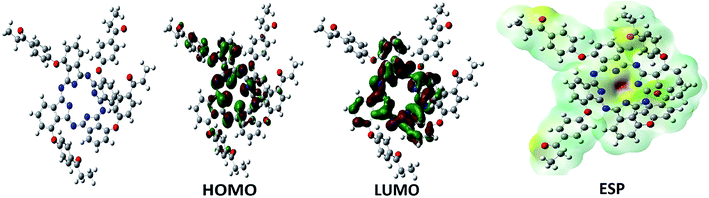Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel type ketone-substituted metallophthalocyanines: synthesis, spectral, structural, computational and anticancer studies
Ceylan Hepokura,
Armağan Günselb,
M. Nilüfer Yarasir b,
Ahmet T. Bilgiçli
b,
Ahmet T. Bilgiçli *b,
Burak Tüzünc,
Gamze Tüzüna and
İlhan Yaylimd
*b,
Burak Tüzünc,
Gamze Tüzüna and
İlhan Yaylimd
aFaculty of Pharmacy, Department of Basic Pharmaceutical Sciences, Division of Biochemistry, Cumhuriyet University, Sivas, Turkey
bDepartment of Chemistry, Sakarya University, TR54187 Serdivan, Sakarya, Turkey. E-mail: abilgicli@sakarya.edu.tr; Tel: +90-264-295-71-16
cDepartment of Chemistry, Cumhuriyet University, Sivas, Turkey
dInstitute of Experimental Medicine, Department of Molecular Medicine, Istanbul, Turkey
First published on 13th December 2017
Abstract
This work reports on the synthesis and characterization of phthalocyanines (M = Cu(II) (2), Zn(II) (3) In(III) (4) and Co(II) (5)) peripherally tetra-substituted with 1-(4-hydroxyphenyl)propan-1-one. Confirmation of the synthesized phthalocyanine structures are performed with a combination of elemental analysis, FTIR, 1H-NMR, 13C-NMR, UV-vis and MALDI-MS SEM and spectral data. Their aggregation properties were examined in THF by UV-vis. Spectral and photophysical (fluorescence quantum yield) properties of complexes (2–4) were reported in THF (tetrahydrofuran). These results suggest that the metal in the core of the phthalocyanine plays an important role in the fluorescence quantum yields ΦF of the synthesized complexes (2–4). Also, the anticancer activities of complexes were studied on MCF-7, MG63, and L929 cell lines. Finally, all synthesized phthalocyanines were investigated by quantum chemical studies. Chemical reactivity parameters such as EHOMO, ELUMO, ΔE (HOMO–LUMO energy gap) were calculated by Gaussian software.
1. Introduction
Cancer is a multi-step process in which cells undergo metabolic and behavioral changes leading to excessive and timeless proliferation. Cancer begins with rapid proliferation of cells and loss of apoptosis functions. According to the International Cancer Research Institute (IARC), an average of 14 million people are diagnosed with cancer each year and 8.2 million people die. It is estimated that 12 million people will lose their lives due to cancer in 2030. There are three basic treatment methods for cancer treatment, namely surgery, chemotherapy and radiotherapy. These three treatment methods are applied to cancer patients in combination with each other, depending on the type of cancer, its stage, its location, the general health of the patient, the sex of the patient, and some other factors.1Phthalocyanines which are synthetic analogues of porphyrin compounds such as hemoglobin and chlorophyll, have visible optical properties and good thermal stability because of having an 18-π aromatic macrocycle.2 Since the discovery of phthalocyanines (Pcs), many scientists have aimed to synthesize/design optimal molecules for a variety of applications such as chemical sensors, display devices, catalysts, biological imaging, and photosensitizers for photodynamic therapy (PDT).3–7 They have been explored to be effective photosensitizers for PDT owing to the strong absorption in the red visible region and high efficiency in producing reactive oxygen species (ROS), e.g. singlet oxygen (1O2), leading to destruction of cancer cells. The variety of metal ion that is inserted to the inner core of phthalocyanines strongly influences the ROS production.8 Especially, scientists have aimed to synthesize the phthalocyanines (Pc) that have diamagnetic ions (Zn2+, Si4+, In3+, Ga3+, etc.) because of high ROS yields for PDT9 such as gallium phthalocyanines (GaPcs)10 and indium phthalocyanines (InPcs).11 The enhancement of the solubility in organic solvents is very indispensable in the applications of phthalocyanine complexes.12 For this, it is necessary to attach some functional groups at the peripheral regions of phthalocyanine ring and/or insert some metal ions to the inner core of phthalocyanines [such as Mn(III), In(III) and Ga(III)]. The features like solubility, aggregation, absorption, photophysical and photochemical strongly depend on the functional groupsat the peripheral and/or nonperipheral regions of phthalocyanines.13,14
George and et al. conjugated Rubus–phthalocyanine apply to MCF-7 cell line for 24 h. Decreased cell viability was observed from 90% to 60%.15 Horne et al. the novel metallo-phthalocyanine was apply to MCF-7 cell line. They were found overall morphology averaging a viability of >85%.16 Ogbodu et al. studied new zinc phthalocyanine–spermine-single walled carbon nanotube conjugate on MCF-7 breast cancer cell line. When dosed at 40 μM cell viability decreased from 100% to 75%.17
Aliphatic and aromatic ketones are the compounds having reactive carbonyl group. They have characteristic flavors, aromas and therapeutic properties. Many synthetic and naturally formed ketones are used in cosmetics, perfumes, food additives, and are important in a variety of medical and biological materials such as antimicrobial activity.14,18,19
Pharmaceutical industries spend billions of dollars for the development of effective agents in the diagnosis and treatment of cancer each year. Therefore, many organic based cytotoxic agents have been discovered, and they are extensively applied for treatment of cancer.20
In this paper, we have firstly submitted the synthesis and characterization of phthalocyanines (M = Cu(II) (2), Zn(II) (3), In(III) (4) and Co(II) (5)) substituted with 1-(4-hydroxyphenyl)propan-1-one at the non-peripheral regions to compare the effect of different metals and the functional group “aromatic ketone” in a variety of medical and biological materials such as new agent for cancer therapeutic. Also, spectral and photophysical (fluorescence quantum yield) properties of phthalocyanine complexes (2–4) were reported in THF (tetrahydrofuran). Additionally, the synthesized phthalocyanines were investigated by quantum chemical studies such as EHOMO, ELUMO, ΔE (HOMO–LUMO energy gap). Finally, the synthesized phthalocyanines were evaluated for anticancer activity on MCF-7, MG63, and L929 cancer cell lines.
2. Materials and methods
2.1. Chemicals
Dimethylsulfoxide (DMSO), Cell Proliferation Kit II (XTT), Dulbecco's Modified Eagle's Medium-high glucose from Sigma (St. Louis, MO, USA). Penicillin, streptomycin and trypsin from Gibco (Paisley, England), Eagle's Minimum, fetal bovine serum (FBS) from Biochrom (Berlin, Germany), phosphate buffer saline (PBS) tablet from Medicago (Uppsala, Sweden). Some chemicals such as chloroform (CHCl3), dichloromethane (CH2Cl2), tetrahydrofuran (THF), dimethylformamide (DMF), dimethyl sulfoxide (DMSO), 4-(4-hydroxyphenyl)butan-2-one, 1-(4-hydroxyphenyl)propan-1-one, CoCI2, CuCl2, Zn(Ac)2 and InCl3 were acquired from Merck and Alfa Aesar and used as received. Cell Proliferation Kit II (XTT), Dulbecco's Modified Eagle's Medium-high glucose from Sigma (St. Louis, MO, USA). Penicillin, streptomycin and trypsin from Gibco (Paisley, England), Eagle's Minimum, fetal bovine serum (FBS) from Biochrom (Berlin, Germany), phosphate buffer saline (PBS) tablet from Medicago (Uppsala, Sweden) were purchased. UV-vis spectra was acquired in a quartz cuvette on an Agilent Model 8453 diode array spectrophotometer. Fluorescence measurement were recorded by Hitachi S-7000 fluorescence spectrophotometer. Perkin-Elmer spectrum two FT-IR spectrophotometer was used to take FT-IR spectra. All the products were purified by chromatography on silica gel (Merck grade 60) from Aldrich. All reactions were achieved under a dry N2 atmosphere. A Bruker 300 spectrometer instruments was used to take 1H-NMR and 13C-NMR spectras. Mass spectras (MS) were analyzed by MALDI SYNAPT G2-Si Mass Spectrometry.2.2. Synthesis
The product was soluble in CHCl3, CH2Cl2, THF, DMF and DMSO. Yield of (1): 1.17 g (65%) mp 113 °C. Anal. calcd for (%) C17H12N2O2 (276.29 g mol−1): C, 73.90; H, 4.38; N, 10.14; found: C, 74.11; H, 4.52; N, 10.93. FT-IR (νmax/cm−1): 3079, 3092 (H-Ar), 2982, 2939 (H-Aliph.), 2234 (CN), 1681 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1570 (Ar C
O), 1570 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1161 (Ar-O-Ar). 1H-NMR (CDCI3): 7.98–7.85 (m, 4H), 7.81 (t, 1H), 7.45–7.30 (d, 2H), 3.12 (m, 2H), 1.18 (t, 3H); 13C-NMR (CDCI3) δ: 203.5, 162.4, 157.3, 139.6, 134.1, 132.1, 124.5, 124.2, 120.7, 118.2, 116.4, 116.0, 108.7, 33.2, 8.2; MS [m/z]: 315.88 [M + K]+.
C), 1161 (Ar-O-Ar). 1H-NMR (CDCI3): 7.98–7.85 (m, 4H), 7.81 (t, 1H), 7.45–7.30 (d, 2H), 3.12 (m, 2H), 1.18 (t, 3H); 13C-NMR (CDCI3) δ: 203.5, 162.4, 157.3, 139.6, 134.1, 132.1, 124.5, 124.2, 120.7, 118.2, 116.4, 116.0, 108.7, 33.2, 8.2; MS [m/z]: 315.88 [M + K]+.
2.2.2.1. 1(4),8(11),15(18),22(25)-Tetrakis (1-(4-hydroxyphenyl)propan-1-one)-phthalocyaninato copper(II) (2). The compound is excellent soluble in THF, CHCl3, CH2Cl2, DMF, DMSO. Yield of (2): 0.043 g, (37%). Anal. calcd for (%) C72H56CuN8O8 (1168.70 g mol−1): C, 69.88; H, 4.14; Cu, 5.44; N, 9.59; O, 10.95; found: C, 70.02; H, 4.05; N, 9.42. FT-IR (νmax/cm−1): 3066, 3030 (H-Ar), 2972, 2936, 2865 (H-Aliph.), 1733 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1681 (Ar C
O), 1681 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1164 (Ar-O-Ar). UV-vis (THF): λmax/nm (log
C), 1164 (Ar-O-Ar). UV-vis (THF): λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 687 (4.71), 621 (4.11), 317 (4.42). 1H-NMR (DMSO-d6) (δ, ppm): 8.95–8.41 (m, 12H, Pc-Ar-H), 7.68–7.11 (m, 16H, Ar-H), 3.82–2.23 (16H, m, –CH2), 1.39 (12H, s, –CH3). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1169.40 [M + H]+.
ε): 687 (4.71), 621 (4.11), 317 (4.42). 1H-NMR (DMSO-d6) (δ, ppm): 8.95–8.41 (m, 12H, Pc-Ar-H), 7.68–7.11 (m, 16H, Ar-H), 3.82–2.23 (16H, m, –CH2), 1.39 (12H, s, –CH3). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1169.40 [M + H]+.
2.2.2.2. 1(4),8(11),15(18),22(25)-Tetrakis (1-(4-hydroxyphenyl)propan-1-one)-phthalocyaninato zinc(II) (3). The compound is excellent soluble in THF, CHCl3, CH2Cl2, DMF, DMSO. Yield of (3): 0.032 g, (32%). Anal. calcd for (%) C68H48ZnN8O8 (1170.57 g mol−1): C, 69.77; H, 4.13; N, 9.57; O, 10.93; Zn, 5.59; found: C, 69.10; H, 4.91; N, 9.85. FT-IR (νmax/cm−1): 3066 (H-Ar), 2939, 2978 (H-Aliph.), 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590 (Ar C
O), 1590 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1161-2 (Ar-O-Ar). UV-vis (THF): λmax/nm (log
C), 1161-2 (Ar-O-Ar). UV-vis (THF): λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 689 (4.79), 624 (4.21), 329 (4.56). 1H-NMR (DMSO-d6) (δ, ppm): 8.97–8.50 (m, 12H, Pc-Ar-H), 7.76–7.23 (m, 16H, Ar-H), 3.88–2.35 (8H, m, –CH2), 1.35 (12H, s, –CH3). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1172.01 [M + H]+.
ε): 689 (4.79), 624 (4.21), 329 (4.56). 1H-NMR (DMSO-d6) (δ, ppm): 8.97–8.50 (m, 12H, Pc-Ar-H), 7.76–7.23 (m, 16H, Ar-H), 3.88–2.35 (8H, m, –CH2), 1.35 (12H, s, –CH3). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1172.01 [M + H]+.
2.2.2.3. 1(4),8(11),15(18),22(25)-Tetrakis (1-(4-hydroxyphenyl)propan-1-one)-phthalocyaninato indium(III) chloride (4). The compound is excellent soluble in THF, CHCl3, CH2Cl2, DMF, DMSO. Yield of (4): 0.034 g, (31%). Anal. calcd for (%) C68H48ClInN8O8 (1255.43 g mol−1): C, 65.06; H, 3.85; Cl, 2.82; In, 9.15; N, 8.93; O, 10.20; found: C, 65.22; H, 3.82; N, 9.02; FT-IR (νmax/cm−1): 3068 (H-Ar), 2977, 2938 (H-Aliph.), 1726 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1682, 1591 (Ar-C
O), 1682, 1591 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1162 (Ar-O-Ar). UV-vis (THF): λmax/nm (log
C), 1162 (Ar-O-Ar). UV-vis (THF): λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 702 (4.21), 639 (3.56), 318 (4.09). 1H-NMR (DMSO-d6) (δ, ppm): 9.12–8.73 (m, 12H, Pc-Ar-H), 7.87–6.98 (m, 16H, Ar-H), 3.09–2.37 (8H, m, –CH2), 1.69 (12H, s, –CH3). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1221.05 [M − CI + H]+.
ε): 702 (4.21), 639 (3.56), 318 (4.09). 1H-NMR (DMSO-d6) (δ, ppm): 9.12–8.73 (m, 12H, Pc-Ar-H), 7.87–6.98 (m, 16H, Ar-H), 3.09–2.37 (8H, m, –CH2), 1.69 (12H, s, –CH3). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1221.05 [M − CI + H]+.
2.2.2.4. 1(4),8(11),15(18),22(25)-Tetrakis (1-(4-hydroxyphenyl)propan-1-one)-phthalocyaninato cobalt(II) (5). The compound is excellent soluble in THF, CHCl3, CH2Cl2, DMF, DMSO. Yield of (5): 0.049 g, (41%). Anal. calcd for (%) C72H56CoN8O8 (1164.09 g mol−1): C, 70.16; H, 4.16; Co, 5.06; N, 9.63; O, 11.00; found: C, 69.98; H, 4.27; N, 9.51; FT-IR (νmax/cm−1): 3062 (H-Ar), 2934, 2865 (H-Aliph.), 1730 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1677 (Ar-C
O), 1677 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1163 (Ar-O-Ar). UV-vis (THF): λmax/nm (log
C), 1163 (Ar-O-Ar). UV-vis (THF): λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 692 (4.71), 618 (4.20), 322 (4.63). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1165.98 [M + H]+.
ε): 692 (4.71), 618 (4.20), 322 (4.63). MS (MALDI-MS, 2,5-dihydroxybenzoic acid as matrix): 1165.98 [M + H]+.
2.3. Cell lines
Human breast adenocarcinoma (MCF-7), human bone osteosarcoma (MG63), and mouse fibroblast (L929) cell lines were supplied by the American Type Culture Collection (ATCC). Cell culture studies of human breast adenocarcinoma cell line (MCF-7), human bone osteosarcoma (MG63), and mouse fibroblast (L929) appropriate conditions and materials required in order to reproduce broth must be provided. In our study 10% fetal bovine serum (FBS), 1% L-glutamine, 100 IU per mL penicillin and 10 mg mL−1 streptomycin in DMEM (low glucose) were used. Cancer cell lines in DMEM medium was produced in 95% humidity and 5% CO2 incubator at 37 °C.2.4. Cytotoxic assay of the synthesized compounds
XTT test which was monitor azo aldehyde thioureas derivatives toxicity was applied on cancer cells. Firstly, the cancer cells were plated in 96-well plate (10 × 104 cells in each well) and the compounds were tested by 4-fold diluting the starting mixture at four different concentration ranging from (12.5; 50; 75; 100 μM) 24 and 48 h. Secondly, for determination of living cells 50 μL XTT reagents were added to each well. After 4 h, at 450 nm micro plate reader was used for absorbance measurements. Finally, the percentage of cell viability was calculated.Anticancer activity of 6 azo chromophore containing new compounds were evaluated in breast cancer cell line and fibroblast cell line was used as control. Cells were exposed to four (12.5; 25; 50; 100 μM) concentration range. XTT protocol was applied after 24 and 48 h and compared with control cell lines. The cell viability of control has been accepted as 100% in order to determined cell viability of compounds. When compounds of [NiL]·H2O, [CoL]·2H2O, [ZnL], [CuL]·3H2O and as 24 and 48 h post-treatment the comparison of the antiproliferative effects were observed to be a more effective treatment for 24 h. If the compounds 48 h after treatment was observed that more antiproliferative effect.
2.5. Statistical analysis
Data were expressed in the form of arithmetic mean ± standard deviation (x ± SD). One way ANOVA and post hoc Tukey analyses were used to reveal the relationships between groups. The differences were accepted as significant for p < 0.05. Statistical analysis were performed with SPSS for windows 22.0 package. All determinations were computed three times.2.6. Computational methodology
With the help of Density Functional Theory, chemical reactivity indices such as chemical hardness (η), electronegativity (χ), chemical potential (μ) are defined as derivatives of the electronic energy (E) and a constant external potential ν(r).25–31 In the conceptual this theory, mentioned chemical properties are given as32,33
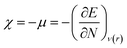 | (1) |
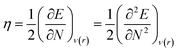 | (2) |
In the conceptual Koopman's theorem,34 the negative of the highest occupied molecular orbital energy and negative of the lowest unoccupied molecular orbital energy corresponds to electron affinity and ionization energy (−ELUMO = A and −EHOMO = I). As a result of Koopman's theorem, chemical potential and chemical hardness can be explained as:35
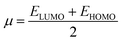 | (3) |
 | (4) |
According to Pearson, the global softness is defined as the inverse of the global hardness and this quantity is given as:36,37
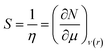 | (5) |
Parr et al. by which the global electrophilicity index (ω) introduced are defined via eqn (6). With the help of this index, electrophilic power of a chemical compound is associated with its electronegativity and chemical hardness. Nucleophilicity (ε) which is the inverse of the electrophilicity, is given via eqn (7).
 | (6) |
| ε = 1/ω | (7) |
3. Results and discussion
3.1. Synthesis and spectroscopic characterization
The starting compounds, 3-(4-propionylphenoxy)phthalonitrile (1) were synthesized from 4-(4-hydroxyphenyl)butan-2-one and 3-nitrophthalonitrile (Scheme 1). The reaction was achieved through base-catalyzed aromatic displacement under N2 atmosphere by using K2CO3 as the base in dry DMF at 45 °C in 72 hours. The formation of the cobalt, copper, zinc and indium metallophthalocyanines (2, 5) were achieved by cyclotetramerisation reaction of the starting compounds (1) in n-hexanol and DBU using anhydrous CoCI2 or CuCl2 or Zn(Ac)2 or InCl3 salts (Scheme 1). The synthesized phthalocyanines (2–5) were purified by chromatography over a silica gel column by CH2Cl2/ethanol 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture as eluent. All phthalocyanine compounds were soluble common organic solvents such as THF, CHCl3, CH2Cl2, DMF, DMSO. After the cyclotetramerization reaction, the disappearance of the sharp –C
1 mixture as eluent. All phthalocyanine compounds were soluble common organic solvents such as THF, CHCl3, CH2Cl2, DMF, DMSO. After the cyclotetramerization reaction, the disappearance of the sharp –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N vibration at around 2234 cm−1 for (1) proved the formation of the phthalocyanines (2–5) (Fig. 1). UV-vis spectra of the phthalocyanine complexes (2–5) exhibited characteristic absorptions in the Q-band region at around 680–700 nm, attributed to the π–π* transition from the HOMO (highest occupied molecular orbital) to the LUMO (lowest unoccupied molecular orbital) of the Pc2− ring, and in the B band region (UV region) at around 350–360 nm, arising from the deeper π–π* transitions. The Q-band absorptions of π–π* transition for all phthalocyanines (2–5) in THF were observed as a single band of high intensity at 687 nm (Q) for (2), 689 nm (Q) for (3), 702 nm (Q) for (4), 683 nm (Q) for (5) (Fig. 2). 3-(4-Propionylphenoxy)phthalonitrile (1) was also characterized by Mass Spectrometry. Mainly protonated ion peak of (1) was at high intensity beside the potassium adduct peak which was compatible with calculated values for (1) (Fig. 3).
N vibration at around 2234 cm−1 for (1) proved the formation of the phthalocyanines (2–5) (Fig. 1). UV-vis spectra of the phthalocyanine complexes (2–5) exhibited characteristic absorptions in the Q-band region at around 680–700 nm, attributed to the π–π* transition from the HOMO (highest occupied molecular orbital) to the LUMO (lowest unoccupied molecular orbital) of the Pc2− ring, and in the B band region (UV region) at around 350–360 nm, arising from the deeper π–π* transitions. The Q-band absorptions of π–π* transition for all phthalocyanines (2–5) in THF were observed as a single band of high intensity at 687 nm (Q) for (2), 689 nm (Q) for (3), 702 nm (Q) for (4), 683 nm (Q) for (5) (Fig. 2). 3-(4-Propionylphenoxy)phthalonitrile (1) was also characterized by Mass Spectrometry. Mainly protonated ion peak of (1) was at high intensity beside the potassium adduct peak which was compatible with calculated values for (1) (Fig. 3).
3.2. Aggregation studies
The aggregation of phthalocyanines affects their application properties such as photophysical, electrochemical and spectroscopic properties. So, the investigation of aggregation properties of phthalocyanine are important. Aggregation is dependent on the nature of the solvent, concentration, nature of substituents, complex metal ions and temperature.38 In this study, the aggregation behaviors of (2–5) were examined in THF (Fig. 4). All synthesized phthalocyanines did not show any aggregation behaviors in THF. When the concentrations of the Pc increased, the intensity of absorption of the Q-band also increased (the Lambert–Beer law was obeyed), and new blue or red shifted band formation were not observed. | ||
| Fig. 4 Absorbance changes of the phthalocyanines (2–5) in THF at different concentrations (inset: plot of absorbance versus concentration). | ||
3.3. Fluorescence measurements
Fluorescence quantum yields were calculated using eqn (8)
 | (8) |
The fluorescence behaviors of (2–4) were studied in THF. The absorption, fluorescence emission and excitation spectra for (2–4) were shown in Fig. 5. Fluorescence emission peaks were observed at 705 nm for (2), 704 nm for (3) and 721 nm for (4) in THF, respectively. The Q bands of the non-peripheral substituted phthalocyanines' luminescent spectra are bathochromic shifted when compared to the corresponding peripheral substituted phthalocyanines. The red shifts observed in emission maxima are 40–60 nm in comparison with peripheral substituted phthalocyanines.40 Fluorescence excitation peaks were observed at 694 nm for (2), 687 nm for (3) and 698 nm for (4) in THF, respectively. Absorption spectra of (2–4) indicated characteristic one absorption band at Q band region that was at 687 nm for (2), 689 nm for (3) and 702 nm for (4) in THF, respectively. Absorption and fluorescence excitation spectra of (2–4) are very similar and mirror images of their emission spectrum in the solvent of THF. The observed Stokes shifts for (2–4) were within the region 15–19 nm in THF which was typical for phthalocyanines.41,42 Absorption, fluorescence excitation and emission peaks for (2–4) in THF were listed in Table 1.
| Solvents | Compounds | Q band, λmax, (nm) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
Excitation, λEx, (nm) | Emission, λEx, (nm) | Stokes shift, ΔStokes, (nm) | Fluorescence spectra and quantum, ΦF |
|---|---|---|---|---|---|---|---|
| THF | (2) | 687 | 4.71 | 694 | 705 | 18 | 0.006 |
| (3) | 689 | 4.79 | 687 | 704 | 15 | 0.115 | |
| (4) | 702 | 4.21 | 698 | 721 | 19 | 0.045 |
Considering different metal effects on ΦF values, the highest ΦF value (0.115) was obtained for the complex (3) containing closed shell diamagnetic ions, such as Zn2+ which is important for PDT. The metal of Zn2+ have been proved as highly promising photosensitizers for PDT (photodynamic therapy) due to their intense absorption in the red region of the visible light.43,44 The other obtained ΦF values were quite low (0.006) for (2) and (0.045) for (4) due to the heavy atom effect.45 The ΦF values of the studied phthalocyanine compounds (2–4) were lower than those of unsubstituted zinc(II) phthalocyanine (ZnPc) in THF. The decrease in the ΦF values is because of the substituents. This implies that the presence of non-peripheral 1-(4-hydroxyphenyl)propan-1-one substituents caused some fluorescence quenching of the parent (2–4).
3.4. Anticancer activity
In this work, anticancer activities of compounds of phthalocyanine were studied on MCF-7, MG63, and L929 cell lines. Anticancer activity of the synthesized compounds were investigated and results are presented in Fig. 6–8. Cells were studied using four different concentrations (12.5; 50; 75; 100 μM). XTT protocol was applied after 24 h and 48 h. Viability of control cells has been accepted as 100%. IC50 values were calculated for MCF-7, MG63, and L929 cell lines for 24 and 48 h (Table 2).| Compounds | L929 | MCF-7 | MG63 |
|---|---|---|---|
| (5) | 19.53 | 8.85 | 12.14 |
| (4) | 39.57 | 44.67 | 22.06 |
| (3) | 26.94 | 5.80 | 20.28 |
| (2) | 15.70 | 12.48 | 15.80 |
The compounds were applied to three different types of cell lines on a 24 h incubation. Complex (5) acted well on MCF-7 and MG63 cell lines. It is not suitable for application to cancer cell lines due to its complex toxicity.
Complex (5) showed anticancer effect on MCF-7 and MG63 cell lines. The complex (4) is not suitable for application to cancer cell lines from the toxic nature of the compound on L929 cell lines. Complex (3) both MCF-7 and MG63 cell lines were anticancer effective; the compound is most likely to have more of an anticancer effect on the MCF-7 cell lines. Complex (2) does not have anticancer effect for MG63 cell lines, whereas MCF-7 cell lines has anticancer effect.
3.5. Computational results
| EHOMO | ELUMO | I | A | ΔE | η | σ | χ | ω | Energy | |
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP/SDD | −6.04315 | −3.66268 | 6.04315 | 3.66268 | 2.38047 | 1.19024 | 0.84017 | 4.85292 | 9.89334 | 0.10108 |
| B3LYP/6-311G | −6.11907 | −3.71085 | 6.11907 | 3.71085 | 2.40823 | 1.20411 | 0.83049 | 4.91496 | 10.03096 | 0.09969 |
| B3LYP/3-21G | −5.64124 | −3.40798 | 5.64124 | 3.40798 | 2.23326 | 1.11663 | 0.89555 | 4.52461 | 9.16692 | 0.10909 |
| HF/SDD | −9.02282 | −0.28980 | 9.02282 | 0.28980 | 8.73302 | 4.36651 | 0.22902 | 4.65631 | 2.48268 | 0.40279 |
| HF/6-311G | −8.89139 | −0.16409 | 8.89139 | 0.16409 | 8.72730 | 4.36365 | 0.22917 | 4.52774 | 2.34900 | 0.42571 |
| HF/3-21G | −8.66227 | −0.08055 | 8.66227 | 0.08055 | 8.58172 | 4.29086 | 0.23305 | 4.37141 | 2.22673 | 0.44909 |
| B3LYP | Hartree–Fock | ||
|---|---|---|---|
| Cu2+ | EHOMO | −32.33173 | −39.96949 |
| ELUMO | −20.06556 | −15.84776 | |
| Thermal free energies (G) | −5308.47607 | −5282.40567 | |
| Co2+ | EHOMO | −29.31397 | −36.02571 |
| ELUMO | −25.61754 | −15.07033 | |
| Thermal free energies (G) | −3918.68202 | −3897.02167 | |
| Zn2+ | EHOMO | −36.16857 | −41.22857 |
| ELUMO | −19.93250 | −16.23553 | |
| Thermal free energies (G) | −1759.01736 | −1704.89964 | |
| InCl2+ | EHOMO | −7.07066 | −9.29412 |
| ELUMO | −2.14700 | 0.29688 | |
| Thermal free energies (G) | −460.56912 | −451.93191 |
In quantum chemical studies, the electrophilicity index (ω) is a very important parameter that indicates the tendency of the active molecule to accept the electrons. As it is well known that this quantity is constantly used in the analysis of chemical reactivity of molecules. Nucleophilicity (ε) is a physically the inverse of electrophilicity (1/ω). For the reason that is should be stated that a molecule have large electrophilicity value that is ineffective of activity of molecule although a molecule that have large nucleophilicity value is a good activity of molecule. As it is well known that molecular electrophilicity are based on both molecular electronegativity and molecular hardness.
The Gibbs free energy (ΔG) was calculated using below equation:
 | (9) |
| Compounds | B3LYP/SDD | B3LYP/6-311G | B3LYP/3-21G | HF/SDD | HF/6-311G | HF/3-21G |
|---|---|---|---|---|---|---|
| (2) | −9238, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 403 403 |
−9208, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473 |
104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326, 65 326, 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447 447 |
−9214, 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 622 |
−9183, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781 781 |
−9076, 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 458 458 |
| (3) | −11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342, 56 342, 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309, 73 309, 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 099 099 |
−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021, 04 021, 04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 892 |
−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283, 07 283, 07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 695 |
−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249, 42 249, 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 331 |
−10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961, 95 961, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 814 |
| (4) | −25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855, 75 855, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 474 |
−25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844, 62 844, 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963 963 |
−24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963, 88 963, 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 578 |
−25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590, 58 590, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
−25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740, 99 740, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754 754 |
−24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862, 43 862, 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 354 354 |
| (5) | −8130, 07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 629 629 |
−8118, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 077 077 |
−7948, 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999 999 |
−8102, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607 607 |
−8071, 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 974 974 |
−8457, 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 356 |
It is clear known that the figure of molecular electrostatic potential (ESP) of six molecules givens an indication of the total charge distribution (electron + nuclei) of the molecule and correlates with dipole moments, electronegativity, partial charges and chemical reactivity of six molecules. It provides that a visual method to understand the relative polarity of the molecules. An electron density isosurface of six molecules mapped with electrostatic potential surface the size, shape, charge density and site of chemical reactivity of molecules.30
The different value of the electrostatic potential represented by different colors: red represents the region of the most negative electrostatic potential, blue represent the regions of the most positive electrostatic potential and green represents the region of zero potential. The potential increases in the order red < orange < yellow < green < blue. From the light of the result given in the mapped have been plotted for title molecules in 6-311++G** basis set using the computer software gauss view (Fig. 9).
4. Conclusion
In this paper, we have synthesized and characterized the phthalocyanines (M = Cu(II) (2), Zn(II) (3), In(III) (4) and Co(II) (5)) which is nonperipherally tetra-substituted with 1-(4-s hydroxyphenyl)propan-1-one. In THF, the fluorescence quantum yields ΦF for complexes (2–4) were found to be lower than unsubstituted zinc(II) phthalocyanine (ZnPc) in THF. This implies that the presence of non-peripheral 1-(4-hydroxyphenyl)propan-1-one substituents caused some fluorescence quenching of the parent (2–4). The most suitable compound for the MCF-7 cell line from the four compounds is the complex (3); the anticancer effect of the complex (5) in the MG63 cell line is good. The synthesized azo chromophore containing compounds might be potentially useful in the field of cancer treatment. Major problem that is chemotherapeutic agents are side-effect, which kill both cancerous and normal cells since they are not selective. So there is new agent for cancer therapeutic. Our studied showed anticancer effect on MCF-7 and MG63 cell line. Complex (3) both MCF-7 and MG63 cell lines were anticancer effective. Complex (3) was applied to MCF-7 cell line and cell viability decreased from 100% to 60%.Conflicts of interest
There are no conflicts to declare.References
- A. Urruticoechea, R. Alemany, J. Balart, A. Villanueva, F. Vinals and G. Capella, Curr. Pharm. Des., 2010, 16, 3 CrossRef CAS PubMed.
- Phthalocyanines: properties and applications, ed. C. C. Leznoff and A. B. P. Lever, New York, 1996, vol. 4 Search PubMed.
- S. Chaure, D. Paul, P. Vadagma and A. K. Ray, J. Hazard. Mater., 2010, 173, 253 CrossRef CAS PubMed.
- M. L. Rodriguez-Mendez and J. A. De Saja, J. Porphyrins Phthalocyanines, 2009, 13, 606 CrossRef CAS.
- X. Chen, X. Hu, L. An, N. Zhang, D. Xia, X. Zuo and X. Wang, Electrocatalysis, 2014, 5, 68 CrossRef CAS.
- K. Mandal, D. Jana, B. K. Ghorai and N. R. Jana, J. Phys. Chem. C, 2016, 120, 5196 CAS.
- S. Swavey and M. Tran, Porphyrin and Phthalocyanine Photosensitizers as PDT Agents: A New Modality for the Treatment of Melanoma, In Tech, Rijeka, Croatia, 2013, p. 253 Search PubMed.
- Ş. Özçelik, G. K. Karaoğlan, G. Gümrükçü, A. Erdogmus and A. Gül, J. Organomet. Chem., 2014, 769, 17 CrossRef.
- H. Ali and J. E. van Lier, Chem. Rev., 1999, 99, 2379 CrossRef CAS PubMed.
- A. T. Bilgiçli, M. Durdaşoglu, E. Kırbaç, M. N. Yarasir and M. Kandaz, Polyhedron, 2015, 100, 1–9 CrossRef.
- B. Kıyak, A. A. Esenpınar and M. Bulut, Polyhedron, 2015, 90, 183–196 CrossRef.
- M. S. Ağırtaş, Dyes Pigm., 2008, 79, 247 CrossRef.
- E. N. Kaya, M. Durmus and M. Bulut, J. Organomet. Chem., 2014, 774, 94 CrossRef CAS.
- A. Günsel, M. N. Yaraşır, M. Kandaz and A. Koca, Synthesis, Polyhedron, 2010, 29, 3394 CrossRef.
- P. G. Blassan, A. Heidi and M. H. Nanjundaswamy, Photodiagn. Photodyn. Ther., 2017, 19, 266 CrossRef PubMed.
- K. H. Tamarisk, A. Heidi and J. C. Marianne, Photodiagn. Photodyn. Ther., 2012, 9, 215 CrossRef PubMed.
- O. Ogbodu, L. L. Janice, P. Earl and T. Nyokong, Synth. Met., 2015, 204, 122 CrossRef.
- J. C. Maruzzela and P. A. Henry, J. Am. Pharm. Assoc., 1958, 47, 294 CrossRef.
- A. J. Papa, in Kirk-Othmer encyclopedia of chemical technology, ed. M. Grayson, H. F. Mark, D. F. Othmer, C. G. Overberger and G. T. Seaborg, John Wiley & Sons, New York, 1981, 13, p. 894 Search PubMed.
- J. Kling, Eur. Mol. Biol. Organ. J., 2007, 8(10), 903 CAS.
- Gauss View 5.0, Gaussian Inc, Wallingford, CT, USA, 2009 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc., Wallingford CT, 2010.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 39, 351 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372 CrossRef CAS.
- S. Kaya, B. Tüzün, C. Kaya and I. B. Obot, J. Taiwan Inst. Chem. Eng., 2016, 58, 528 CrossRef CAS.
- S. Kaya, C. Kaya, L. Guo, F. Kandemirli, B. Tüzün, İ. Uğurlu, L. H. Madkour and M. Saraçoğlu, J. Mol. Liq., 2016, 219, 497 CrossRef CAS.
- S. Kaya, P. Banerjee, S. K. Saha, B. Tüzün and C. Kaya, RSC Adv., 2016, 6(78), 74550 RSC.
- I. B. Obot, S. Kaya, C. Kaya and B. Tüzün, Phys. E, 2016, 80, 82 CrossRef CAS.
- I. B. Obot, S. Kaya, C. Kaya and B. Tüzün, Res. Chem. Intermed., 2016, 42(5), 4963 CrossRef CAS.
- B. Tüzün, Journal of New Results in Science, 2014, 5, 67 Search PubMed.
- S. Kaya, L. Guo, C. Kaya, B. Tüzün, I. B. Obot, R. Touir and N. Islam, J. Taiwan Inst. Chem. Eng., 2016, 65, 522 CrossRef CAS.
- H. Chermette, J. Comput. Chem., 1999, 20, 129 CrossRef CAS.
- R. G. Parr and P. K. Chattaraj, J. Am. Chem. Soc., 1991, 113, 1854 CrossRef CAS.
- T. Koopmans, Physica, 1933, 1, 104 CrossRef CAS.
- N. Islam and D. C. Ghosh, Int. J. Quantum Chem., 2011, 109, 917 CAS.
- W. Yang, C. Lee and S. K. Ghosh, J. Phys. Chem., 1985, 89, 5412 CrossRef CAS.
- W. Yang and R. G. Parr, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 6723 CrossRef CAS.
- H. Enkelkamp and R. J. M. Nolte, J. Porphyrins Phthalocyanines, 2000, 4, 454 CrossRef.
- P. Zimcik, V. Novakova, K. Kopecky, M. Miletin, R. Z. Uslu Kobak and E. Svandrlikova, Inorg. Chem., 2012, 51, 4215 CrossRef CAS PubMed.
- S. Makhseed, M. Al-Sawah, J. Samuel and H. Manaa, Tetrahedron Lett., 2009, 50, 165 CrossRef CAS.
- I. Ozçeşmeci, A. K. Burat and Z. A. Bayır, J. Organomet. Chem., 2014, 750, 125 CrossRef.
- Y. Zorlu, F. Dumoulin, M. Durmus and V. Ahsen, Tetrahedron, 2010, 66, 3248 CrossRef CAS.
- E. Kirbaç, G. Y. Atmaca and A. Erdoğmuş, J. Organomet. Chem., 2014, 752, 115 CrossRef.
- I. Acar, Z. Bıyıklıoğlu, M. Durmus and H. Kantekin, J. Organomet. Chem., 2012, 65, 708 Search PubMed.
- I. Ozçeşmeci, A. Gelir and A. Gül, J. Lumin., 2014, 147, 141 CrossRef.
- G. Makov, J. Phys. Chem., 1995, 99, 9337 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- R. G. Pearson, J. Chem. Educ., 1968, 45, 581 CrossRef CAS.
- I. B. Obot, N. O. Obi-Egbedi and A. O. Eselo, Ind. Eng. Chem. Res., 2011, 50, 2098 CrossRef CAS.
- R. G. Pearson, J. Chem. Educ., 1987, 64, 561 CrossRef CAS.
- A. Üngördü and N. Tezer, J. Mol. Graphics Modell., 2017, 74, 256 CrossRef PubMed.
- A. Üngördü and N. Tezer, J. Saudi Chem. Soc., 2017, 21, 837 CrossRef.
- A. C. Ekennia, A. A. Osowole, L. O. Olasunkanmi, D. C. Onwudiwe and E. E. Ebenso, Res. Chem. Intermed., 2017, 43(7), 3787 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |