Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Core-shell structured NaMnF3: Yb, Er nanoparticles for bioimaging applications†
Shuai Ye ,
Mengjie Zhao,
Jun Song
,
Mengjie Zhao,
Jun Song * and
Junle Qu*
* and
Junle Qu*
Key Lab of Optoelectronic Devices and Systems of Ministry of Education/Guangdong Province, College of Optoelectronic Engineering, Shenzhen University, Shenzhen 518060, China. E-mail: songjun@szu.edu.cn; jlqu@szu.edu.cn
First published on 13th November 2017
Abstract
NaMnF3: Yb,Er upconversion nanoparticles (UCNPs) have received considerable attention due to their single-band emission. In this paper, a modified thermal decomposition method to synthesize single or core/shell structured lanthanide-doped NaMnF3 nanoparticles is proposed. The NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles displayed pure red emission under 980 nm laser excitation, and the emission intensity could be significantly enhanced by coating a NaMnF3 or NaMnF3: 20% Yb3+ shell layer on their surfaces. Moreover, NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles shelled with NaMnF3: 20% Nd3+ or NaMnF3: 20% Yb3+, 20% Nd3+ were synthesized for the first time, and displayed pure red emission when excited by an 808 nm laser. The core/shell structured NaMnF3: Yb/Er@NaMnF3: Yb or NaMnF3: Yb/Er@NaMnF3: Yb/Nd nanoparticles were applied to bioimaging. The NaMnF3 based UCNPs exhibited good photostability and biocompatibility in HeLa cells. The obtained images indicated that the UCNPs could mainly exist in the cytoplasmic regions in the cells. Besides, images from the same region exposed to laser irradiations at 808 nm and 980 nm were found to be comparable. This result indicated that, for NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+, 20% Nd3+ nanoparticles, laser excitation at 808 nm was as efficient as that at 980 nm for bioimaging.
1. Introduction
Lanthanide-doped upconversion nanoparticles (UCNPs) have received considerable attention due to their intriguing features including non-autofluorescence, low photobleaching, strong penetration abilities, and low toxicity.1–10 UCNPs have been widely used in solar cells, biolabeling, bioimaging, optical data storage, drug delivery, etc.11–15 UCNPs with red single-band emission are preferred for biological imaging applications, as red light can penetrate deeper than other visible wavelengths through most tissues.16,17 Besides, UCNPs with only red single-band emission are ideal candidates for photodynamic therapy applications, as the absorption peak of most commercial photosensitizers is located in the red region at ∼650–670 nm.18,19 Therefore, to satisfy the requirements of several applications, the synthesis of UCNPs with pure red emission is crucial.20Until now, most research studies on the synthesis of UCNPs with strong red or near-infrared (NIR) emission have focused on Yb/Er or Yb/Tm doped NaYF4 nanoparticles, as strong green (∼540 nm) and weak red (∼654 nm) emissions are produced by Yb/Er doped NaYF4 nanoparticles, whereas blue (∼480 nm) and strong NIR (∼800 nm) emissions are produced by Yb/Tm doped NaYF4 nanoparticles.21–24 Although each trivalent lanthanide activator ion has a unique energy level structure to produce emission peaks at specific wavelengths, the excitation and relaxation dynamics of energy levels involved in UC processes can be manipulated to vary the relative luminescence intensity between different UC bands.25–32 The UC emission color may be tuned by incorporating other ions such as Mn2+, Zr4+, Mg2+ or Ce3+ in the host lattice.33–38 Mn2+ ion doped NaYF4 nanoparticles show very promising performances, because the 4T1 energy states of Mn2+ ions can facilitate non-radiative energy transfer from the 2H9/2 and 4S3/2 levels to the 4F9/2 level of Er3+ ions (from 1D2 and 1G4 levels to 3F4 levels of Tm3+ ions), which can tune the color emission from green to red or from blue to NIR for the Yb/Er and Yb/Tm doped UCNPs, respectively. Thus, UCNPs with single red or NIR band emissions can be obtained.
Besides, UCNPs with Yb3+ as sensitizer are generally excited by a 980 nm laser. However, the use of 980 nm NIR photons has an intrinsic disadvantage due to the strong absorption of water molecules in biological tissues at that wavelength, resulting in the risk of local temperature rise or even tissue overheating under continual irradiation.39–41 To avoid water absorption in tissues, the wavelength of the exciting light source should be shorter than 800 nm. A promising strategy was proposed to incorporate Nd3+ ions into UCNPs as sensitizers to absorb the energy of the 800 nm photons, followed by transfer of non-radiative energy to Yb3+ ions.42 Owing to the quenching effect between them, Nd3+ and activator ions (Er3+, Tm3+) needed to be separated by a core/shell structure to obtain effective Nd3+ sensitized UCNPs.43,44
To maintain the penetration depth and simultaneously avoid overheating during in vivo bioimaging, it was necessary to develop UCNPs which can upconvert from ∼800 nm NIR laser to red light. Obviously, Nd3+ sensitized NaMnF3: Yb/Er nanoparticles were the most promising candidate, although Nd3+ sensitized Ho3+ single band UCNPs have been reported.45 Until now, solvothermal routes have been typically used to synthesize NaMnF3: Yb/Er nanoparticles;46,47 the syntheses have been conducted in teflon-lined autoclaves under high pressure, and core/shell UCNPs with perfect morphologies have been hardly obtained. In this work, a novel method to synthesize single or core/shell structured NaMnF3: Yb, Er nanoparticles with pure red emission under 808 or 980 nm laser excitation for bioimaging was developed. Then, bioimaging of core/shell structured NaMnF3: Yb/Er nanoparticles in HeLa cells was investigated.
2. Experimental
2.1 Materials
For the proposed procedure, appropriate selection of the raw materials is critical. MnCl2 hardly reacts with oleic acid to produce the necessary metal-oleic complex. The frequently used rare earth chloride must be abandoned, because chloride ions could hinder the complex reaction of Mn2+ ions. Rather, rare earth acetate hexahydrate and manganese(II) pentanedionate were used. Chemicals were purchased from Alfa Aesar and used without further purification. The acetate hexahydrate had a trace metal basis of 99.9%.2.2 Synthesis of single nanoparticles
In this work, NaMnF3: 20% Yb3+, A (A = Er3+, Ho3+, or Tm3+) nanoparticles were prepared by a modified one-step thermal decomposition. A mixture containing 0.2 mmol of Yb(CH3COO)3·4H2O, (0.8 − x) mmol of manganese(II) pentanedionate, and x mmol of A(CH3COO)3·4H2O was placed in a 100 ml flask. Then, 7 ml of oleic acid and 15 ml of 1-octadecene were added, and the solution was heated to 160 °C for 60 min under Ar atmosphere and vigorous stirring. After cooling to room temperature, a solution comprising 4 mmol of NH4F and 2.5 mmol of NaOH in 10 ml of methanol was added, and the mixture was heated to 100 °C for 30 min to evaporate methanol. Finally, the solution was heated to 300 °C under Ar atmosphere for 60 min, and then cooled to room temperature. The nanoparticles were precipitated with 50 ml of ethanol, collected after centrifugation (7000 rpm, 5 min), and redispersed in 10 ml of hexane for later use.2.3 Synthesis of core/shell structured nanoparticles
To prepare core/shell structured NaMnF3: 20% Yb3+, A nanoparticles, 0.5 mmol of manganese(II) pentanedionate was added to a 100 ml flask, followed by 7 ml of oleic acid and 15 ml of 1-octadecene. The solution was heated to 160 °C for 60 min under Ar atmosphere and vigorous stirring. After cooling to room temperature, 5 ml of colloidal NaMnF3: 20% Yb3+/A, synthesized as in Section 2.2, was added, followed by a solution consisting of 2 mmol of NH4F and 1.25 mmol of NaOH in 5 ml of methanol; the mixture was then heated to 100 °C for 30 min to evaporate methanol and hexane. Finally, the solution was heated to 290 °C under Ar atmosphere for 60 min, and subsequently cooled to room temperature. The nanoparticles were precipitated with 50 ml of ethanol, collected after centrifugation (7000 rpm, 5 min), and redispersed in 5 ml of hexane for characterization.2.4 Surface modification of oleic acid (OA) UCNPs by polymethacrylamide (PAAM)
To obtain hydrophilic UCNPs for biological application, PAAM was used to modify OA-capped UCNPs. Firstly, 0.5 ml of a solution of hexane-dispersed OA-UCNPs was added to 5 ml of anhydrous ethanol containing 0.5 ml of PAAM, and stirred vigorously at ambient temperature for 24 h. The UNCPs were precipitated by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm; 15 min) and redispersed in phosphate buffer saline solution by sonication for further research.
000 rpm; 15 min) and redispersed in phosphate buffer saline solution by sonication for further research.
2.5 Characterization
The sample morphologies were observed by transmission electron microscopy (TEM; Joel JEM-2100; acceleration voltage: 200 kV). The UC luminescence spectra were recorded using an Ocean Optics QE65000 spectrofluorometer with a slit width defining a spectral resolution of 1 nm. The colloidal UCNPs were excited at 980 or 808 nm by using a continuous-wave laser diode with tunable laser power in the scale of 0–0.8 W. The spectra of colloidal UCNPs were measured with 0.5 W laser irradiation if no special requirement was mentioned. The emission decay time was measured by using the proposed system illustrated in.483. Results and discussion
Fig. 1a shows the TEM morphologies of the synthesized NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles. Ultrasmall lanthanide-doped NaMnF3 nanoparticles were synthesized following the proposed procedure; all the nanoparticles were uniformly monodispersed. The average size of the NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles was approximately 9.5 nm. The XRD pattern shown in Fig. S1† was in accord with the standard NaMnF3 host lattice of JCPDS 18-1224, indicating the prepared UCNPs were NaMnF3 phase. Fig. 1b shows the UC emission spectrum of the as-synthesized NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles. Only a single emission band centered at 654 nm, which corresponds to the energy transfer 4F9/2 → 4F15/2 of Er3+ ions, is visible in the spectrum. The emission band corresponding to the energy transfer 4S3/2 → 4F15/2 of Er3+ ions—present in the NaYF4: 20% Yb3+, 2% Er3+ nanoparticle spectrum—completely disappears in the NaMnF3: 20% Yb3+, 2% Er3+ nanoparticle spectrum. This is ascribed to the non-radiative energy transfer from the 4S3/2 level of Er3+ ions to the 4T1 level of Mn2+ ions, followed by back energy transfer to the 4F9/2 level of Er3+ ions. Hence, the color output of the NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles is pure red, as shown in Fig. S2.† Additionally, Yb/Ho and Yb/Tm doped NaMnF3 nanoparticles were synthesized. TEM images and emission spectra are shown in Figs. S3 and S4.† The Yb/Ho and Yb/Tm doped NaMnF3 nanoparticles were also monodispersed with average sizes of approximately 9.5 and 9.8 nm, respectively. The NaMnF3: 20% Yb3+, 1% Tm3+ nanoparticles displayed a single-band emission centered at 800 nm, corresponding to the energy transfer 3H4 → 3H6. However, the NaMnF3: 20% Yb3+, 2% Ho3+ nanoparticles exhibited a multiband output including a weak green emission band at 540 nm and a strong emission band at 640 nm.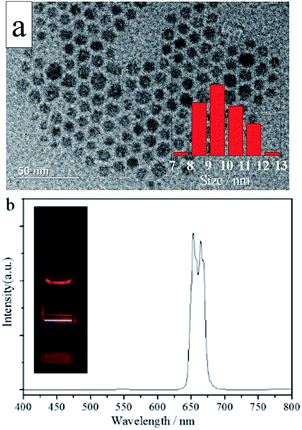 | ||
| Fig. 1 (a) Typical TEM image and (b) photoluminescence spectrum of NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles. The inset in (a) shows the size distribution of NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles. | ||
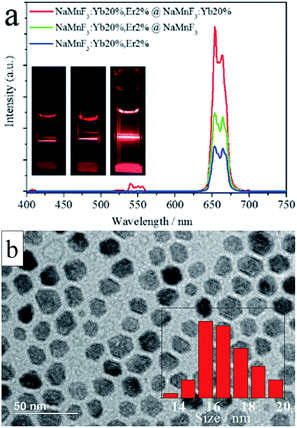
Notably, the UC emission intensity can be significantly enhanced by coating effective shell layers on the surface of the UCNPs.4,49 Layers of different types (i.e., an inert NaMnF3 layer or an active NaMnF3: 20% Yb3+ layer) were coated on the surfaces of NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles to boost the UC emission intensity, as shown in Fig. 2. The red emission intensity was enhanced by a factor of 1.75 in the presence of the inert NaMnF3 layer, whereas an enhancement factor of 3 was obtained by coating with the active NaMnF3: 20% Yb3+ layer. The output color of all these coated nanoparticles was red, as shown in the inset images; however, a very weak peak centered at 540 nm appeared in the spectrum of the NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+ nanoparticles, whose TEM image is shown in Fig. 2b. The average size was approximately 16 nm, almost twice that of the NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles.
To investigate the mechanism behind the intensity enhancement of the red emission in core/shell structured lanthanide-doped NaMnF3 nanoparticles, the dependence of the red emission intensity on the pump laser power was measured for NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles with or without shell layer (Fig. 3). Generally, the number of photons required to populate the upper emitting state under unsaturated conditions is related to the intensity by:
| If ∝ Pn | (1) |
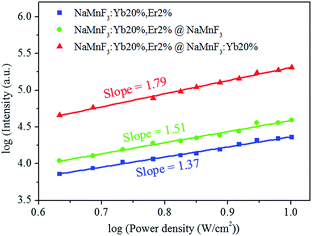 | ||
| Fig. 3 Dependence of red emission intensity on laser pumping power for NaMnF3: 20% Yb3+, 2% Er3+, NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3, and NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+ nanoparticles. | ||
The decay profiles of the 4S3/2 → 4F15/2 transition of Er3+ ions at 540 nm and 4F9/2 → 4F15/2 transition of Er3+ ions at 654 nm were also measured, as shown in Fig. 4. The decay curves were non-exponential, owing to enhanced non-radiative energy transfer processes, as well as effects of the surrounding environment. The effective lifetime τm is
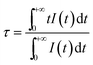 | (2) |
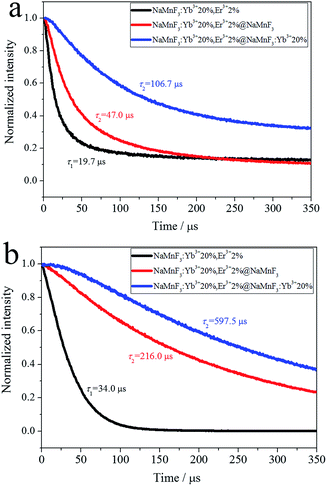 | ||
| Fig. 4 Decay profiles of transitions of Er3+ ions: (a) 4S3/2 → 4I15/2 at 540 nm and (b) 4F9/2 → 4I15/2 at 654 nm. | ||
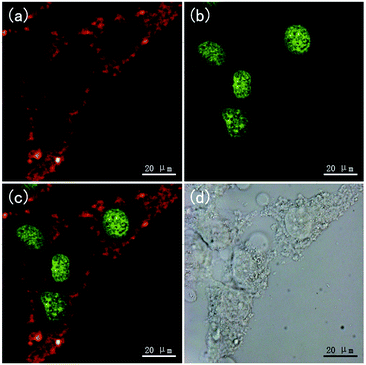
The NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+ nanoparticles were applied to bioimaging, as shown in Fig. 5. HeLa cells were incubated with PAAM-UCNPs for 4 h. After washing the unbound nanoparticles, the HeLa cells were imaged in bright field and fluorescence field by using a Leica confocal microscope equipped with a 980 nm NIR laser. Clear red UC luminescence from the UCNPs under 980 nm excitation (Fig. 5a) and green downconversion fluorescence from the DNA staining dye (SYTO®11, Invitrogen) under 514 nm excitation (Fig. 5b) were simultaneously observed in the HeLa cells. The merged images (Fig. 5c) show that the UCNP fluorescence mainly occurred in the cytoplasmic regions. Furthermore, the toxicity of the PAAM-UCNPs was investigated in HeLa cells, as shown in Fig. S5.† After 24 h of incubation in PAAM-UCNPs with different concentrations, the cell viability was still above 80% for UCNP concentrations up to 400 μg ml−1, indicating a good biocompatibility of the NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+ nanoparticles with the cells. The photostability of the PAAM-UCNPs was also measured, as shown in Fig. S6.† The photoluminescence intensity of the NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+ nanoparticles was reduced by only 2.3% after irradiation for 10 min.
In addition, NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles shelled with Nd3+ sensitized layers (NaMnF3: 20% Nd3+ or NaMnF3: 20% Yb3+, 20% Nd3+) were synthesized to obtain pure red emission under 808 nm laser excitation; the photoluminescence spectra are shown in Fig. 6. The NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles shelled with Nd3+ sensitized layers exhibited pure red emission when excited by 808 nm or 980 nm laser. However, the NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+, 20% Nd3+ nanoparticles exhibited considerably higher emission intensity than the NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Nd3+ nanoparticles under 808 nm laser excitation, which indicated that the incorporation of Yb3+ ions into the Nd3+ sensitized shell could facilitate the energy transfer from Nd3+ to Yb3+ ions.39,44 The TEM image of the NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+, 20% Nd3+ nanoparticles is shown in Fig. S7.† The average size was approximately 17 nm.
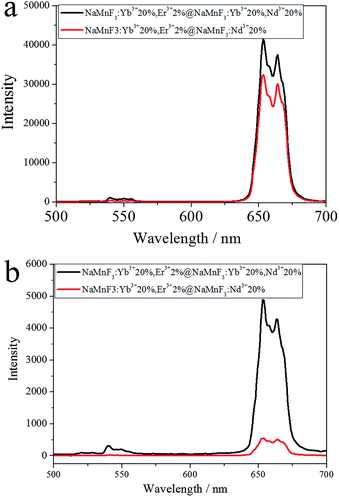 | ||
| Fig. 6 Spectra of NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles shelled with Nd3+ sensitized layers excited by (a) 980 nm or (b) 808 nm laser. | ||
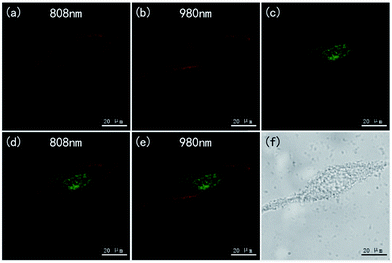
The NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+, 20% Nd3+ nanoparticles were also applied to bioimaging, as shown in Fig. 7. The HeLa cells incubated with PAAM-UCNPs were imaged in bright field and fluorescence field by using a Leica confocal microscope equipped with 808 nm and 980 nm NIR lasers. The merged images (Fig. 7d and e) indicate that the UCNP fluorescence mainly occurred in the cytoplasmic regions. The images obtained for the same region by using 808 nm and 980 nm laser excitations (Fig. 7a and b) appear comparable, which implied that, for NaMnF3: 20% Yb3+, 2% Er3+@NaMnF3: 20% Yb3+, 20% Nd3+ nanoparticles, the 808 nm laser excitation was as efficient as the 980 nm laser excitation for bioimaging. Notably, it has been confirmed39,43 that, compared with the popular excitation wavelength of 980 nm, the 808 nm excitation wavelength minimized the overheating effect for in vivo tissues.
4. Conclusions
A novel strategy was proposed to synthesize single or core/shell structured lanthanide-doped NaMnF3 nanoparticles. The synthesized NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles displayed pure red emission under 980 nm laser excitation, and the luminescence intensity could be significantly enhanced by coating their surface with a NaMnF3: 20% Yb3+ shell layer. Moreover, NaMnF3: 20% Yb3+, 2% Er3+ nanoparticles shelled with NaMnF3: 20% Yb3+, 20% Nd3+ were synthesized, and displayed pure red emission under 808 nm laser excitation. The NaMnF3: 20% Yb3+ NaMnF3: 20% Yb3+, 20% Nd3+ were applied to bioimaging under excitation of 808 nm and 980 nm laser excitations, revealing that the laser excitation at 808 nm was as efficient as that at 980 nm.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Parts of this work were supported by the National Basic Research Program of China (2015CB352005);the National Natural Science Foundation of China (61605124/61525503/ 61378091/ 61405123/61405062); Guangdong Natural Science Foundation Innovation Team (2014A030312008); Hong Kong, Macao and Taiwan cooperation innovation platform & major projects of international cooperation in Colleges and Universities in Guangdong Province (2015KGJHZ002); and Shenzhen Basic Research Project (JCYJ20150324141711561/JCYJ2015093010 4948169/ZDSYS20140430164957663/KQCX20140509172719 305); the Training Plan of Guangdong Province Outstanding Young Teachers in Higher Education Institutions (Yq2013142).References
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- X. Liu, R. Deng, Y. Zhang, Y. Wang, H. Chang, L. Huang and X. Liu, Probing the Nature of Upconversion Nanocrystals: Instrumentation Matters, Chem. Soc. Rev., 2015, 44, 1479–1508 RSC.
- W. Zheng, P. Huang, D. Tu, E. Ma, H. Zhu and X. Chen, Lanthanide-doped Upconversion Nano-bioprobes: Electronic Structures, Optical Properties and Biodetection, Chem. Soc. Rev., 2015, 44, 1379–1415 RSC.
- G. Chen, H. Ågren, T. Y. Ohulchanskyya and P. N. Prasad, Light Upconverting Core–shell Nanostructures: Nanophotonic Control for Emerging Applications, Chem. Soc. Rev., 2015, 44, 1680–1713 RSC.
- X. Li, F. Zhang and D. Zhao, Lab on Upconversion Nanoparticles: Optical Properties and Applications Engineering via Designed Nanostructure, Chem. Soc. Rev., 2015, 44, 1346–1378 RSC.
- H. Dong, S. Du, X. Zheng, G. Lu, L. Sun, L. Li, P. Zhang, C. Zhang and C. Yan, Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy, Chem. Rev., 2015, 115(19), 10725–10815 CrossRef CAS PubMed.
- Y. Sun, W. Feng, P. Yang, C. Huang and F. Li, The Biosafety of Lanthanide Upconversion Nanomaterials, Chem. Soc. Rev., 2015, 44, 1509–1525 RSC.
- S. Gai, C. Li, P. Yang and J. Lin, Recent Progress in Rare Earth Micro/Nanocrystals: Soft Chemical Synthesis, Luminescent Properties, and Biomedical Applications, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- X. Liu, C. Yan and J. A. Capobianco, Photon Upconversion Nanomaterials, Chem. Soc. Rev., 2015, 44, 1299–1301 RSC.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Upconversion nanoparticles as versatile light nanotransducers for photoactivation applications, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- A. Paulino, O. Olalla, M. Diego, L. Enrique, B. Isabel and R. Jorge, Synthesis, Characterization, and Application in HeLa Cells of an NIR Light Responsive Doxorubicin Delivery System Based on NaYF4:Yb, Tm@SiO2-PEG Nanoparticles, ACS Appl. Mater. Interfaces, 2015, 7, 14992–14999 Search PubMed.
- J. Shen, L. Zhao and G. Han, Lanthanide-doped Upconverting Luminescent Nanoparticle Platforms for Optical Imaging-guided Drug Delivery and Therapy, Adv. Drug Delivery Rev., 2013, 5, 744–755 CrossRef PubMed.
- L. Xiong, T. Yang, Y. Yang, C. Xu and F. Li, Long-term in vivo Biodistribution Imaging and Toxicity of Polyacrylic Acid-coated Upconversion Nanophosphors, Biomaterials, 2010, 3, 7078–7085 CrossRef PubMed.
- Q. Zhan, X. Zhang, Y. Zhao, J. Liu and S. He, Tens of Thousands-fold Upconversion Luminescence Enhancement Induced by a Single Gold Nanorod, Laser Photonics Rev., 2015, 9, 479–487 CrossRef CAS.
- G. Tian, X. Zheng, X. Zhang, W. Yin, J. Yu, D. Wang, Z. Zhang, X. Yang, Z. Gu and Y. Zhao, TPGS-stabilized NaYbF4:Er Upconversion Nanoparticles for Dual-modal Fluorescent/CT Imaging and Anticancer Drug Delivery to Overcome Multi-drug Resistance, Biomaterials, 2015, 8, 107–116 CrossRef PubMed.
- G. Chen, J. Shen, T. Ohulchanskyy, N. J. Patel, A. Kutikov, Z. Li, J. Song, R. Pandey, H. Agren, P. Prasad and G. Han, (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging, ACS Nano, 2012, 9, 8280–8287 CrossRef PubMed.
- L. Zhou, R. Wang, C. Yao, X. Li, C. Wang, X. Zhang, C. Xu, A. Zeng, D. Zhao and F. Zhang, Single-band upconversion nanoprobes for multiplexed simultaneous in situ molecular mapping of cancer biomarkers, Nat. Commun., 2015, 6938 CrossRef CAS PubMed.
- T. Dougherty, C. Gomer, B. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic Therapy, J. Natl. Cancer Inst., 1998, 12, 889–905 CrossRef.
- G. Tian, W. Ren, L. Yan, S. Jian, Z. Gu, L. Zhou, S. Jin, W. Yin, S. Li and Y. Zhao, Red-Emitting Upconverting Nanoparticles for Photodynamic Therapy in Cancer Cells Under Near-Infrared Excitation, Small, 2013, 11, 1929–1938 CrossRef PubMed.
- T. Wen, Y. Zhou, Y. Guo, C. Zhao, B. Yang and Y. Wang, Color-tunable and single-band red upconversion luminescence from rare-earth doped Vernier phase ytterbium oxyfluoride nanoparticles, J. Mater. Chem. C, 2016, 4, 684–690 RSC.
- J. Zeng, J. Su, Z. Li, R. Yan and Y. Li, Synthesis and Upconversion Luminescence of Hexagonal-Phase NaYF4:Yb,Er3+ Phosphors of Controlled Size and Morphology, Adv. Mater., 2005, 17, 2119–2123 CrossRef CAS.
- J. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, Synthesis of Colloidal Upconverting NaYF4 Nanocrystals Doped with Er3+, Yb3+ and Tm3+, Yb3+ via Thermal Decomposition of Lanthanide Trifluoroacetate Precursors, J. Am. Chem. Soc., 2006, 128, 7444–7445 CrossRef CAS PubMed.
- J. Shan, X. Qin, N. Yao and Y. Ju, Synthesis of Monodisperse Hexagonal NaYF4:Yb, Ln (Ln = Er, Ho and Tm) Upconversion Nanocrystals in TOPO, Nanotechnology, 2007, 18, 445607 CrossRef.
- Z. Li and Y. Zhang, An Efficient and User-friendly Method for the Synthesis of Hexagonal-phase NaYF4:Yb, Er/Tm Nanocrystals with Controllable Shape and Upconversion Fluorescence, Nanotechnology, 2008, 19, 345606 CrossRef PubMed.
- G. Yi, H. Lu, S. Zhao, Y. Ge, W. Yang, D. Chen and L. Guo, Synthesis, Characterization, and Biological Application of Size-Controlled Nanocrystalline NaYF4:Yb, Er Infrared-to-Visible Up-Conversion Phosphors, Nano Lett., 2004, 11, 2191–2196 CrossRef.
- H. Mai, Y. Zhang, L. Sun and C. Yan, Highly Efficient Multicolor Up-Conversion Emissions and Their Mechanisms of Monodisperse NaYF4:Yb,Er Core and Core/Shell-Structured Nanocrystals, J. Phys. Chem. C, 2007, 111, 13721–13729 CAS.
- F. Wang and X. Liu, Upconversion Multicolor Fine-Tuning: Visible to Near-Infrared Emission from Lanthanide-Doped NaYF4 Nanoparticles, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- A. Punjabi, X. Wu, A. Tokatli-Apollon, M. El-Rifai, H. Lee, Y. Zhang, C. Wang, Z. Liu, E. M. Chan, C. Duan and G. Han, Amplifying the Red-Emission of Upconverting Nanoparticles for Biocompatible Clinically Used Prodrug-Induced Photodynamic Therapy, ACS Nano, 2014, 10, 10621–10630 CrossRef PubMed.
- W. Wei, Y. Zhang, R. Chen, J. Goggi, N. Ren, L. Huang, K. K. Bhakoo, H. Sun and T. T. Y. Tan, Cross Relaxation Induced Pure Red Upconversion in Activator- and Sensitizer-Rich Lanthanide Nanoparticles, Chem. Mater., 2014, 26, 5183–5186 CrossRef CAS.
- W. Niu, S. Wu and S. Zhang, A Facile and General Approach for the Multicolor Tuning of Lanthanide-ion Doped NaYF4 Upconversion Nanoparticles within a Fixed Composition, J. Mater. Chem., 2010, 20, 9113–9117 RSC.
- G. Tian, W. Ren, L. Yan, S. Jian, Z. Gu, L. Zhou, S. Jin, W. Yin, S. Li and Y. Zhao, Red-Emitting Upconverting Nanoparticles for Photodynamic Therapy in Cancer Cells Under Near-Infrared Excitation, Small, 2013, 11, 1929–1938 CrossRef PubMed.
- S. Ye, G. Chen, W. Shao, J. Qu and P. N. Prasad, Tuning Upconversion through a Sensitizer/activator-isolated NaYF4 Core/shell Structure, Nanoscale, 2015, 7, 3976–3984 RSC.
- G. Tian, Z. Gu, L. Zhou, W. Yin, X. Liu, L. Yan, S. Jin, W. Ren, G. Xing, S. Li and Y. Zhao, Mn2+ Dopant-Controlled Synthesis of NaYF4:Yb/Er Upconversion Nanoparticles for in vivo Imaging and Drug Delivery, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS PubMed.
- J. Wang, F. Wang, C. Wang, Z. Liu and X. Liu, Single-Band Upconversion Emission in Lanthanide-Doped KMnF3 Nanocrystals, Angew. Chem., Int. Ed., 2011, 50, 10369–10372 CrossRef CAS PubMed.
- D. Chen, L. Lei, R. Zhang, A. Yang, J. Xu and Y. Wang, Intrinsic Single-Band Upconversion Emission in Colloidal Yb/Er(Tm):Na3Zr(Hf)F7 Nanocrystals, Chem. Commun., 2012, 48, 10630–10632 RSC.
- J. Song, G. Wang, S. Ye, Y. Tian, M. Xiong, D. Wang, H. Niu and J. Qu, Lanthanide-doped Na3ZrF7 upconversion nanoparticles synthesized by a facile method, J. Alloys Compd., 2016, 658, 914–919 CrossRef CAS.
- M. Wu, E. Song, Z. Chen, S. Ding, S. Ye, J. Zhou, S. Xu and Q. Zhang, Single-band red upconversion luminescence of Yb3+–Er3+ via nonequivalent substitution in perovskite KMgF3 nanocrystals, J. Mater. Chem. C, 2016, 4, 1675–1684 RSC.
- G. Chen, H. Liu, G. Somesfalean, H. Liang and Z. Zhang, Upconversion Emission Tuning from Green to Red in Yb3+/Ho3+-codoped NaYF4 Nanocrystals by Tridoping with Ce3+ Ions, Nanotechnology, 2009, 20, 385704 CrossRef PubMed.
- Y. Wang, G. Liu, L. Sun, J. Xiao, J. Zhou and C. Yan, Nd3+-Sensitized Upconversion Nanophosphors: Efficient In Vivo Bioimaging Probes with Minimized Heating Effect, ACS Nano, 2013, 7, 7200–7206 CrossRef CAS PubMed.
- X. Li, R. Wang, F. Zhang, L. Zhou, D. Shen, C. Yao and D. Zhao, Nd3+ Sensitized Up/Down Converting Dual-Mode Nanomaterials for Efficient In-vitro and In-vivo Bioimaging Excited at 800 nm, Sci. Rep., 2013, 3(3536), 1–4 CAS.
- Q. Zhan, J. Qian, H. Liang, G. Somesfalean, D. Wang, S. He, Z. Zhang and S. Andersson-Engels, Using 915 nm Laser Excited Tm3+/Er3+/Ho3+-Doped NaYbF4 Upconversion Nanoparticles for in Vitro and Deeper in Vivo Bioimaging without Overheating Irradiation, ACS Nano, 2011, 5, 3744–3757 CrossRef CAS PubMed.
- J. Shen, G. Chen, A. Vu, W. Fan, O. S. Bilsel, C. Chang and G. Han, Engineering the Upconversion Nanoparticle Excitation Wavelength: Cascade Sensitization of Tri-doped Upconversion Colloidal Nanoparticles at 800 nm, Adv. Opt. Mater., 2013, 7, 644–650 CrossRef.
- X. Xie, N. Gao, R. Deng, Q. Sun, Q. Xu and X. Liu, Mechanistic Investigation of Photon Upconversion in Nd3+-Sensitized Core−Shell Nanoparticles, J. Am. Chem. Soc., 2013, 135, 12608–12611 CrossRef CAS PubMed.
- X. Huang and J. Lin, Active-core/active-shell nanostructured design: an effective strategy to enhance Nd3+/Yb3+ cascade sensitized upconversion luminescence in lanthanide-doped nanoparticles, J. Mater. Chem. C, 2015, 3, 7652–7657 RSC.
- D. Chen, L. Liu, P. Huang, M. Ding, J. Zhong and Z. Ji, Nd3+-Sensitized Ho3+ Single-Band Red Upconversion Luminescence in Core−Shell Nanoarchitecture, J. Phys. Chem. Lett., 2015, 6, 2833–2840 CrossRef CAS PubMed.
- Y. Zhang, J. Lin, V. Vijayaragavan, K. K. Bhakoo and T. T. Y. Tan, Tuning sub-10 nm Single-phase NaMnF3 Nanocrystals as Ultrasensitive Hosts for Pure Intense Fluorescence and Excellent T1 Magnetic Resonance Imaging, Chem. Commun., 2012, 48, 10322–10324 RSC.
- Z. Wang, J. Feng, S. Song, Z. Sun, S. Yao, X. Ge, M. Pang and H. Zhang, Pure and Intense Orange Upconversion Luminescence of Eu3+ from the Sensitization of Yb3+–Mn2+ Dimer in NaY(Lu)F4 Nanocrystals, J. Mater. Chem. C, 2014, 2, 9004–9011 RSC.
- J. Liu, N. Li, R. Wu, Y. Zhao and Q. Zhan, Sailing He. Sub-5-nm lanthanide-doped ZrO2@NaYF4 nanodots as efficient upconverting probes for rapid scanning microscopy and aptamer-mediated bioimaging, Opt. Mater. Express, 2015, 5, 1759–1771 CrossRef CAS.
- F. Vetrone, R. Naccache, V. Mahalingam, C. G. Morgan and J. A. Capobianco, The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles, Adv. Funct. Mater., 2009, 19, 2924–2929 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10393j |
| This journal is © The Royal Society of Chemistry 2017 |