Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual valence Eu-doped phospho-alumino-silicate glass-ceramics containing Ba3AlO3PO4 nanocrystals for W-LEDs
Xiaoman Liab,
Dengke Xua,
XueYun Liuc and
Hai Guo *a
*a
aDepartment of Physics, Zhejiang Normal University, Jinhua, Zhejiang 321004, China. E-mail: ghh@zjnu.cn
bState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, Guangdong 510640, China
cLaboratory of Infrared Materials and Devices, The Advanced Technology Research Institute, Ningbo University, Ningbo, Zhejiang 315211, China
First published on 23rd November 2017
Abstract
Novel dual valence Eu-doped phospho-alumino-silicate glass-ceramics containing orthorhombic Ba3AlO3PO4 nanocrystals were first fabricated by a traditional melt-quenching method and subsequent heat-treatment in an air atmosphere. Their structural and luminescent properties were systemically investigated by XRD, TEM analysis, and spectroscopic and fluorescence lifetime measurements. The incorporation of Eu3+ into Ba3AlO3PO4 crystallites and the reduction mechanism of Eu3+ to Eu2+ were also discussed based on the optical analyses. Simultaneously, perfect white light emission was obtained under 325 nm excitation. An improved anti-thermal quenching property was achieved resulting from the successful enrichment of Eu3+ and Eu2+ into Ba3AlO3PO4 crystallites, which was evidenced by active energy calculation. Our results indicate that these dual valence Eu-doped transparent Ba3AlO3PO4 glass-ceramics may have potential applications in W-LEDs.
Introduction
White light-emitting diodes (W-LEDs), the next generation of solid-state light source, have attracted more attention recently due to not only their long lifetime, environment benefits and energy saving but also their wide applications for lighting and displays, such as general illumination, device indicators, backlights, and automobile headlights.1–5 However, the efficiency of W-LEDs fabricated by ultraviolet (UV) LED chips coupled with tri-color phosphors is limited because of the strong re-absorption of blue light by green and red phosphors.6–10 Compared to the conventional phosphors used for W-LEDs, white emitting rare earth ion (REI)-doped glass-ceramics not only present excellent luminescent properties and better mechanical properties but also show an epoxy-resin free assembly process11–20 with a lower production cost and simpler manufacturing procedures. Therefore, it is of urgency to design novel single-phased multi-activator co-doped systems capable of emitting white light under UV chip excitation, which is based on the luminescence and (or) energy transfer between multi-activators.20–25Among the luminescent activators, dual valence europium ions have been widely used in luminescent materials. It is well-known that the emission of Eu3+ ion consists of sharp lines in the orange-red spectra region, while that of Eu2+ ion is typically broad in the blue-green spectral region.26–32 Consequently, a perfect white light emission may be achieved in appropriate glass-ceramics by combining the sharp emission of Eu3+ and the broad emission of Eu2+.33,34
For the glassy host, silicate glasses have attracted considerable interest due to their low-cost, large tensile strength, high chemical durability, and excellent thermal stability. In addition, with the introduction of Al2O3, the solubility of REI will be increased and the concentration quenching of luminescence will be limited. Besides, P2O5, a kind of glass network modifier, may be conducive to lower viscosity and improve the crystallization process of glass-ceramics.35 Therefore, highly transparent phospho-alumino-silicate glasses and glass-ceramics are considered as suitable hosts to obtain practically visible luminescence.
Herein, dual valence Eu-doped Ba3AlO3PO4 based phospho-alumino-silicate glass-ceramics were first fabricated by a traditional melt-quenching method in an air atmosphere. Their structure and luminescent properties were systematically investigated. Perfect white light emission and large active energy were achieved under the excitation of UV light, which makes these glass and glass-ceramics good candidates for W-LED phosphors even at high temperatures.
Experimental
The glass samples with nominal composition 35SiO2–17Al2O3–20BaCO3–20BaF2–8P2O5–0.5EuF3 (in mol%) were prepared by the melt-quenching method. SiO2, Al2O3, P2O5, BaCO3, BaF2 (A.R., all from Sinopharm Chemical Reagent Co., Ltd., China), and high purity EuF3 (99.99%, from AnSheng Inorganic Materials Co., Ltd., China) were used as starting materials. The well-ground stoichiometric chemicals were put into a covered alumina crucible and melted at 1500 °C for 1 h in an air atmosphere. The melt was poured onto a 300 °C preheated stainless-steel plate and then pressed by another plate to form the precursor glasses (labeled as PG), followed by annealing at 450 °C for 5 h to release the internal stresses. Subsequently, the PG samples were heat-treated for 2 h at 670 and 680 °C to form the transparent glass-ceramics, which were labeled as GC670 and GC680, respectively. All samples were cut and polished optically with a thickness of 2 mm for further characterization and measurements.The differential scanning calorimeter (DSC) curve of the PG (powder, 10.0 mg) was collected on a STA449C Jupiter (Netzsch, Germany) apparatus at a heating rate of 10 °C min−1 in the range of 40–950 °C in an air atmosphere. X-ray diffraction (XRD) patterns were recorded on a Philips X'Pert PRO SUPER X-ray diffraction apparatus with a Cu Kα radiation. Transmittance spectra were measured on a U-3900 Ultraviolet-Visible (UV-VIS) spectrophotometer (Hitachi). The microstructure of the glass-ceramics was analyzed by a JEM-2010 transmission electron microscope (TEM) (JOEL Ltd., Tokyo, Japan). The excitation, emission spectra, and decay curves were recorded on an Edinburgh FLS920 spectrofluorometer equipped with a continuous wave 450 W Xe lamp, a microsecond flashlamp (μF900), and a nanosecond flashlamp (nF900) as excitation sources. Thermal degradation experiments were also recorded on an Edinburgh FLS920 spectrofluorometer by utilizing a homemade high-temperature unit. All above measurements were carried out at room temperature, except thermal degradation experiments.
Results and discussion
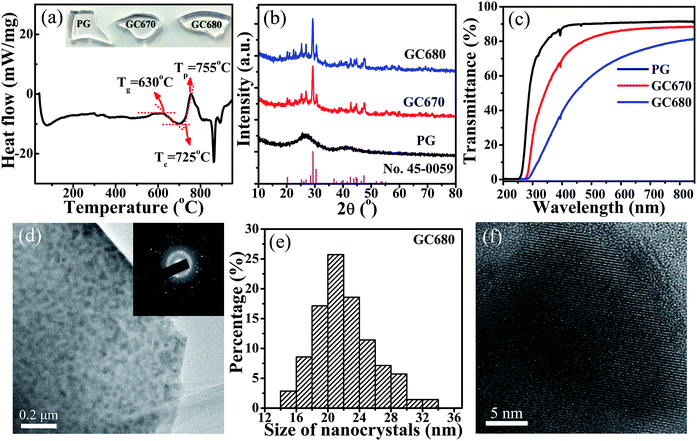
In order to determine the crystallization temperature of this Eu-doped phospho-alumino-silicate glass, the DSC curve of the PG sample was collected at a heating rate of 10 °C min−1 in the air atmosphere, as exhibited in Fig. 1(a). An obvious crystallization peak at 755 °C (Tp) with a FWHM (full width at half maximum) of 52.6 °C is observed. The glass transition temperature (Tg) and the onset of crystallization temperature (Tc) are about 630 and 725 °C, respectively. Because of a large difference of 95 °C between Tg and Tc, 670 to 680 °C (40–50 °C beyond Tg) can be used as the crystallization temperature to obtain the glass-ceramics. In this work, 670 and 680 °C were chosen as the heat treatment temperatures for crystallization.Fig. 1(b) shows the XRD patterns of PG, GC670, and GC680. PG does not exhibit any discrete diffraction peaks, confirming its amorphous nature. Moreover, as shown in the photographic images of the samples (inset in Fig. 1(a)), PG exhibits good transparency. The diffraction peaks of GC670 and GC680 match well with that of orthorhombic Ba3AlO3PO4 (JCPDS card no. 45-0059), indicating that the Ba3AlO3PO4 based glass-ceramics were elaborated. The size of Ba3AlO3PO4 nanocrystals can be estimated by the following Scherrer's equation:36
D = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
The UV-VIS transmittance spectra of PG, GC670, and GC680 in the range of 200–850 nm are shown in Fig. 1(c). The characteristic absorption peaks observed at 393 and 464 nm are ascribed to the transitions from the 7F0 ground state to the 5L6 and 5D2 excited states of Eu3+,34 respectively. The cut-off wavelength of GC gets a red-shift compared to that of PG, which may be attributed to the optical scattering of Ba3AlO3PO4 nanocrystals. Similar to PG, the GC samples maintain a perfect transparency (up to 70%) owing to the smaller size of the precipitated Ba3AlO3PO4 nanocrystals compared to the wavelength of visible-near infrared (VIS-NIR) light.34 The transmittance decreases gradually with elevating heat-treatment temperature because of the larger size of nanocrystals and crystallinity after further crystallization, which is reflected in the photographs of the glass samples shown in Fig. 1(a).
The microstructure of the GC680 sample is characterized by TEM and high-resolution TEM (HRTEM) images, as displayed in Fig. 1(d) and (f), respectively. The TEM bright-field micrograph and selected area electron diffraction (SAED) patterns reveal that Ba3AlO3PO4 nanocrystals with a polycrystalline diffraction feature are homogeneously dispersed in the amorphous glassy phase. The particle size distribution of Ba3AlO3PO4 nanocrystals in the GC680 sample is shown in Fig. 1(e). The results show that the size of Ba3AlO3PO4 nanocrystals is at a range of 14–34 nm and the average size is about 21 nm, in accordance with that estimated by the Scherrer's equation. HRTEM image displays the resolved lattice fringes of nano-sized single-crystalline Ba3AlO3PO4 clearly and the value of the associated interplanar spacing d is about 0.314 nm, which is corresponding to the (311) crystal plane of orthorhombic Ba3AlO3PO4 (d(311) = 0.313 nm).
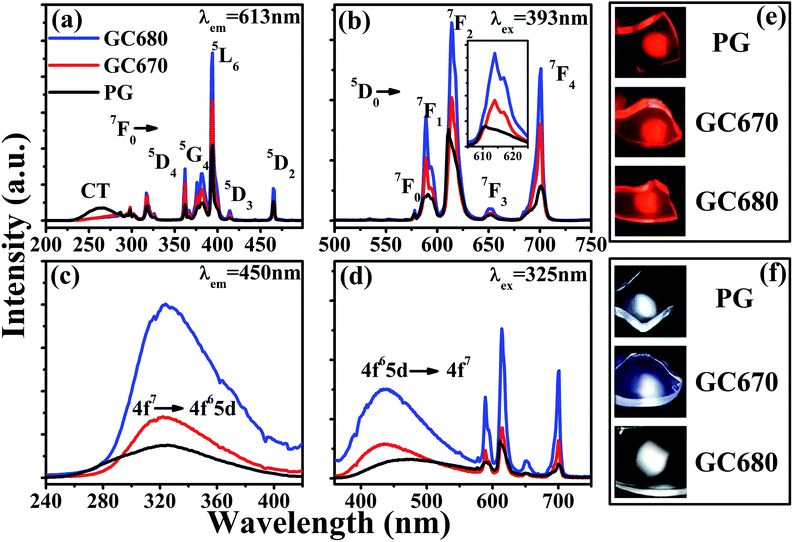
The excitation (λem = 613 nm) and emission (λex = 393 nm) spectra of Eu3+ ions in the PG and GC samples are given in Fig. 2(a) and (b), respectively. Besides several characteristic sharp peaks assigned to the 4f–4f transitions of Eu3+ ions (350–500 nm) from the ground state to the marked excited levels, there is also a broadband at 263 nm originated from the well-known O2−–Eu3+ charge-transfer (CT) band in the excitation spectra monitored at the 613 nm emission. The strongest excitation peak at 393 nm corresponds to the 7F0 → 5L6 transition of Eu3+ (ref. 33 and 34) and the excitation efficiency is apparently intensified for the GC samples compared with that of the PG sample. In the emission spectra excited by 393 nm, the characteristic red emission peaks originating from 5D0 → 7FJ (J = 0–4) transitions of Eu3+ ions (570–720 nm) are detected.37 The luminescent properties of Eu3+ undergo significant changes after crystallization. Compared to those of PG, the red emissions at 613 and 702 nm of Eu3+ ions of GC680 are enhanced by 3 and 6 times, respectively. Stark splitting for 5D0 → 7F2 transitions of Eu3+ ions is also observed after crystallization, as shown in the inset of Fig. 2(b). These phenomena generally confirm the incorporation of Eu3+ ions into Ba3AlO3PO4 nanocrystals with increasing the symmetrical environment for Eu3+.34,36 Fig. 2(e) shows the photographs of the PG and GC samples under the excitation of 393 nm light. It can be seen that all glass samples emit red light.
It is well-known that 5D0 → 7F2 electric dipole transition of Eu3+ is forbidden in the centrosymmetric environment, while 5D0 → 7F1 magnetic dipole transition does not depend on the symmetry of the environment that Eu3+ is located in. As a result, the intensity ratio R of 5D0 → 7F2 to 5D0 → 7F1 emissions can be taken as a spectral probe to detect the symmetry of local crystal field that Eu3+ is located in. A lower value of R suggests a higher crystal symmetry.13–15 In this work, the integrated intensity ratios R calculated are 3.3, 2.5, and 2.4 for PG, GC670, and GC680, respectively. Hence, the decrease of the R value after heat-treatment is another evidence for the incorporation of Eu3+ into the symmetric environment of Ba3AlO3PO4 nanocrystals.
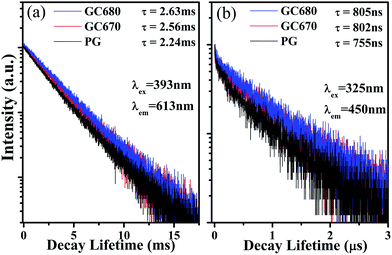
The fluorescence decay curves of Eu3+ emission can further evaluate the surroundings that Eu3+ is located in. Fig. 3(a) shows the decay curves of the 613 nm (5D0 → 7F2 transition) emission of Eu3+ ions (λex = 393 nm) in the PG and GC samples. For all samples, the observed decay curves approximately follow a single exponential function. The lifetimes of PG, GC670, and GC680 are about 2.25, 2.56, and 2.63 ms, respectively. Slightly longer lifetimes for the glass-ceramics indicate that Eu3+ ions are partially incorporated into the Ba3AlO3PO4 crystalline phase.
 | ||
| Fig. 3 Luminescent decay curves of (a) 5D0 → 7F2 transition (613 nm) of Eu3+ (λex = 393 nm), and (b) 4f65d → 4f7 transition (450 nm) of Eu2+ (λex = 325 nm). | ||
Briefly summarized, the increased luminescence intensities of Eu3+ ions, the obvious Stark splitting, the decreased intensity ratio R, and the longer fluorescence lifetimes of the GC samples indicate that Eu3+ ions have been partially incorporated into Ba3AlO3PO4 nanocrystals after crystallization. Such phenomena may be attributed to the smaller ion radii of Eu3+ (0.0947 nm) compared to that of Ba2+ (0.1350 nm).38
Noteworthy, besides the sharp emissions of Eu3+, a pronounced broad emission centered at about 450 nm from Eu2+ is also detected, implying that a part of Eu3+ ions is reduced to Eu2+ in the present host. The Eu2+-related characteristic excitation (λem = 450 nm) and emission (λex = 325 nm) spectra in PG, GC670, and GC680 are presented in Fig. 2(c) and (d), respectively. The excitation band at 270–390 nm is ascribed to the 4f7 → 4f65d transition of Eu2+. The intense broad emission band in the range of 390–560 nm is due to the 4f65d → 4f7 transition of Eu2+.33,34 For PG, the emission intensity of Eu2+ is very weak, implying that only small quantity of Eu3+ is reduced to Eu2+. After crystallization, the intensity of the Eu2+ emission is enhanced remarkably with increasing the crystallization temperature, which reveals that more Eu3+ ions are reduced to Eu2+ ions during the crystallization process. The resultant Eu2+ (0.1170 nm)38 ions undergo more ordered local crystal field environments with the incorporation into Ba3AlO3PO4 nanocrystals by displacing the positions of Ba2+,39 reflected by the narrowed full width of half maximum (FWHM) of 63 nm in GC670 (or GC680) compared to 77 nm in PG. Fig. 2(f) presents the glass photographs under 325 nm radiation. Intriguingly, the emitting light colors of the glass samples vary from greenish-white in PG to bluish-white and then to perfect white in GC670 and GC680, respectively.
The fluorescence decay curves of Eu2+ (λex = 325 nm and λem = 450 nm), as shown in Fig. 3(b), can further illustrate the above point. Decay curves of Eu2+ emission in all glass samples do not follow the single exponential decay equation, but a double exponential decay equation with a shorter and a longer lifetime components. Hence, here, the lifetimes of Eu2+ ions are characterized uniformly by the average lifetime (![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) ) derived by:21
) derived by:21
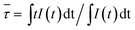 | (2) |
![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) are 755, 802, and 805 ns for PG, GC670, and GC680, respectively. The longer lifetime of Eu2+ in the GC samples strongly proves the partial incorporation of Eu2+ into orthorhombic Ba3AlO3PO4 nanocrystals.
are 755, 802, and 805 ns for PG, GC670, and GC680, respectively. The longer lifetime of Eu2+ in the GC samples strongly proves the partial incorporation of Eu2+ into orthorhombic Ba3AlO3PO4 nanocrystals.
The reduction mechanism of Eu3+ to Eu2+ in the air atmosphere can be illustrated perfectly by a charge compensation model.28,40 When doped into Ba3AlO3PO4 nanocrystals, Eu3+ will replace Ba2+ ions. Meanwhile, two Eu3+ ions are needed to substitute three Ba2+ ions to maintain the charge balance. Consequently, one cation vacancy [V′′Ba] and two defects 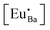 would be created simultaneously. Here, V′′Ba holds two negative charges, while
would be created simultaneously. Here, V′′Ba holds two negative charges, while  bears one positive charge. V′′Ba acts as an electron donor, while
bears one positive charge. V′′Ba acts as an electron donor, while  becomes an electron acceptor. By continuous thermal stimulation, the electrons in vacancy defect V′′Ba are released and then trapped by
becomes an electron acceptor. By continuous thermal stimulation, the electrons in vacancy defect V′′Ba are released and then trapped by  , reducing Eu3+ ions to Eu2+ ions. As a result, Eu3+ ions are efficiently reduced to Eu2+ ions within the precipitated Ba3AlO3PO4 nanocrystals in the glass-ceramics.
, reducing Eu3+ ions to Eu2+ ions. As a result, Eu3+ ions are efficiently reduced to Eu2+ ions within the precipitated Ba3AlO3PO4 nanocrystals in the glass-ceramics.
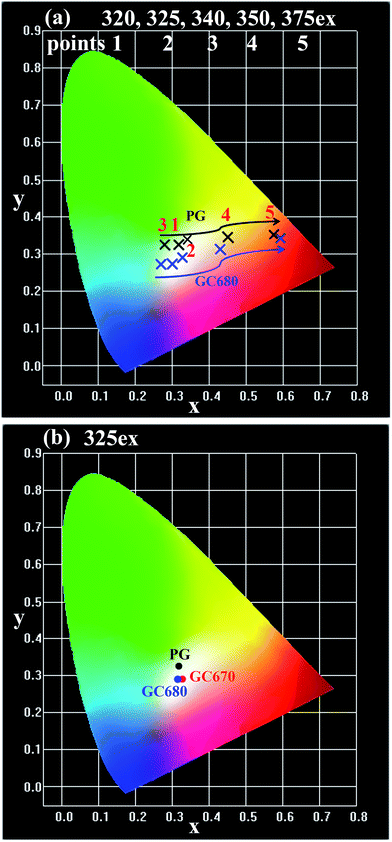
Due to the great difference in the excitation spectrum characteristics of Eu2+ and Eu3+, a tunable visible emission ranging from blue to red can be achieved by simply altering the excitation wavelength. Fig. 4(a) presents the Commission International de I'Eclairage (CIE) chromaticity coordinates of the PG and GC680 samples excited by 320–375 nm. Both PG and GC680 samples exhibit a tunable luminescent color, covering green-white, blue-white light to warm-white and then red light. It needs to be mentioned that good white light emissions can be achieved in the PG, GC670, and GC680 samples excited by 325 nm UV light, which was exhibited in Fig. 2(f). And the related CIE chromaticity coordinates of the PG and GC samples are shown in Fig. 4(b). Obviously, the CIE chromaticity coordinates of all samples are in the white region and close to the standard equal energy white light illuminate (X = 0.333, Y = 0.333). The emitting light color of the glass sample can be tuned from green-white light in PG to red-white light in the GC samples after crystallization, which suggests that the mixed-valence Eu-doped Ba3AlO3PO4 glass-ceramics can act as white-emitting phosphors for UV LED chips.
In practical applications, the thermal quenching property is an important technological parameter for a luminescent glass in the solid-state lighting field because it greatly affects the color rendering index and the light output of LEDs,41–43 which will finally determine whether glass phosphors can sustain a long-term emission efficiency at temperatures over 423 K in LED. Therefore, it is necessary to evaluate the thermal quenching property of the glass particularly at temperatures higher than room temperatures. In order to ensure the results as accurate as possible, 330 nm was chosen as the most effective excitation wavelength for these dual valence europium doped glass and glass-ceramics. Fig. 5(a) and (b) show the emission spectra of the PG and GC680 sample excited by 330 nm at different temperatures, respectively. The insets in the figures are the partially enlarged view of Eu2+ emission. Clearly, both emission intensities of Eu2+ and Eu3+ decrease with increasing temperature. The intensities of Eu2+ and Eu3+ in GC680 at 423 K remain about 79% and 55% of the initial intensity at 303 K, respectively.
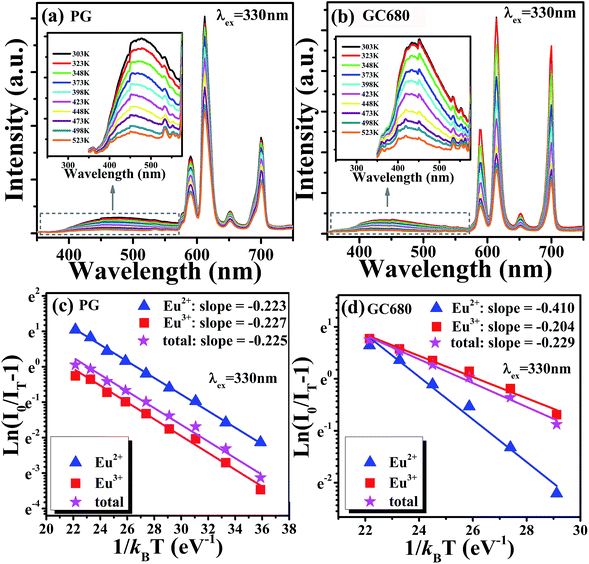 | ||
| Fig. 5 Temperature-dependent emission spectra (λex = 330 nm) of (a) PG and (b) GC680 samples, the inset is the corresponding partial enlarged view of Eu2+ emission spectra; plot of ln(I0/IT − 1) v.s. 1/kBT, and the linear fit of the data through eqn (4) of (c) PG and (d) GC680. | ||
Generally, the thermal quenching with increasing temperature is caused by the enhancement of thermally active multi-phonon nonradiative rate from the luminescent state to a lower state. The thermally activated nonradiative transition rate knr is given by:44,45
knr = s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔEa/kBT) exp(−ΔEa/kBT)
| (3) |
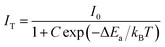 | (4) |
The slightly larger value of ΔEa in GC680 implies better anti-thermal degradation property of GC680 than that of PG. This result indicates that our glass-ceramics have a better thermal stability than PG, which makes the glass-ceramics promising as phosphors for W-LEDs in practice.
Conclusions
Highly transparent dual valence Eu-doped orthorhombic Ba3AlO3PO4 based glass-ceramics were first fabricated by a melt-quenching method. The luminescent properties of Eu3+ and Eu2+ ions in the precursor glass and glass-ceramics revealed the incorporation of Eu3+ and Eu2+ ions into the Ba3AlO3PO4 crystalline phase by displacing Ba2+ lattice sites after crystallization. By combining the sharp orange-red emission of Eu3+ and the broad blue-green emission of Eu2+, perfect white light emissions can be achieved by the excitation of 325 nm UV light. A larger active energy of Eu2+ than Eu3+ after crystallization further manifests the incorporation of Eu2+ into Ba3AlO3PO4 nanocrystals with a better anti-thermal quenching property. All results indicate that such dual valence Eu-doped transparent Ba3AlO3PO4 glass and glass-ceramics can be considered as good candidates for phosphor in W-LEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11374269 and 51702172).Notes and references
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS PubMed.
- P. F. Smet, A. B. Parmentier and D. Poelman, J. Electrochem. Soc., 2011, 158, R37–R54 CrossRef CAS.
- D. Chen, Y. Yu, H. Lin, P. Huang, F. Weng, Z. Shan and Y. Wang, Opt. Lett., 2009, 34, 2882–2884 CrossRef CAS PubMed.
- B. Zhu, S. Zhang, S. Zhou, N. Jiang and J. Qiu, Opt. Lett., 2007, 32, 653–655 CrossRef CAS PubMed.
- D. Ramachari, L. R. Moorthy and C. K. Jayasankar, Ceram. Int., 2014, 40, 11115–11121 CrossRef CAS.
- Y. Narukawa, I. Niki, K. Izuno, M. Yamada, Y. Murazaki and T. Mukai, Jpn. J. Appl. Phys., Part 2, 2002, 41, L371–L373 CAS.
- X. Piao, T. Horikawa, H. Hanzawa and K. I. MacHida, Appl. Phys. Lett., 2006, 88, 161908 CrossRef.
- F. W. Kang, H. S. Zhang, L. Wondraczek, X. B. Yang, Y. Zhang, D. Y. Lei and M. Y. Peng, Chem. Mater., 2016, 28, 2692–2703 CrossRef CAS.
- F. W. Kang, M. Y. Peng, D. Y. Lei and Q. Y. Zhang, Chem. Mater., 2016, 28, 7807–7815 CrossRef CAS.
- M. Y. Peng, X. W. Yin, P. A. Tanner, M. G. Brik and P. F. Li, Chem. Mater., 2015, 27, 2938–2945 CrossRef CAS.
- S. Yi, W. J. Chung and J. Heo, J. Am. Ceram. Soc., 2014, 97, 342–345 CrossRef CAS.
- R. Zhang, H. Lin, Y. Yu, D. Chen, J. Xu and Y. Wang, Laser Photonics Rev., 2014, 8, 158–164 CrossRef CAS.
- G. Gao, N. Da, S. Reibstein and L. Wondraczek, Opt. Express, 2010, 18, A575–A583 CrossRef CAS PubMed.
- G. Gao, S. Reibstein, M. Peng and L. Wondraczek, J. Mater. Chem., 2011, 21, 3156–3161 RSC.
- G. Gao and L. Wondraczek, Opt. Mater. Express, 2014, 4, 476–485 CrossRef CAS.
- D. Chen, Y. Yu, P. Huang, H. Lin, Z. Shan and Y. Wang, Acta Mater., 2010, 58, 3035–3041 CrossRef CAS.
- Q. Luo, X. Qiao, X. Fan, H. Yang, X. Zhang, S. Cui, L. Wang and G. Wang, J. Appl. Phys., 2009, 105, 043506 CrossRef.
- Z. Lin, X. Liang, Y. Ou, C. Fan, S. Yuan, H. Zeng and G. Chen, J. Alloys Compd., 2010, 496, L33–L37 CrossRef CAS.
- H. Guo, F. Li, R. Wei, H. Zhang and C. Ma, J. Am. Ceram. Soc., 2012, 95, 1178–1181 CrossRef CAS.
- X. M. Li, J. K. Cao, F. F. Hu, R. F. Wei and H. Guo, RSC Adv., 2017, 7, 35147–35153 RSC.
- H. Guo, R. F. Wei and X. Y. Liu, Opt. Lett., 2012, 37, 1670–1672 CrossRef CAS PubMed.
- H. Guo, X. Wang, J. Chen and F. Li, Opt. Express, 2010, 18, 18900–18905 CrossRef CAS PubMed.
- H. Zeng, Z. Lin, Q. Zhang, D. Chen, X. Liang, Y. Xu and G. Chen, Mater. Res. Bull., 2011, 46, 319–322 CrossRef CAS.
- H. Lin, D. Chen, Y. Yu, A. Yang, R. Zhang and Y. Wang, Mater. Res. Bull., 2012, 47, 469–472 CrossRef CAS.
- M. L. C. Jota, A. G. Murillo, F. C. Romo, M. G. Hernandez, A. D. M. Ramirez, S. Velumani, E. D. Cruz and A. Kassiba, Mater. Res. Bull., 2014, 51, 418–425 CrossRef.
- S. Liu, G. Zhao, W. Ruan, Z. Yao, T. Xie, J. Jin, H. Ying, J. Wang and G. Han, J. Am. Ceram. Soc., 2008, 91, 2740–2742 CrossRef CAS.
- C. Zhu, Y. Yang, X. Liang, S. Yuan and G. Chen, J. Am. Ceram. Soc., 2007, 90, 2984–2986 CrossRef CAS.
- S. Taruta, M. Matsuki, H. Nishikiori, T. Yamakami, T. Yamaguchi and K. Kitajima, Ceram. Int., 2010, 36, 1303–1309 CrossRef CAS.
- G. J. Gao, J. X. Wei, Y. Shen, M. Y. Peng and L. Wondraczek, J. Mater. Chem. C, 2014, 2, 8678–8682 RSC.
- G. J. Gao and L. Wondraczek, J. Mater. Chem. C, 2014, 2, 691–695 RSC.
- M. Chowdhury and S. K. Sharma, RSC Adv., 2015, 5, 51102–51109 RSC.
- A. A. Reddy, S. Das, S. Ahmad, S. S. Babu, J. M. F. Ferreira and G. V. Prakash, RSC Adv., 2012, 2, 8768–8776 RSC.
- H. Guo, X. Liu, F. Li, R. Wei, Y. Wei and C. Ma, J. Electrochem. Soc., 2012, 159, J223–J226 CrossRef CAS.
- X. Liu, Y. Wei and H. Guo, J. Am. Ceram. Soc., 2013, 96, 369–371 CAS.
- F. Fu, B. Chen, L. Shen, E. Y. B. Pun and H. Lin, J. Alloys Compd., 2014, 582, 265–272 CrossRef CAS.
- X. Liu, Y. Wei, R. Wei, J. Yang and H. Guo, J. Am. Ceram. Soc., 2013, 96, 798–800 CrossRef CAS.
- C. Zhang, S. Zhao, D. Deng, L. Huang, Y. Tian and S. Xu, Ceram. Int., 2014, 40, 2737–2740 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- Q. Luo, X. Fan, X. Qiao, H. Yang, M. Wang and X. Zhang, J. Am. Ceram. Soc., 2009, 92, 942–944 CrossRef CAS.
- M. Peng, Z. Pei, G. Hong and Q. Su, J. Mater. Chem., 2003, 13, 1202–1205 RSC.
- Z. G. Xia, Z. H. Xu, M. Y. Chen and Q. L. Liu, Dalton Trans., 2016, 45, 11214–11232 RSC.
- X. J. Zhang, L. Huang, F. J. Pan, M. M. Wu, J. Wang, Y. Chen and Q. Su, ACS Appl. Mater. Interfaces, 2014, 6, 2709–2717 CAS.
- X. Y. Liu, H. Guo, S. X. Dai, M. Y. Peng and Q. Y. Zhang, Opt. Mater. Express, 2016, 6, 3574–3585 CrossRef.
- S. Saha, S. Das, U. K. Ghorai, N. Mazumder, D. Ganguly and K. K. Chattopadhyay, J. Phys. Chem. C, 2015, 119, 16824–16835 CAS.
- J. H. Li, J. Yan, D. W. Wen, W. U. Khan, J. X. Shi, M. M. Wu, Q. Su and P. A. Tanner, J. Mater. Chem. C, 2016, 4, 8611–8623 RSC.
- K. J. Laidler, J. Chem. Educ., 1984, 61, 494 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |