materials. We believe that results of this work will guide experimental studies for the design and synthesis of new MOFs with better separation performances for C2H6 separations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. K. acknowledges ERC-2017-Starting Grant. This study has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC-2017-Starting Grant, grant agreement No 756489-COSMOS).References
- D. Banerjee, J. Liu and P. K. Thallapally, Comments Inorg. Chem., 2015, 35, 18–38 CrossRef CAS.
- M. Shi, C. C. H. Lin, T. M. Kuznicki, Z. Hashisho and S. M. Kuznicki, Chem. Eng. Sci., 2010, 65, 3494–3498 CrossRef CAS.
- F. X. Coudert and A. H. Fuchs, Coord. Chem. Rev., 2016, 307, 211–236 CrossRef CAS.
- M. Eddaoudi, H. Li and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391–1397 CrossRef CAS.
- R. B. Getman, Y. S. Bae, C. E. Wilmer and R. Q. Snurr, Chem. Rev., 2012, 112, 703–723 CrossRef CAS PubMed.
- S. Keskin, in Molecular Dynamics-Theoretical Developments and Applications in Nanotechnology and Energy, ed. L. Wang, InTech, 2012 Search PubMed.
- S. Xiang, Y. He, Z. Zhang, H. Wu, W. Zhou, R. Krishna and B. Chen, Nat. Commun., 2012, 3, 954–962 CrossRef PubMed.
- A. Battisti, S. Taioli and G. Garberoglio, Microporous Mesoporous Mater., 2011, 143, 46–53 CrossRef CAS.
- R. Babarao and J. Jiang, Langmuir, 2008, 24, 5474–5484 CrossRef CAS PubMed.
- Y. Liu, D. Liu, Q. Yang, C. Zhong and J. Mi, Ind. Eng. Chem. Res., 2010, 49, 2902–2906 CrossRef CAS.
- R. Krishna, RSC Adv., 2015, 5, 52269–52295 RSC.
- J. McEwen, J. D. Hayman and A. O. Yazaydin, Chem. Phys., 2013, 412, 72–76 CrossRef CAS.
- Z. B. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. L. Ren, X. Y. Lu and S. G. Deng, Langmuir, 2011, 27, 13554–13562 CrossRef CAS PubMed.
- U. Böhme, B. Barth, C. Paula, A. Kuhnt, W. Schwieger, A. Mundstock, J. Caro and M. Hartmann, Langmuir, 2013, 29, 8592–8600 CrossRef PubMed.
- H. Bux, C. Chmelik, R. Krishna and J. Caro, J. Membr. Sci., 2011, 369, 284–289 CrossRef CAS.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- Y. He, R. Krishna and B. Chen, Energy Environ. Sci., 2012, 5, 9107–9120 CAS.
- Y. He, Z. Zhang, S. Xiang, F. R. Fronczek, R. Krishna and B. Chen, Chem.–Eur. J., 2012, 18, 613–619 CrossRef CAS PubMed.
- C. E. Wilmer, O. K. Farha, T. Yildirim, I. Eryazici, V. Krungleviciute, A. A. Sarjeant, R. Q. Snurr and J. T. Hupp, Energy Environ. Sci., 2013, 6, 1158–1163 CAS.
- Y. Zhang, B. Li, R. Krishna, Z. Wu, D. Ma, Z. Shi, T. Pham, K. Forrest, B. Space and S. Ma, Chem. Commun., 2015, 51, 2714–2717 RSC.
- M. Hartmann, U. Böhme, M. Hovestadt and C. Paula, Langmuir, 2015, 31, 12382–12389 CrossRef CAS PubMed.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 1–9 Search PubMed.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed.
- H. C. Guo, F. Shi, Z. F. Ma and X. Q. Liu, Mol. Simul., 2014, 40, 349–360 CrossRef CAS.
- Y. Wu, H. Y. Chen, D. F. Liu, Y. Qian and H. X. Xi, Chem. Eng. Sci., 2015, 124, 144–153 CrossRef CAS.
- R. S. Pillai, M. L. Pinto, J. Pires, M. Jorge and J. R. B. Gomes, ACS Appl. Mater. Interfaces, 2015, 7, 624–637 CAS.
- C. Altintas and S. Keskin, Chem. Eng. Sci., 2016, 139, 49–60 CrossRef CAS.
- S. S. Chan, T.-S. Chung, Y. Liu and R. Wang, J. Membr. Sci., 2003, 218, 235–245 CrossRef CAS.
- S. S. Chan, R. Wang, T.-S. Chung and Y. Liu, J. Membr. Sci., 2002, 210, 55–64 CrossRef CAS.
- C. Staudt-Bickel and W. J. Koros, J. Membr. Sci., 2000, 170, 205–214 CrossRef CAS.
- K. Tanaka, A. Taguchi, J. Hao, H. Kita and K. Okamoto, J. Membr. Sci., 1996, 121, 197–207 CrossRef CAS.
- Y. Pan and Z. Lai, Chem. Commun., 2011, 47, 10275–10277 RSC.
- A. Huang, N. Wang, C. Kong and J. Caro, Angew. Chem., Int. Ed., 2012, 51, 10551–10555 CrossRef PubMed.
- S. Japip, H. Wang, Y. Xiao and T. Shung Chung, J. Membr. Sci., 2014, 467, 162–174 CrossRef CAS.
- R. Mueller, V. Hariharan, C. Zhang, R. Lively and S. Vasenkov, J. Membr. Sci., 2016, 499, 12–19 CrossRef CAS.
- J. Ploegmakers, S. Japip and K. Nijmeijer, J. Membr. Sci., 2013, 428, 445–453 CrossRef CAS.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Nat. Mater., 2016, 15, 845–849 CrossRef CAS PubMed.
- F. Stallmach, S. Gröger, V. Künzel, J. Kärger, O. Yaghi, M. Hesse and U. Müller, Angew. Chem., Int. Ed., 2006, 45, 2123–2126 CrossRef CAS PubMed.
- D. C. Ford, D. Dubbeldam, R. Q. Snurr, V. Künzel, M. Wehring, F. Stallmach, J. Kärger and U. Müller, J. Phys. Chem. Lett., 2012, 3, 930–933 CrossRef CAS PubMed.
- C. Chmelik, D. Freude, H. Bux and J. Haase, Microporous Mesoporous Mater., 2012, 147, 135–141 CrossRef.
- A. I. Skoulidas and D. S. Sholl, J. Phys. Chem. B, 2005, 109, 15760–15768 CrossRef CAS PubMed.
- L. Sarkisov, T. Düren and R. Q. Snurr, Mol. Phys., 2004, 102, 211–221 CrossRef CAS.
- B. Borah, H. Zhang and R. Q. Snurr, Chem. Eng. Sci., 2015, 124, 135–143 CrossRef CAS.
- T. Chokbunpiam, R. Chanajaree, O. Saengsawang, S. Reimann, C. Chmelik, S. Fritzsche, J. Caro, T. Remsungnen and S. Hannongbua, Microporous Mesoporous Mater., 2013, 174, 126–134 CrossRef CAS.
- P. Krokidas, M. Castier, S. Moncho, E. Brothers and I. G. Economou, J. Phys. Chem. C, 2015, 119, 27028–27037 CAS.
- R. J. Verploegh, S. Nair and D. S. Sholl, J. Am. Chem. Soc., 2015, 137, 15760–15771 CrossRef CAS PubMed.
- Y. G. Chung, J. Camp, M. Haranczyk, B. J. Sikora, W. Bury, V. Krungleviciute, T. Yildirim, O. K. Farha, D. S. Sholl and R. Q. Snurr, Chem. Mater., 2014, 26, 6185–6192 CrossRef CAS.
- K. B. Sezginel, A. Uzun and S. Keskin, Chem. Eng. Sci., 2015, 124, 125–134 CrossRef CAS.
- M. Fischer, J. R. B. Gomes and M. Jorge, Mol. Simul., 2014, 40, 537–556 CrossRef CAS.
- F. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef.
- L. Sarkisov and A. Harrison, Mol. Simul., 2011, 37, 1248–1257 CrossRef CAS.
- S. L. Mayo, B. D. Olafson and W. A. Goddard, J. Phys. Chem. C, 1990, 94, 8897–8909 CrossRef CAS.
- D. Frenkel and B. Smit, Understanding Molecular Simulation: From Algorithms to Applications, Academic Press, San Diego, 2nd edn, 2002 Search PubMed.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. Goddard and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- M. G. Martin and J. I. Siepmann, J. Phys. Chem. B, 1998, 102, 2569–2577 CrossRef CAS.
- S. Keskin and D. S. Sholl, Langmuir, 2009, 25, 11786–11795 CrossRef CAS PubMed.
- I. Erucar and S. Keskin, Ind. Eng. Chem. Res., 2013, 52, 3462–3472 CrossRef CAS.
- J. Perez-Pellitero, H. Amrouche, F. R. Siperstein, G. Pirngruber, C. Nieto-Draghi, G. Chaplais, A. Simon-Masseron, D. Bazer-Bachi, D. Peralta and N. Bats, Chem.–Eur. J., 2010, 16, 1560–1571 CrossRef CAS PubMed.
- J. A. Greathouse and M. D. Allendorf, J. Phys. Chem. C, 2008, 112, 5795–5802 CAS.
- E. Haldoupis, T. Watanabe, S. Nair and D. S. Sholl, ChemPhysChem, 2012, 13, 1–4 CrossRef PubMed.
- I. Erucar and S. Keskin, J. Membr. Sci., 2016, 514, 313–321 CrossRef CAS.
- M. Witman, S. Ling, S. Jawahery, P. G. Boyd, M. Haranczyk, B. Slater and B. Smit, J. Am. Chem. Soc., 2017, 139, 5547–5557 CrossRef CAS PubMed.
- R. Krishna and D. Paschek, Phys. Chem. Chem. Phys., 2002, 4, 1891–1898 RSC.
- Y. Basdogan, K. B. Sezginel and S. Keskin, Ind. Eng. Chem. Res., 2015, 54, 8479–8491 CrossRef CAS.
- E. Adatoz and S. Keskin, J. Nanomater., 2015, 2015, 1–9 CrossRef.
- G. Garberoglio and R. Vallauri, Microporous Mesoporous Mater., 2008, 116, 540–547 CrossRef CAS.
- M. Rungta, C. Zhang, W. J. Koros and L. R. Xu, AIChE J., 2013, 59, 3475–3489 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- T. A. Centeno and A. B. Fuertes, J. Membr. Sci., 1999, 160, 201–211 CrossRef CAS.
- A. B. Fuertes and I. Menendez, Sep. Purif. Technol., 2002, 28, 29–41 CrossRef CAS.
- J.-i. Hayashi, H. Mizuta, M. Yamamoto, K. Kusakabe, S. Morooka and S.-H. Suh, Ind. Eng. Chem. Res., 1996, 35, 4176–4181 CrossRef CAS.
- C. Zhang, R. P. Lively, K. Zhang, J. R. Johnson, O. Karvan and W. J. Koros, J. Phys. Chem. Lett., 2012, 3, 2130–2134 CrossRef CAS PubMed.
- L. Starannikova, Y. Yampolskii, K. Makovetskii and T. Golenko, Desalination, 2006, 200, 18–19 CrossRef CAS.
- I. Pinnau and Z. He, J. Membr. Sci., 2004, 244, 227–233 CrossRef CAS.
- W. Dai, M. Scheibe, L. Li, N. Guan and M. Hunger, J. Phys. Chem. C, 2012, 116, 2469–2476 CAS.
- A. Romero-Pérez and G. Aguilar-Armenta, J. Chem. Eng. Data, 2010, 55, 3625–3630 CrossRef.
- D. M. Ruthven and S. C. Reyes, Microporous Mesoporous Mater., 2007, 104, 59–66 CrossRef CAS.
- A. Khosravi and M. Sadeghi, J. Membr. Sci., 2013, 434, 171–183 CrossRef CAS.
- I. Tirouni, M. Sadeghi and M. Pakizeh, Sep. Purif. Technol., 2015, 141, 394–402 CrossRef CAS.
- A. Alentiev, I. G. Economou, E. Finkelshtein, J. Petrou, V. E. Raptis, M. Sanopoulou, S. Soloviev, N. Ushakov and Y. Yampolskii, Polymer, 2004, 45, 6933–6944 CrossRef CAS.
- W. Robb, Ann. N. Y. Acad. Sci., 1968, 146, 119–137 CrossRef CAS PubMed.
- T. N. Ozturk and S. Keskin, J. Phys. Chem. C, 2014, 118, 13988–13997 CAS.
- K.-H. Cui, S.-Y. Yao, H.-Q. Li, Y.-T. Li, H.-P. Zhao, C.-J. Jiang and Y.-Q. Tian, CrystEngComm, 2011, 13, 3432–3437 RSC.
- T. K. Kim, J. H. Lee, D. Moon and H. R. Moon, Inorg. Chem., 2013, 52, 589–595 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: List of the MOFs studied in this work and their structural properties. Potential parameters of gas molecules used in the simulations. See DOI: 10.1039/c7ra11562h |
| This journal is © The Royal Society of Chemistry 2017 |