Open Access Article
Open Access ArticleInvestigating the tribological behavior of PEGylated MoS2 nanocomposites as additives in polyalkylene glycol at elevated temperature
Zhuang Xuab,
Wenjing Loua,
Xinhu Wuab,
Xiaobo Wang *a and
Junying Hao*a
*a and
Junying Hao*a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: wangxb@lzb.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, PR China
First published on 20th November 2017
Abstract
MoS2–polydopamine–methoxypolyethyleneglycol amine (MoS2–PDA–MGA) was synthesized through the combination of mussel-inspired chemistry and the Michael addition reaction. The modification of MoS2 via PDA and MGA enhanced its dispersion stability in oil. The tribological properties of MoS2–PDA–MGA for use as additives in polyalkylene glycol (PAG) base oil were investigated using an oscillating reciprocating tribometer at elevated temperatures. The results indicated that the addition of 0.7 wt% MoS2–PDA–MGA in PAG resulted in excellent friction-reducing and anti-wear (AW) performances compared with PAG base oil. The tribological results of MoS2–PDA–MGA and MoS2 dispersed in PAG base oil after settling for 7 days indicate that MoS2–PDA–MGA, with its good dispersion stability, has stable friction-reducing and AW properties. XPS analysis suggested that a protective boundary film formed on the wear surfaces during the friction and wearing process, which was believed to be responsible for the excellent tribological performance of MoS2–PDA–MGA dispersed in PAG at elevated temperature.
1. Introduction
Almost all equipment requires the use of lubricating oil. Additives play a very important role in lubricating oils and are used to improve, modify or impart desired properties in base oils. However, the rapid development of industrial equipment requires lubricants to operate under severe conditions, such as high temperatures and high loads. In recent years, extensive research has been carried out on the application of nanomaterials as friction-reducing and AW additives.1–5 Particularly, layered two-dimensional (2D) materials, such as molybdenum disulphide (MoS2), tungsten disulphide (WS2), hexagonal boron nitride (h-BN), and so on, have garnered tremendous attention in the lubrication field.6–11 These materials, based on weak van der Waals forces, are capable of sliding between layers, and the formation of a tribo-film on the interface has been emphasized as the key mechanism for decreasing friction and wear. Due to the excellent tribological properties, MoS2 and WS2 have shown great potential for lubrication applications. For instance, Alazemi et al. investigated sub-micrometre-sized carbon spheres coated with a MoS2 nanolayer (CS–MoS2) as an additive in conventional oil lubricants (SAE 5W30) to improve the tribological properties.12 Friction with 1 wt% CS–MoS2 particle-containing oil was 15–35% lower compared to the base oil. Aldana et al. explored the mechanism of WS2 nanoparticles with ZDDP additive in poly-alpha-olefin under the boundary lubrication regime. The results indicated that the AW performance of the ZDDP was enhanced when WS2 nanoparticles were added to the system.13Despite the excellent tribological properties of MoS2/WS2 nanomaterials, their development has been hindered by their poor dispersibility in lubricating oils. Fortunately, previous reports have shown that surface modification can improve the dispersion stability of nanomaterials in lubricating oil, and the modified nanomaterials exhibit significantly better friction-reducing and AW properties compared to the base oil.14–19 Chen et al. synthesized oil-soluble ultrathin MoS2 nanocomposites modified by oleylamine, and its addition could improve the load carrying property of liquid paraffin from less than 50 N to 2000 N at 120 °C.
Mussels display adhesive properties and have the ability to attach to almost all types of organic and inorganic surfaces, using dopamine (3,4-dihydroxy-phenylalanine) and other catechol compounds. Inspired by this property, dopamine undergoes oxidative self-polymerization under defined conditions, creating a polymerized layer on almost any type of material. Moreover, PDA film can act as a platform for easy and diverse secondary reactions to immobilize different functional groups.20 Recently, surface modification of MoS2-based materials by mussel-inspired dopamine chemistry and the Michael addition reaction has been studied.21,22 Modified MoS2 nanosheets consist of a core (MoS2) and PEG chains densely tethered by a chemical bond on the MoS2 surfaces. PEG contains polar groups and has good dispersibility in polar liquids, the special molecular structure allowed the modified MoS2 to disperse well in polar liquids. However, the potential of PEGylated MoS2 nanocomposites as additives for lubricating oils has never been demonstrated. Moreover, polyalkylene glycol (PAG) lubricants are unique among synthetic lubricants due to their high oxygen content and inherent polarity. More advanced technologies will demand the application of PAG synthetic lubricants. Consequently, it is very important to study the tribological properties of nanomaterial additives in PAG.
To demonstrate this concept, we first synthesized PEGylated MoS2 through a combination of mussel-inspired chemistry and the Michael addition reaction.22 The resulting product (MoS2–PDA–MGA) was characterized by various characterization techniques, and when dispersed in PAG, it demonstrated an excellent dispersion stability. Its tribological behaviors were studied by a ball-on-disk tribometer for steel/steel contact at elevated temperature. Furthermore, the lubrication mechanism of PAG lubricants with MoS2–PDA–MGA was explored by X-ray photoelectron spectroscopy (XPS).
2. Experimental
2.1. Materials
MoS2 was purchased from XFNANO Materials Tech Co., Ltd. (XF-184-1, Nanjing, China). Other feedstocks, including dopamine hydrochloride (DA, 98%), Tris-hydroxylmethylaminomethane (Tris, >99.9%), methoxypolyethylene glycol amine (MGA, MW: 2000 Da, 99.25%) were obtained from Aladdin (Shanghai, China). Polyalkylene glycol (PAG) was obtained from Weier Chemical Corporation (Nanjing, China), and its typical properties are presented in Table 1. Deionized (DI) water was used in the whole experiment process. All chemical reagents were used without any further purification.| Item | Value |
|---|---|
| Kinematic viscosity at 40 °C (mm2 s−1) | 98.64 |
| Kinematic viscosity at 100 °C (mm2 s−1) | 18.48 |
| Viscosity index (VI) | 209 |
| Pour point (°C) | −40 |
| Flash point (°C) | 220 |
| Acid value (mg KOH g−1) | 0.05 |
2.2. Synthesis of MoS2–PDA–MGA
MoS2–PDA–MGA was synthesized according to the procedures described by Zeng et al.,22 with some modifications.First, 300 mg MoS2 was dispersed in 100 ml of a 5 g l−1 dopamine solution. Then, 1 ml of 1 M Tris buffer solution was added and stirred for 6 h at room temperature. After that, the product was separated by centrifuging at 6000 rpm for 10 min. It was then washed with water and ethanol several times and dried under vacuum at 60 °C to obtain the MoS2–PDA (MoS2 coated with polydopamine (PDA) thin films).
Second, 200 mg of MoS2–PDA was first dispersed in 100 ml of Tris buffer solution (10 mM, pH = 8.5), 300 mg of MGA was added into the reaction system, and this reaction was carried out at room temperature (25 °C) for 12 h. The final product was separated by centrifuging at 6000 rpm for 10 min, washed with water and ethanol several times to remove the unreacted polymers and dried at 50 °C for 12 h. MoS2–PDA–MGA was obtained.
2.3. Characterizations and measurements
X-ray diffraction (XRD) patterns were collected by a Bruker AXS D8-ADVANCE diffractometer. The morphology of MoS2–PDA–MGA was obtained by a JEOL TEM-2100 Plus high-resolution transmission electron microscope (HRTEM) and a scanning electron microscope (SEM, HITACHI S-4800) equipped with an energy dispersive X-ray (EDX) detector. The Fourier transform infrared (FT-IR) spectra were collected on a Bruker Tensor 27 FT-IR spectrometer using the KBr disk method. The thermogravimetric analysis (TGA) was carried out on a TGA Netzsch STA 449 F5 instrument from ambient temperatures to approximately 650 °C with a heating rate of 10 °C min−1 in N2 flow. The dispersion stability of MoS2–PDA–MGA in PAG base oil was investigated using an ultraviolet-visible light (UV-vis) spectrophotometer (PERSEE, TU-1810) at different time intervals.The tribological properties of MoS2–PDA–MGA as an additive were evaluated by an Optimal SRV-IV oscillating reciprocating. The upper ball (ø 10 mm, AISI 52100 bearing steel, hardness 700 HV, surface roughness Ra = 25 nm) slides reciprocally against the stationary lower steel discs (AISI 52100 bearing steel, ø 24.00 × 7.88 mm, hardness of approximately 625 HV, surface roughness Ra = 41 nm). The wear volumes of the lower discs were measured using a MicroXAM-3D non-contact surface mapping profilometer. The morphology state and chemical composition of the wear scar surfaces were analysed by scanning electron microscopy (SEM, JSM-5600LV) and X-ray photoelectron spectroscopy (XPS, Thermos scientific, ESCALAB 250Xi).
3. Results and discussion
3.1. XRD, HR-TEM and EDS analysis of MoS2–PDA–MGA
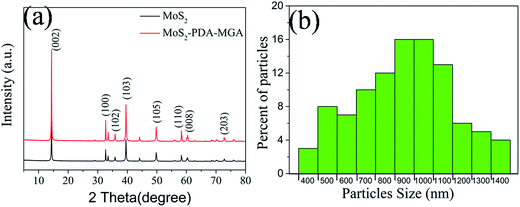
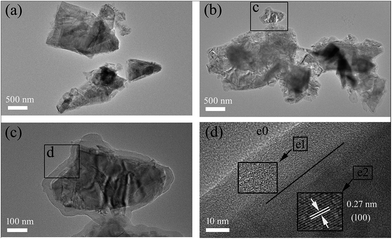
The XRD patterns of MoS2–PDA–MGA and MoS2 are shown in Fig. 1(a), and the characteristic peaks located at 14.25, 32.7, 35.95, 39.5, 49.8, 58.35, 60.36 and 72.8 are attributed to the (002), (100), (102), (103), (105), (110), (008) and (203) planes of MoS2, respectively (PDF37-1492). The peak of MoS2–PDA–MGA is consistent with MoS2. The size distribution of MoS2–PDA–MGA is shown in Fig. 1(b). The data were obtained by sampling a relevant number of particles and measuring the dimensions by FE-SEM. Their mean diameter is approximately 900 nm.The morphologies of MoS2 and MoS2–PDA–MGA were characterized by HRTEM, as shown in Fig. 2. Fig. 2(a) and (b) show the low-magnification TEM images, and Fig. 2(c) is the magnified TEM images from Fig. 2(b). Compared with the morphology of the MoS2, a thick organic film could be obviously observed in MoS2–PDA–MGA. Fig. 2(d) shows the HRTEM image of the latter, and it is clear that there are three regions with different lattice fringes: MoS2 (e2), organic layer (e1), and amorphous carbon film (e0). In addition, HRTEM displays the hexagonal lattice structure of the MoS2, giving a lattice spacing of 0.27 nm (inset in Fig. 2(d), the areas were marked by black box in Fig. 2(d)). Therefore, the results clearly show that the MoS2 have been successfully modified with PDA and MGA.
Furthermore, the EDS element mapping (Fig. 3) analysis of MoS2–PDA–MGA displayed a homogeneous distribution of elemental C, O, N, Mo and S. It is clear that C, O and N appeared on the MoS2–PDA–MGA. Thus, MoS2–PDA–MGA was successfully synthesized.
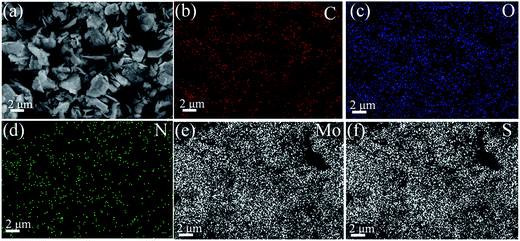 | ||
| Fig. 3 FE-SEM image (a) of MoS2–PDA–MGA and the corresponding element mapping for (b) C, (c) O, (d) N, (e) Mo and (f) S. | ||
3.2. FT-IR spectra and thermal properties of MoS2–PDA–MGA
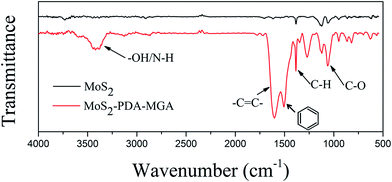
To further confirm structure and surface state of MoS2–PDA–MGA, it was characterized by FT-IR and TGA. The FT-IR spectra of MoS2 and MoS2–PDA–MGA are shown in Fig. 3 and the main absorption peaks of MoS2–PDA–MGA are all present in the IR spectra. Compared with the spectrum of the MoS2, the new absorption peak at approximately 3450 cm−1 corresponds to the –OH and N–H stretching vibration. The absorption bands located at 1500 cm−1 and 1600 cm−1 are attributed to the aromatic rings and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, suggesting that PDA has been grafted on the MoS2 surface. Moreover, the obvious peak at 1080 cm−1 assigned to the stretching vibration of C–O demonstrates that the polymer (MGA) is present on the surface of MoS2. Consequently, these results indicate that MoS2 was successfully modified with PDA and MGA.
C–, suggesting that PDA has been grafted on the MoS2 surface. Moreover, the obvious peak at 1080 cm−1 assigned to the stretching vibration of C–O demonstrates that the polymer (MGA) is present on the surface of MoS2. Consequently, these results indicate that MoS2 was successfully modified with PDA and MGA.
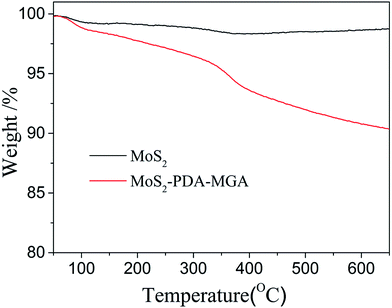
Fig. 4 shows their TGA curves. Moreover, the weight percentage of polymer on the surface of MoS2 was calculated. It could be found that the weight loss of MoS2 and MoS2–PDA–MGA is 0.9% and 10.1%, respectively. The figures show that the weight percentage of polymer is approximately 9.2%. It is obvious that MoS2 was modified with PDA and MGA (Fig. 5).
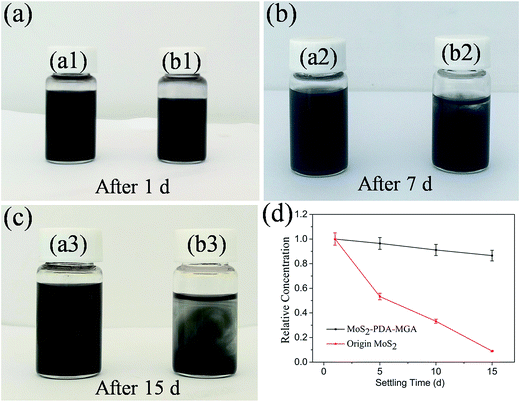
The dispersion stability of MoS2 and MoS2–PDA–MGA in PAG base oil has been estimated using pictures and UV-vis spectrophotometer. For comparison, a hybrid lubricant with 0.7 wt% MoS2 was prepared as well. As shown in Fig. 6(a3), MoS2–PDA–MGA retained good stability in PAG oils for two weeks after preparation. In contrast, the MoS2 dispersion had the apparent precipitation after settling for 7 days (Fig. 6(b2)). Moreover, a large amount of MoS2 precipitate was observed after settling for 15 days (Fig. 6(b3)). The variation of the relative concentration of the sample with time also demonstrates the excellent stability of MoS2–PDA–MGA in the base oil (Fig. 6(d)). After 15 days, the relative concentration of MoS2–PDA–MGA remained at 86.6%. However, the relative concentration of the MoS2 suspensions in the oil significantly decreased with increasing settling time and was only at 8.9% after 15 days. The results indicate that MoS2–PDA–MGA has better dispersion stability than MoS2.
3.3. Tribological properties of MoS2–PDA–MGA
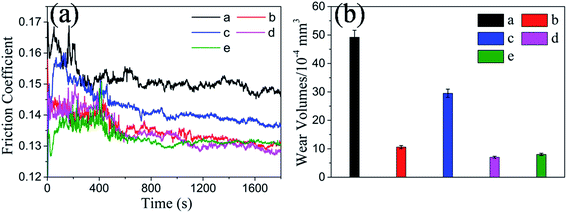
The friction-reducing and AW performances of MoS2–PDA–MGA as an additive in PAG were investigated for steel/steel contact under different loads at 150 °C. The duration for each test was 30 min, as shown in Fig. 7(a) and (b). The average friction coefficients decreased continuously with increasing MoS2–PDA–MGA content. When the content of MoS2–PDA–MGA reached to 0.7 wt%, the lubricant had the lowest average friction coefficient (Fig. 7(a)). However, further increasing the content above 0.7 wt% demonstrated that the friction coefficient could not be further improved. It can be concluded that, at a low concentration of MoS2–PDA–MGA, the lubricant cannot sufficiently form an adequate tribo-film to prevent direct contact of the friction pairs, thereby leading to a relatively high friction coefficient and high wear. When the additive concentration was 0.7 wt%, MoS2–PDA–MGA could form a sufficient tribo-film to provide optimum friction-reducing and AW properties. After the contact area becomes saturated with the tribo-film, further tribo-film formation would not be effective.23 The addition of 0.7 wt% MoS2–PDA–MGA could reduce the average friction coefficients to approximately 3.16%, 12.5% and 12.2% compared with the base oil under loads of 50 N, 100 N and 150 N, respectively. In addition, PAG containing 0.7 wt% MoS2–PDA–MGA also exhibits the lowest wear loss during the sliding process (Fig. 7(b)). In fact, the AW properties of the PAG base oil were improved by approximately 77.6%, 83.7% and 70.9% under loads of 50 N, 100 N and 150 N, respectively. Based on these values, it can be concluded that the addition of 0.7 wt% MoS2–PDA–MGA in PAG is the optimum amount to provide significant friction reduction and AW properties for steel/steel contacts under a load of 100 N at 150 °C. Moreover, the effects of PDA and MGA on the tribological properties of PAG were also investigated, the results indicate that the tribological properties of PAG containing 0.7 wt% organics (0.35 wt% PDA and 0.35 wt% MGA) are similar to those of the PAG base oil under a load of 100 N at 150 °C, with no obvious improvement (Fig. 7(a) and (b)).To investigate the effect of dispersion stability of nano-additives on the tribological properties, the tribological performance of MoS2–PDA–MGA and MoS2 dispersed in PAG base oil after settling over different time intervals were evaluated under a load of 100 N at 150 °C. All tests were carried out at an optimized concentration (0.7 wt%). As shown in (Fig. 8(a) and (b)), after settling 1 day, the friction coefficient and wear volumes of the MoS2–PDA–MGA and MoS2 dispersed in PAG base oil were similar. However, after settling for 7 days, the friction coefficient and wear volumes of MoS2 dispersed in PAG base oil increased significantly. In contrast, the friction coefficient and wear volumes of MoS2–PDA–MGA dispersed in PAG base oil were almost unchanged compared to the sample left to settle for 1 day. These results indicate that MoS2–PDA–MGA, with its good dispersion stability, has stable friction-reducing and AW properties after settling for 7 days. The excellent tribological performances of MoS2–PDA–MGA can be explained by the fact that MoS2–PDA–MGA, with its good dispersion stability in PAG base oil, is able to easily access the contact interface and form an effective film that leads to a low friction coefficient and low wear volumes.
Then, the friction-reducing and AW properties of PAG containing MoS2–PDA–MGA with optimized concentration (0.7 wt%) were further studied at various temperatures under a constant load of 100 N. As shown in Fig. 9(a), the average friction coefficient of 0.7 wt% MoS2–PDA–MGA added in PAG demonstrated almost no change compared to the base oil at 50 °C. However, 0.7 wt% MoS2–PDA–MGA in PAG dramatically improved the friction-reducing properties of the system as the temperature increased from 90 °C to 170 °C. For example, 0.7 wt% MoS2–PDA–MGA reduced the friction coefficient by approximately 6.52% at 90 °C, 10.73% at 130 °C, 12.5% at 150 °C, and 12.0% at 170 °C. Furthermore, increasing the temperature may play an active role in improving the friction-reducing of PAG with the additives.24 The addition of MoS2–PDA–MGA in PAG may form a lubrication film that maintains lower friction when the temperature is increased. Moreover, as shown in Fig. 9(b), compared with the base oil, the addition of 0.7 wt% MoS2–PDA–MGA could reduce the wear volume by approximately 35.3%, 77.4%, 81.3%, 82.1%, and 83.7% at 50 °C, 90 °C, 130 °C, 150 °C, and 170 °C, respectively. This result indicates that the percentage of wear volume reduction increased when the temperature increased from 50 °C to 170 °C. Considering the results mentioned above, MoS2–PDA–MGA added in PAG exhibits excellent friction-reducing and AW properties at elevated temperature, which might be due to the fact that raising the temperature played an active role in improving the tribological behavior of the base oil in the presence of nanoparticle additives.24 Under higher temperature, thermal activation may promote the chemical reaction between MoS2–PDA–MGA and the interfaces, which is more conducive to the formation of lubricating film.25
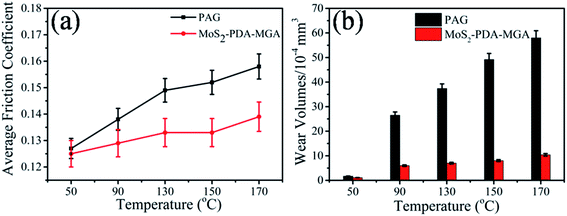 | ||
| Fig. 9 Average friction coefficient and wear volumes of the discs lubricated by PAG and PAG containing 0.7 wt% MoS2–PDA–MGA at various temperatures (SRV load, 100 N; stroke, 1 mm; frequency, 25 Hz). | ||
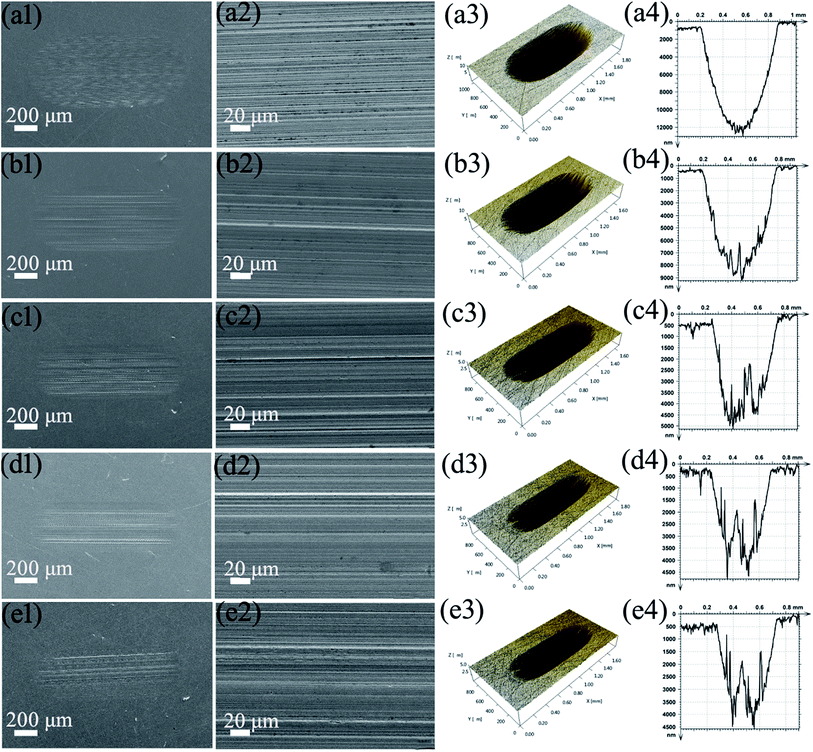
Fig. 10 shows SEM images and a 3D profile curve of the wear surfaces lubricated by PAG and PAG with different concentrations of MoS2–PDA–MGA under loads of 100 N at 150 °C. It could be seen that the wear scar is wide and deep when the wear surfaces were lubricated by only PAG base oil, which indicates that severe wear has occurred. The surface of the friction pair lubricated by the PAG lubricants containing MoS2–PDA–MGA was much narrower and much shallower than that lubricated with PAG base oil, especially when the percentage of MoS2–PDA–MGA is 0.7 wt%, the lowest wear is obtained. Those results are consistent with the results in Fig. 7(b). The results indicate that MoS2–PDA–MGA obviously enhanced the AW performance of PAG base oil, which is consistent with the wear volume results in Fig. 7(b). However, there were a few parallel grooves on the worn surfaces lubricated by PAG lubricants containing MoS2–PDA–MGA, which might be due to the presence of a few nanocomposite aggregates. When the MoS2–PDA–MGA were added into the PAG base oil, the additives tended to form a few aggregates and did not properly fill the furrows between the contact surfaces, and so, the parallel grooves increased in depth.25,26
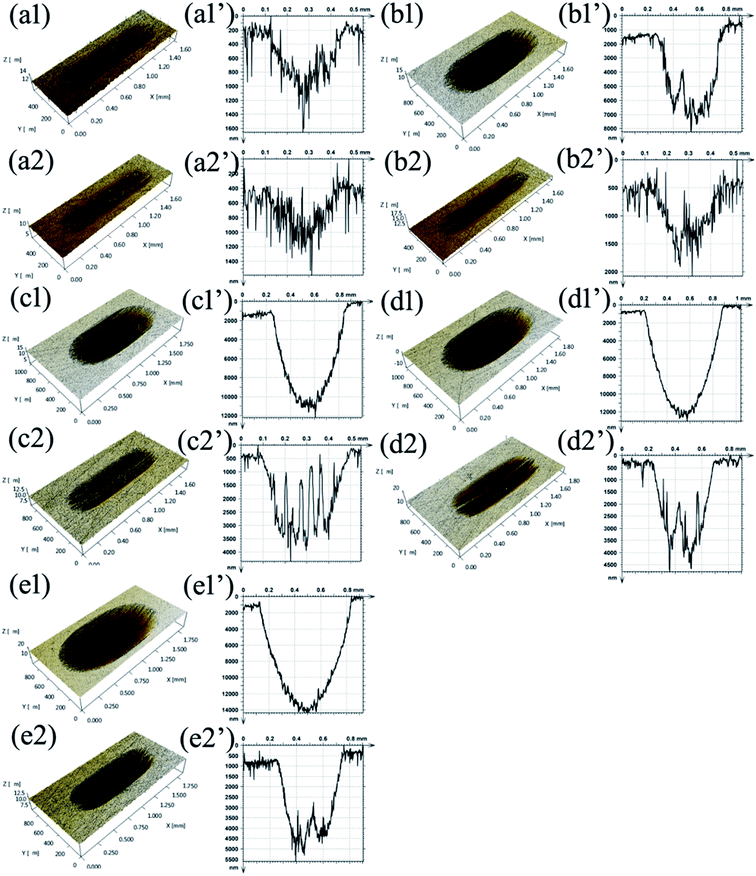
Fig. 11 also shows the 3D morphologies and 3D profile curves of wear scars lubricated by PAG and PAG containing 0.7 wt% MoS2–PDA–MGA at various temperatures under a load of 100 N. It is clear that the PAG lubricants containing MoS2–PDA–MGA displayed smaller and shallower wear scars than the PAG base oil at elevated temperature. This result further confirms that the addition of MoS2–PDA–MGA significantly enhanced the AW property of PAG base oil.
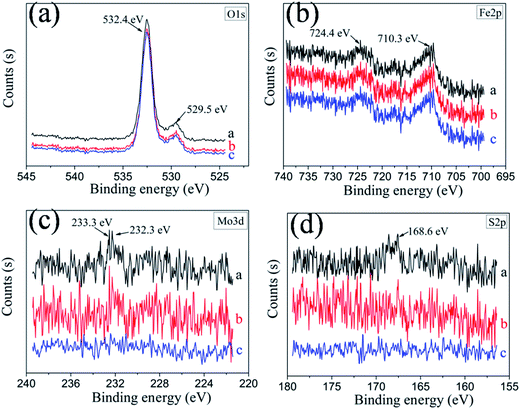
The worn surface of the discs lubricated by PAG containing 0.7 wt% MoS2–PDA–MGA or MoS2 (0.7 wt%) after different settling time intervals were characterized by XPS to explore the friction-reducing and AW mechanism. The chemical composition of the wear scar is shown in Fig. 12. After settling for 1 day, compared with the worn surface lubricated by MoS2–PDA–MGA, the XPS spectra of the worn surfaces lubricated by MoS2 showed no significant difference. However, after settling for 7 days, the peaks of Mo 3d and S 2p of the worn surfaces lubricated by MoS2 were significantly reduced. This result corresponds to the results that show the significant increase in the friction coefficient and wear volumes of MoS2 dispersed in PAG base oil after settling 7 days (Fig. 8). The XPS peaks of O 1s were located at approximately 529.5 eV and 532.4 eV (Fig. 12(a)), corresponding to FeO or Fe2O3, FeOOH, FeSO4 or Fe2(SO4)3, and C–O bonding.27 The Fe 2p peaks appeared at binding energies of 710.3 eV and 724.4 eV (Fig. 12(b)), demonstrating that Fe2O3 and Fe2(SO4)3 formed during the tribo-chemical reactions. The peaks at 233.3 eV and 232.3 eV of the friction surface are attributed to Mo 3d of MoS2 and MoO3 (Fig. 12(c)), indicating that MoS2–PDA–MGA was absorbed on the friction pair surfaces and were partially oxidized during the sliding process to form MoO3. The peaks of S 2p emerged at the binding energy of 168.6 eV (Fig. 12(d)),28 which proves that FeSO4 or Fe2(SO4)3 was formed at the friction surface. These results confirmed that MoS2–PDA–MGA, with its good dispersion stability, has stable friction-reducing and AW properties. XPS results suggest the deposition of MoS2–PDA–MGA lamellae. Deposited MoS2–PDA–MGA is speared on the contact interfaces and the weak van der Waals interaction between atomic lamellae of MoS2–PDA–MGA facilitates their sliding on the friction interfaces. Therefore, MoS2–PDA–MGA plays an important role in friction-reducing and AW properties.11 On the other hand, MoS2–PDA–MGA participated in a tribo-chemical reaction during the sliding process, and the boundary adsorption film and tribo-chemical reaction film were formed on the worn surfaces of the discs. The lubrication film can improve the friction-reducing and AW properties of PAG base oil.
4. Conclusions
MoS2–PDA–MGA was synthesized through the combination of mussel-inspired chemistry and the Michael addition reaction. This modification could be used as an efficient method for improving the dispersion stability of MoS2 in PAG base oil. The tribological results illustrated that the addition of 0.7 wt% MoS2–PDA–MGA in PAG was the optimum content to provide significant friction-reducing and AW properties for steel/steel contacts under a load of 100 N at 150 °C. The tribological results of MoS2–PDA–MGA and MoS2 dispersed in PAG base oil after settling for 7 days indicate that MoS2–PDA–MGA, with its good dispersion stability, has stable friction-reducing and AW properties. The tribological testing results demonstrated that MoS2–PDA–MGA was an effective additive for improving the friction-reducing and AW performance of PAG oil at various temperatures, especially at elevated reaction temperatures. XPS analysis indicated that a boundary lubrication film composed of FeO or Fe2O3, FeOOH, FeSO4 or Fe2(SO4)3, MoS2, and C–O binding formed on the worn surface, contributing to the excellent friction-reducing and AW behaviors of MoS2–PDA–MGA as an additive in PAG base oil at elevated temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for financial support of this work by National Key Basic Research Program of China (973 Program) (No. 2013CB632301) and National Natural Science Foundation of China (NSFC 51475445 and 51775536).References
- Z. Z. Wu, D. Z. Wang, Y. Wang and A. K. Sun, Adv. Eng. Mater., 2010, 12, 534–538 CrossRef CAS.
- A. Greco, K. Mistry, V. Sista, O. Eryilmaz and A. Erdemir, Wear, 2011, 271, 1754–1760 CrossRef CAS.
- G. Y. Bai, J. Q. Wang, Z. G. Yang, H. G. Wang, Z. F. Wang and S. R. Yang, RSC Adv., 2014, 4, 47096–47105 RSC.
- M. Gulzar, H. H. Masjuki, M. Varman, M. A. Kalam, R. A. Mufti, N. W. M. Zulkifli and R. Yunus, Tribol. Int., 2015, 88, 271–279 CrossRef CAS.
- N. Salah, M. S. Abdel wahab, A. Alshahrie, N. D. Alharbi and Z. H. Khan, RSC Adv., 2017, 7, 40295–40302 RSC.
- W. Li, Z. L. Cheng and Z. Liu, RSC Adv., 2016, 6, 110866–110873 RSC.
- M. Kalin, J. Kogovšek and M. Remškar, Wear, 2012, 280, 36–45 CrossRef.
- I. Lahouij, B. Vacher, J. M. Martin and F. Dassenoy, Wear, 2012, 296, 558–567 CrossRef CAS.
- L. N. Zhu, C. B. Wang, H. D. Wang, B. S. Xu, D. M. Zhuang, J. J. Liu and G. L. Li, Appl. Surf. Sci., 2012, 258, 1944–1948 CrossRef CAS.
- Y. B. Zhang, C. H. Li, D. Z. Jia, D. K. Zhang and X. W. Zhang, J. Cleaner Prod., 2015, 87, 930–940 CrossRef CAS.
- S. Kumari, R. Gusain, N. Kumar and O. Khatri, J. Ind. Eng. Chem., 2016, 42, 87–94 CrossRef CAS.
- A. Alazemi, A. Dysart, X. L. Phuah, V. Pol and F. Sadeghi, Carbon, 2016, 110, 367–377 CrossRef CAS.
- P. U. Aldana, B. Vacher, T. L. Mogne, M. Belin, B. Thiebaut and F. Dassenoy, Tribol. Int., 2014, 56, 249–258 CAS.
- Z. Chen, X. W. Liu, Y. H. Liu, S. Gunsel and J. B. Luo, Sci. Rep., 2015, 5, 12869–12876 CrossRef CAS PubMed.
- Z. Y. Xu, Y. Xu, K. H. Hu, Y. F. Xu and X. G. Hu, Tribol. Int., 2015, 81, 139–148 CrossRef CAS.
- Q. M. Wan, Y. Jin, P. C. Sun and Y. L. Ding, J. Nanopart. Res., 2014, 16, 2386–2395 CrossRef.
- S. Kumari, O. P. Sharma, R. Gusain, H. P. Mungse, A. Kukrety, N. Kumar, H. Sugimura and O. P. Khatri, ACS Appl. Mater. Interfaces, 2015, 7, 3708–3716 CAS.
- P. F. Du, G. X. Chen, S. Y. Song, H. L. Chen, J. Li and Y. Shao, Tribol. Int., 2016, 62, 29–38 Search PubMed.
- L. Liu, Z. B. Huang and P. Huang, Tribol. Int., 2016, 104, 303–308 CrossRef CAS.
- S. K. Madhurakkat Perikamana, J. K. Lee, Y. B. Lee, Y. M. Shin, E. J. Lee, A. G. Mikos and H. Shin, Biomacromolecules, 2015, 16, 2541–2555 CrossRef CAS PubMed.
- K. Chen, X. Q. Xu, J. W. Guo, X. L. Zhang, S. L. Han, R. B. Wang, X. H. Li and J. X. Zhang, Biomacromolecules, 2015, 16, 3574–3583 CrossRef CAS PubMed.
- G. J. Zeng, M. Y. Liu, X. H. Liu, Q. Huang, D. Z. Xu, L. C. Mao, H. Y. Huang, F. J. Deng, X. Y. Zhang and Y. We, Appl. Surf. Sci., 2016, 387, 399–405 CrossRef CAS.
- X. J. Zheng, Y. F. Xu, J. Geng, Y. B. Peng, D. Olson and X. G. Hu, Tribol. Int., 2016, 102, 79–87 CrossRef CAS.
- X. H. Wu, G. Q. Zhao, Q. Zhao, K. L. Gong, X. B. Wang, W. M. Liu and W. S. Liu, RSC Adv., 2016, 101, 98606–98610 RSC.
- J. Zhao, Y. Y. He, Y. F. Wang, W. Wang, L. Yan and J. B. Luo, Tribol. Int., 2016, 97, 14–20 CrossRef CAS.
- A. Moshkovith, V. Perfiliev, I. Lapsker, N. Fleischer, R. Tenne and L. Rapoport, Tribol. Int., 2006, 24, 225–228 CAS.
- X. H. Wu, J. M. Liu, Q. Zhao, M. Zhang, G. Q. Zhao and X. B. Wang, ACS Sustainable Chem. Eng., 2015, 3, 2281–2290 CrossRef CAS.
- K. H. Hu, X. G. Hu, Y. F. Xu, F. Huang and J. S. Liu, Tribol. Int., 2010, 40, 225–228 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |