Open Access Article
Open Access ArticleSynthesis and structure–activity relationship of N4-benzylamine-N2-isopropyl-quinazoline-2,4-diamines derivatives as potential antibacterial agents
Zhengyun Jianga,
W. David Hongbcde,
Xiping Cuia,
Hongcan Gaoa,
Panpan Wuad,
Yingshan Chena,
Ding Shena,
Yang Yanga,
Bingjie Zhanga,
Mark J. Taylorb,
Stephen A. Wardb,
Paul M. O'Neillc,
Suqing Zhao *a and
Kun Zhang*ade
*a and
Kun Zhang*ade
aDepartment of Pharmaceutical Engineering, School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, China. E-mail: sqzhao@gdut.edu.cn; kzhang@gdut.edu.cn
bResearch Centre for Drugs & Diagnostics, Department of Parasitology, Liverpool School of Tropical Medicine, Liverpool, L3 5QA, UK
cDepartment of Chemistry, University of Liverpool, Liverpool, L69 7ZD, UK
dFaculty of Chemical & Environmental Engineering, Wuyi University, Jiangmen, 529020, China
eInternational Healthcare Innovation Institute (Jiangmen), Jiangmen, 529000, China
First published on 10th November 2017
Abstract
A series of N4-benzylamine-N2-isopropyl-quinazoline-2,4-diamine derivatives has been synthesized and tested for antibacterial activity against five bacterial strains. Twelve different substituents on the N4-benzylamine group have been investigated along with replacement of the quinazoline core (with either a benzothiophene or regioisomeric pyridopyrimidine ring systems). In order to develop structure activity relationships, all derivatives were tested for their antibacterial activities against Escherichia coli and Staphylococcus aureus via Kirby–Bauer assays and minimum inhibitory concentration assays. Eight of the most potent compounds against S. aureus and E. coli were also screened against one strain of methicillin-resistant S. aureus (MRSA), Staphylococcus epidermidis and Salmonella typhimurium to further examine their antibacterial activities. Lead compound A5 showed good activities with MICs of 3.9 μg mL−1 against E. coli, S. aureus and S. epidermidis and 7.8 μg mL−1 against MRSA. Selected front runners were also screened for their DMPK properties in vitro to assess their potential for further development.
Introduction
Novel antibacterial chemotypes are urgently needed because of the emergence and spread of multidrug resistant microorganisms and pathogenic bacterial infections.1,2 Staphylococcus aureus is the most common pathogen of surgical site and wound infections.3–6 Antibiotic-resistant strains of S. aureus infections often occur in epidemic waves that are initiated by one, or a few, successful clones.7–9 Every year in the United States approximately two million people fall ill and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die because of antibiotic-resistant bacterial infections, according to a report from the Center for Diseases Control and Prevention.10,11 Antibiotic-resistant bacterial strains, in particular methicillin-resistant S. aureus (MRSA) infection rates remain constant within the community and hospital settings in U.S.12–14 Worryingly, strains of S. aureus showing extended resistance to vancomycin, daptomycin, linezolid and ceftaroline, which are used to treat MRSA infections, have been reported recently in the literature.15–18 MRSA is on the list of bacteria for which new antibiotics are urgently needed as recognized by World Health Organization in 2017.19
000 people die because of antibiotic-resistant bacterial infections, according to a report from the Center for Diseases Control and Prevention.10,11 Antibiotic-resistant bacterial strains, in particular methicillin-resistant S. aureus (MRSA) infection rates remain constant within the community and hospital settings in U.S.12–14 Worryingly, strains of S. aureus showing extended resistance to vancomycin, daptomycin, linezolid and ceftaroline, which are used to treat MRSA infections, have been reported recently in the literature.15–18 MRSA is on the list of bacteria for which new antibiotics are urgently needed as recognized by World Health Organization in 2017.19
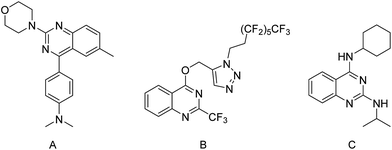
Quinazolines and derivatives have shown attractive antibacterial activity.20–22 In previous studies, quinazoline-based compounds have been investigated for their potential antibacterial activity, especially anti-MRSA activity. Bedi et al. (2004) reported 2,4-disubstituted quinazoline, such as compound A (Fig. 1), displayed antibacterial activity against a wide spectrum of bacteria including S. aureus, and E. coli.23 Chandrika et al. (2010) reported the in vitro activity of multiple fluoro-substituted triazol-4-yl substituted quinazoline B (Fig. 1) against S. aureus and S. epidermidis with an MIC of 9.375 μg mL.24 Van Horn et al. (2014) have reported N2,N4-disubstituted quinazoline-2,4-diamines such as C (Fig. 1) that displayed in vitro and in vivo activities against S. aureus, a low potential for spontaneous resistance and low toxicity.25 Furthermore, this proof-of-concept work opened up opportunities for further investigation of quinazoline-2,4-diamines in terms of structural activity relationships against a broader spectrum of bacteria strains and DMPK related parameters. In this work, we designed a library of 2,4-diaminoquinazoline analogues and closely related derivatives, including thirteen 2,4-diaminoquinazolines, thirteen 2,4-diaminothieno[3,2-d]pyrimidines, six 2,4-diaminopyrido[3,2-d]pyrimidine derivatives and six 2,4-diaminopyrido[2,3-d]pyrimidine derivatives. These compounds are structurally distinct from previously reported quinazoline core antibacterials. These compounds have been used to further explore how the structural variation affects antibacterial activity. Herein, a structure–activity relationship (SAR) study is reported which focuses on the substituent of N4-benzylamine and the variations of the quinazoline scaffold.
All derivatives were tested against S. aureus and E. coli in Kirby–Bauer assays initially. The antibacterial potency of active compounds was further determined by minimum inhibitory concentrations (MICs) assays. Further MICs assays were performed against methicillin-resistant S. aureus (MRSA), Staphylococcus epidermidis and Salmonella typhimurium to evaluate the spectrum of antibacterial activity of the most active compounds. In addition, DMPK related properties, such as lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4), aqueous solubility and in vitro metabolic stability were also assessed for a selected group of compounds to illustrate how the structural modifications can potentially influence the pharmacokinetics of these new lead molecules.
D7.4), aqueous solubility and in vitro metabolic stability were also assessed for a selected group of compounds to illustrate how the structural modifications can potentially influence the pharmacokinetics of these new lead molecules.
Results and discussion
Chemistry
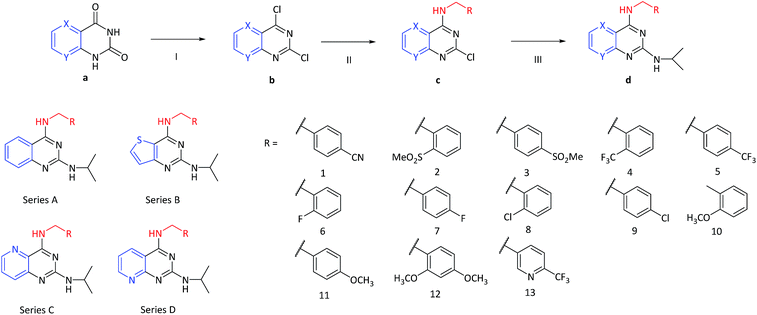
To systematically explore structure–activity relationships, a group of N4-benzylamine-N2-isopropyl-quinazoline-2,4-diamine derivatives which include four chemical subsets of compounds with different heterocyclic aromatic rings fused to the pyrimidine ring in the scaffold were designed and then subsequently synthesized via adapted methods from the literature (Fig. 2).25–30 Commercially available compound (a) was reacted with phosphorus oxychloride and phosphorus pentachloride resulting in the corresponding 2,4-dichloro intermediates (b). Substitution with benzylamines occurred selectively at the 4-position, yielding 4-benzylamino-2-chloroquinazoline (c) under mild conditions. Next, substitution at the 2-position with isopropyl amine gave the desired 2,4-diamino products (d). The synthesis of compounds from series A and B were started from the commercially available as quinazoline-2,4(1H,3H)-dione and thieno[3,2-d]pyrimidine-2,4(1H,3H)-dione, respectively. However, 2,4-dichloropyrido[3,2-d]-pyrimidine and 2,4-dichloropyrido[2,3-d]pyrimidine, exemplified as intermediate (b) which were readily acquired commercially have been used in the synthesis of series C and D compounds. Throughout the synthetic routes, all intermediates have been purified and the final N4-benzylamine-N2-isopropyl-quinazoline-2,4-diamines derivatives have been characterized by 1H NMR, 13C NMR and high resolution mass spectrum.Structure–activity relationship study
Initially all 4 chemical subsets (series A–D) were tested for their activities in inhibiting bacterial growth in the Kirby–Bauer assay against E. coli and S. aureus. Active compounds with zones of inhibitions (ZOIs) ≥ 7.0 mm in the Kirby–Bauer assay were then tested for their minimum inhibitory concentrations (MICs) to quantify their potency against E. coli and S. aureus (Table 1). The data of ZOIs and MICs presented are the average results from three independent experiments.| Compound code | R | E. colia | S. aureusb | ||
|---|---|---|---|---|---|
| ZoIs (mm) | MICs (μg mL−1) | ZoIs (mm) | MICs (μg mL−1) | ||
| a Escherichia coli, CMCC 44102.b Staphylococcus aureus, ATCC 6538.c No zone of inhibition was determined.d Not tested. | |||||
| A1 | 4-CN–phenyl– | 20.2 ± 0.6 | 31.2 | 11.8 ± 0.5 | 31.2 |
| A2 | 2-SO2Me–phenyl– | 8.3 ± 0.2 | 125.0 | 8.1 ± 0.2 | 62.5 |
| A3 | 4-SO2Me–phenyl– | 7.5 ± 0.3 | ≥125.0 | None | NT |
| A4 | 2-CF3–phenyl– | 20.4 ± 0.7 | 15.6 | 16.2 ± 0.7 | 3.9 |
| A5 | 4-CF3–phenyl– | 22.4 ± 0.1 | 3.9 | 13.2 ± 0.5 | 3.9 |
| A6 | 2-F–phenyl– | 23.8 ± 0.8 | 15.6 | 12.4 ± 0.3 | 15.6 |
| A7 | 4-F–phenyl– | 22.3 ± 0.5 | 15.6 | 14.5 ± 0.3 | 7.8 |
| A8 | 2-Cl–phenyl– | 21.2 ± 0.7 | 15.6 | 12.0 ± 0.5 | 3.9 |
| A9 | 4-Cl–phenyl– | 18.6 ± 0.2 | 7.8 | 11.6 ± 0.2 | 3.9 |
| A10 | 2-OCH3–phenyl– | 15.7 ± 0.6 | 31.2 | 17.4 ± 0.5 | 31.2 |
| A11 | 4-OCH3–phenyl– | 17.1 ± 0.9 | 31.2 | 15.4 ± 0.4 | 7.8 |
| A12 | 3,4-Di–OCH3–phenyl– | 16.2 ± 0.4 | 31.2 | 14.6 ± 0.5 | 15.6 |
| A13 | 4-CN–phenyl– | 21.0 ± 0.4 | 31.2 | 11.6 ± 0.3 | 31.2 |
| B1 | 2-SO2Me–phenyl– | 7.2 ± 0.1 | ≥125.0 | 8.9 ± 0.3 | 62.5 |
| B2 | 4-SO2Me–phenyl– | 7.9 ± 0.2 | ≥125.0 | None | NT |
| B3 | 2-CF3–phenyl– | Nonec | NTd | None | NT |
| B4 | 4-CF3–phenyl– | 16.8 ± 0.8 | 31.2 | 12.1 ± 0.3 | 7.8 |
| B5 | 2-F–phenyl– | 8.6 ± 0.1 | 125.0 | 12.7 ± 0.3 | 7.8 |
| B6 | 4-F–phenyl– | 8.0 ± 0.3 | 62.5 | 11.1 ± 0.5 | 31.2 |
| B7 | 2-Cl–phenyl– | 8.5 ± 0.4 | 62.5 | 10.9 ± 0.3 | 31.2 |
| B8 | 4-Cl–phenyl– | 11.4 ± 0.4 | 62.5 | 13.6 ± 0.6 | 15.6 |
| B9 | 2-OCH3–phenyl– | 8.1 ± 0.5 | 62.5 | 12.9 ± 0.4 | 7.8 |
| B10 | 4-OCH3–phenyl– | None | NT | None | NT |
| B11 | 3,4-Di–OCH3–phenyl– | None | NT | None | NT |
| B12 | 4-CN–phenyl– | None | NT | 9.2 ± 0.4 | 62.5 |
| B13 | 2-SO2Me–phenyl– | 7.3 ± 0.1 | ≥125.0 | 8.2 ± 0.1 | 62.5 |
| C1 | 4-SO2Me–phenyl– | None | NT | 7.6 ± 0.3 | 62.5 |
| C2 | 4-CF3–phenyl– | None | NT | None | NT |
| C3 | 4-CN–phenyl– | None | NT | None | NT |
| C5 | 2-SO2Me–phenyl– | None | NT | 8.5 ± 0.6 | 15.6 |
| C13 | 4-SO2Me–phenyl– | None | NT | None | NT |
| D1 | 4-CF3–phenyl– | None | NT | None | NT |
| D2 | 4-CF3–pyridyl– | None | NT | 8.1 ± 0.2 | 31.2 |
| D3 | 4-CF3–pyridyl– | None | NT | None | NT |
| D5 | 4-CF3–pyridyl– | None | NT | 10.2 ± 0.1 | 7.8 |
| D13 | 4-CF3–pyridyl– | None | NT | 8.1 ± 0.8 | 62.5 |
| Norfloxacin | NT | ≤0.12 | NT | 0.24 | |
| Vancomycin | NT | >31.2 | NT | 0.98 | |
| Methicillin | NT | >31.2 | NT | 0.49 | |
Kirby–Bauer assay
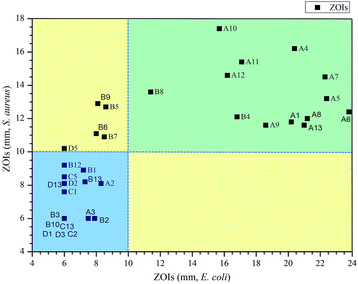
Kirby–Bauer assay was used to test the compounds at a single concentration of 5 mg mL−1 to determine whether they inhibited bacterial growth. The data are exhibited in Table 1. In this assay, sterile filter disks with a diameter of 6 mm were used. The diameter of ZOIs greater than 10.0 mm was defined as strongly active, 7.0–10.0 mm was weakly active, and less than 7.0 mm was inactive. As shown in Fig. 3, 13 compounds all from series A or B appear in the green area which signifies compounds showing potent activity against both Gram-negative bacteria, E. coli and Gram-positive bacteria S. aureus. In the left-hand side yellow area, 5 compounds from series B and D showed some activity against Gram-positive S. aureus but weak activity against Gram-negative E. coli. No compound showed noticeably stronger activity against Gram-negative E. coli than Gram-positive S. aureus, represented by the blue coloured area in Fig. 3. From this analysis, it also indicated that while a number of series A quinazoline analogues have activities against both Gram-positive and Gram-negative bacteria, series B thieno[3,2-d]pyrimidines were more biased to Gram-positive bacteria and series C and D compounds had limited activities in inhibiting the growth of these bacterial strains.Minimum inhibitory concentrations (MICs) assay
All N4-benzylamine-N2-isopropyl-quinazoline-2,4-diamines derivatives with measurable ZOIs in the Kirby–Bauer assays were tested in minimum inhibitory concentration (MIC) assays against the same two strains of bacteria to quantitatively determine their antibacterial potency. Norfloxacin, vancomycin and methicillin were used as positive controls.In general, the results of the MIC assay mirrored the results from the single concentration Kirby–Bauer assay, and the antibacterial potency of the chemical series are in the order of quinazolines > thieno[3,2-d]pyrimidines > pyrido[3,2-d]pyrimidines ≈ pyrido[2,3-d]pyrimidines, while higher potency was observed against Gram-positive S. aureus than against Gram-negative E. coli. (Table 1). In the most active subset, series A, substitutions on the benzene ring in the N4-benzylamin side-chain had a significant effect of the potency of compounds. Lipophilic electron withdrawing group substitution in this side-chain, such as CF3 and Cl were beneficial for antibacterial activity. On the other hand, the more polar substitutions i.e. SO2Me or CN were not tolerated (A1–3). Similar observations were also seen after the incorporation of an additional nitrogen in this side-chain (A13) which reduced both lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D) and potency compared with the corresponding analogue A5 without the nitrogen (further discussion regarding the effect of the structural modifications to the physiochemical properties are described in a following session). Series A analogues, with substitutions at the para-position of the benzene ring, were more potent against Gram-negative E. coli than the corresponding ortho-substituted analogues, but these substitutions had little effect on potency against Gram-positive S. aureus (A4 vs. A5 and A8 vs. A9). Quinazoline derivative A5 with a trifluoromethyl group at the para-position of the benzene ring was the most active compound in this subset of compounds and indeed in this whole set of compounds with MICs = 3.9 μg mL−1 against both Gram-positive and negative bacteria, S. aureus and E. coli. It was closely followed by the chloro-substituted analogue at the same position (A9) that has the same potency against S. aureus but slightly reduced potency (MIC = 7.8 μg mL−1) against Gram-negative E. coli. The series B thieno[3,2-d]pyrimidine analogues also showed some activity against both strains of bacteria, but they were less active than their corresponding quinazoline core analogues in general. The SAR observed in series B compounds is very similar to the SAR of series A. While the replacement of the quinazoline core to a theino[3,2-d]pyrimidine core had less effect on the potency against Gram-positive S. aureus (2–4 fold reductions), this modification resulted in a noticeable reduction of antibacterial activity against Gram-negative E. coli (2–32 fold reductions). Compounds in both series C and D with an additional nitrogen incorporated into the 5- or 8-position of the quinazoline core showed significantly reduced potency against both strains of bacteria. Two compounds with p-CF3 substitutions on the N4-benzylamine side-chain (C5 and D5) in these two series showed moderate activities against S. aureus (MIC = 15.6 μg mL−1 and 7.8 μg mL−1, respectively), but no activity against E. coli.
D) and potency compared with the corresponding analogue A5 without the nitrogen (further discussion regarding the effect of the structural modifications to the physiochemical properties are described in a following session). Series A analogues, with substitutions at the para-position of the benzene ring, were more potent against Gram-negative E. coli than the corresponding ortho-substituted analogues, but these substitutions had little effect on potency against Gram-positive S. aureus (A4 vs. A5 and A8 vs. A9). Quinazoline derivative A5 with a trifluoromethyl group at the para-position of the benzene ring was the most active compound in this subset of compounds and indeed in this whole set of compounds with MICs = 3.9 μg mL−1 against both Gram-positive and negative bacteria, S. aureus and E. coli. It was closely followed by the chloro-substituted analogue at the same position (A9) that has the same potency against S. aureus but slightly reduced potency (MIC = 7.8 μg mL−1) against Gram-negative E. coli. The series B thieno[3,2-d]pyrimidine analogues also showed some activity against both strains of bacteria, but they were less active than their corresponding quinazoline core analogues in general. The SAR observed in series B compounds is very similar to the SAR of series A. While the replacement of the quinazoline core to a theino[3,2-d]pyrimidine core had less effect on the potency against Gram-positive S. aureus (2–4 fold reductions), this modification resulted in a noticeable reduction of antibacterial activity against Gram-negative E. coli (2–32 fold reductions). Compounds in both series C and D with an additional nitrogen incorporated into the 5- or 8-position of the quinazoline core showed significantly reduced potency against both strains of bacteria. Two compounds with p-CF3 substitutions on the N4-benzylamine side-chain (C5 and D5) in these two series showed moderate activities against S. aureus (MIC = 15.6 μg mL−1 and 7.8 μg mL−1, respectively), but no activity against E. coli.
The MIC assay was used to evaluate the eight most active compounds against S. aureus (as described above) namely, A4, A5, A7–9, A11, B5 and, B9 against a strain of MRSA. MICs of these selected compounds against MRSA were only two to four folds higher than the corresponding MICs against the susceptible strain of S. aureus. The MIC difference seen with this group of compounds is similar to the MIC differences seen with the two positive controls, i.e. Norfloxacin and vancomycin (2–3 folds), but is significantly less than that seen with methicillin (∼16 folds). These results suggest that this new class of compound has minimal cross resistance with this strain of MRSA although further studies are required to formally confirm this using a broader range of MRSA lines with unique and well characterized resistance mechanisms. The most potent compound against this strain of MRSA was A5, the p-CF3 substituted analogue from series A.
In order to extend our understanding of the antibacterial activities of this class of compounds, the same group of eight active compounds from series A and B were also tested against additional strains of Gram-positive and Gram-negative bacteria, namely S. epidermidis and S. typhimurium using the MIC assay. Overall the antibacterial activities of these eight selected compounds against these additional bacterial lines matched very well with those against S. aureus and E. coli (Table 2). Most of the tested compounds showed good activities against Gram-positive S. epidermidis at similar levels to those observed with S. aureus, but their potency against Gram-negative S. typhimurium was considerably lower than their potency against other tested strains of bacteria. Both quinazoline analogues with CF3 substituted at either ortho- or para-positions in the N4-benzylamine side-chain (A4 and A5) were the most potent compounds from the assays against S. epidermidis and S. typhimurium with MIC = 3.9 μg mL−1 and 15.6 μg mL−1, respectively.
| Compound | MRSAa | S. epidermidisb | S. typhimuriumc |
|---|---|---|---|
| MICs (μg mL−1) | MICs (μg mL−1) | MICs (μg mL−1) | |
| a Methicillin-resistant Staphylococcus aureus (MRSA), ATCC 43300.b Staphylococcus epidermidis, ATCC 12228.c Salmonella typhimurium, CMCC 50115. | |||
| A4 | 15.6 | 3.9 | 15.6 |
| A5 | 7.8 | 3.9 | 15.6 |
| A7 | 31.2 | 7.8 | 15.6 |
| A8 | 15.6 | 7.8 | 62.5 |
| A9 | 15.6 | 7.8 | 15.6 |
| A11 | 31.2 | 15.6 | 31.2 |
| B5 | 15.6 | 7.8 | ≥125.0 |
| B9 | 15.6 | 7.8 | 31.2 |
| Norfloxacin | 0.97 | 1.95 | ≤0.12 |
| Vancomycin | 1.95 | 3.9 | ≥31.2 |
| Methicillin | 7.8 | 0.24 | ≥31.2 |
Physicochemical properties and in vitro metabolic stability studies
Along with the SAR studies of these compounds, selected derivatives were also evaluated for their DMPK properties including physicochemical properties such as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4, aqueous solubility and plasma protein binding, and in vitro metabolic stability against human microsome and rat hepatocytes (Table 3). Overall, the more active compounds, such as A4, A5, A8 and A9 showed high lipophilicity (log
D7.4, aqueous solubility and plasma protein binding, and in vitro metabolic stability against human microsome and rat hepatocytes (Table 3). Overall, the more active compounds, such as A4, A5, A8 and A9 showed high lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4) and low aqueous solubility in PBS buffer. Replacing the benzene ring of quinazoline scaffold with a thiophene ring (B5 vs. A5 and B9 vs. A9) did not alter lipophilicity but increased the aqueous solubility slightly. Incorporation of nitrogens in different parts of the molecules (A5 vs. A13 or D5) or replacement of CF3 group with an OMe group (A5 vs. A11) resulted in reductions of lipophilicity and improvement of solubility, but all manipulations resulted in decreased antibacterial activities. Based on the available SAR and the data of log
D7.4) and low aqueous solubility in PBS buffer. Replacing the benzene ring of quinazoline scaffold with a thiophene ring (B5 vs. A5 and B9 vs. A9) did not alter lipophilicity but increased the aqueous solubility slightly. Incorporation of nitrogens in different parts of the molecules (A5 vs. A13 or D5) or replacement of CF3 group with an OMe group (A5 vs. A11) resulted in reductions of lipophilicity and improvement of solubility, but all manipulations resulted in decreased antibacterial activities. Based on the available SAR and the data of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 in Table 3, it suggested there is a potential positive correlation between lipophilicity and antibacterial activity. Compounds A4, A5, A8, A9, B5 and B9, which have log
D7.4 in Table 3, it suggested there is a potential positive correlation between lipophilicity and antibacterial activity. Compounds A4, A5, A8, A9, B5 and B9, which have log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 values higher than 4, showed good activities against S. aureus with MICs = 3.9 μg mL−1. On the contrary, compounds A7, A11 and A13, which have log
D7.4 values higher than 4, showed good activities against S. aureus with MICs = 3.9 μg mL−1. On the contrary, compounds A7, A11 and A13, which have log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 values from 3.3 to 3.6, showed a 2-fold or 8-fold less active against the same bacterium. The percentage of human plasma protein binding for those measured compounds also showed positive correlation with lipophilicity. Although the plasma protein bindings are in the relatively high range of percentage (96.5–99.9%) it is not uncommon for anti-infective agents. In terms of metabolic stability, most of the quinazoline analogues showed acceptable stability in vitro against both human microsome and rat hepatocytes, and are more stable than the corresponding thienopyrimidine analogues (A5 vs. B5 and A9 vs. B9). The azaquinazoline analogue D5 had the best in vitro metabolic stability against both human microsome and rat hepatocytes, but this modification was not well tolerated in the antibacterial SAR.
D7.4 values from 3.3 to 3.6, showed a 2-fold or 8-fold less active against the same bacterium. The percentage of human plasma protein binding for those measured compounds also showed positive correlation with lipophilicity. Although the plasma protein bindings are in the relatively high range of percentage (96.5–99.9%) it is not uncommon for anti-infective agents. In terms of metabolic stability, most of the quinazoline analogues showed acceptable stability in vitro against both human microsome and rat hepatocytes, and are more stable than the corresponding thienopyrimidine analogues (A5 vs. B5 and A9 vs. B9). The azaquinazoline analogue D5 had the best in vitro metabolic stability against both human microsome and rat hepatocytes, but this modification was not well tolerated in the antibacterial SAR.
| Compound | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 D7.4 |
Aq. Sol.a (μM) | H. Mics CLintb (μl min−1 mg) | R. Heps CLintc (μl min−1 per 106 cells) | H. PPBd % |
|---|---|---|---|---|---|
| a Aqueous solubility in pH 7.4 PBS.b Human microsomes intrinsic clearance.c Rat hepatocytes intrinsic clearance.d Human plasma protein binding. | |||||
| A4 | 4.6 | 0.2 | 9.2 | 25 | 99.6 |
| A5 | 4.7 | 0.5 | 29 | 45 | 99.8 |
| A7 | 3.6 | 27 | 32 | 37 | 98.0 |
| A8 | 4.2 | 5 | 19 | 98 | 98.9 |
| A9 | 4.3 | 4 | 36 | 51 | 99.2 |
| A11 | 3.3 | 40 | 37 | >300 | 97.4 |
| A13 | 3.3 | 17 | 45 | 7.8 | 96.5 |
| B5 | 4.5 | 3.7 | 70 | 76 | 99.9 |
| B9 | 4.8 | 11 | 156 | 217 | 99.8 |
| D5 | 4.4 | 3 | 7.6 | 6.5 | 98.5 |
Conclusions
In this article, 36 N2,N4-disubstituted quinazoline-2,4-diamines and closely related derivatives with potential antibacterial activity were designed and synthesized. Our SAR study demonstrated that lipophilic electron withdrawing groups, such as trifluoromethyl and chloro, in the N4-benzylamine side-chain were beneficial for antibacterial potency, while substitution at the 4-position of the benzene ring was advantageous compared with substitutions at the 2-position. Changing the quinazoline scaffold to a thieno[3,2-d]pyrimidine, series B compounds, still showed limited antibacterial activity compared with the corresponding quinazoline analogues. Scaffold morphing to pyrido[3,2-d]pyrimidine or pyrido[2,3-d]pyrimidine was not tolerated in the SAR and most of the compounds bearing these two cores were significantly less potent than the parent quinazolines. Screens against one strain of MRSA suggested this class of compounds had limited cross resistance to methicillin although the exact protein target(s) for this class of compounds needs to be further investigated against a broader selection of MRSA bacteria. The results from the screens against S. aureus and E. coli in the SAR study and the additional screens against S. epidermidis and S. typhimurium demonstrated a broad spectrum of antibacterial activity against both Gram-positive and Gram-negative bacteria while the potency against Gram-positive bacteria was generally higher than the latter. The in vitro DMPK screening results suggested that some of the lead series A quinazolines had acceptable metabolic stability in vitro, their physiochemical properties, aqueous solubility in particular would need to be improved if they were to be developed further. The most active compound A5 showed good potency against E. coli, S. aureus, S. epidermidis, S. typhimurium and MRSA. Although the cytotoxicity of these series of compounds were not determined yet, the cytotoxicity of some closed analogues were reported by our group and others (Van Horn 2014, Devine 2015 and Johnston 2017), and there is no obvious risk of cytotoxicity for these chemotypes in general.25,31,32 Further investigation of cytotoxicity of selected leads will be carried out in the next stage of optimisation and evaluation to mitigate any potential risk in this area. The results from this work indicate the potential of this chemical series and directions for further investigation of this chemotype as potential antibacterial agents.Experimental
All chemicals, reagents and solvents were obtained from commercial sources and used without further purification. 1H and 13C NMR spectra were recorded on a Bruker Avance III 400 MHz Superconducting Fourier system in the solvent indicated. Chemical shifts were reported in units of ppm on the delta (δ) scale and coupling constants (J) were reported in units of Hz. Data for 1H NMR were reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), integration and coupling constant (Hz), while 13C NMR analyses were reported in terms of chemical shift. Thin-layer chromatography was performed on silica gel 60 F254 aluminium sheets from Merck KGaA. Melting points were determined by using a microscopic melting point instrument and were uncorrected. High resolution mass spectra (HRMS) were performed on a Bruker maXis impact system.General method for the synthesis of compounds A, B, C, D
A1 N4-(4-Cyanobenzyl)-N2-isopropylquinazoline-2,4-diamine. Yellow solid; yield, 75%; Rf = 0.36 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, DMSO-d6) δ 10.35 (s, 1H), 8.38 (s, 1H), 7.80 (d, J = 8.3 Hz, 2H), 7.78–7.72 (m, 1H), 7.59 (d, J = 8.2 Hz, 2H), 7.37 (s, 1H), 4.84 (d, J = 5.5 Hz, 2H), 4.06 (s, 1H), 1.08 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 159.90, 158.94, 144.21, 139.90, 138.89, 135.05, 132.28, 128.26, 128.17, 124.24, 123.70, 118.75, 109.73, 62.76, 42.89, 22.03. HRMS: m/z calcd for C19H20N5 [M + H]+ 318.1713; found 318.1719. Melting point 257–260 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, DMSO-d6) δ 10.35 (s, 1H), 8.38 (s, 1H), 7.80 (d, J = 8.3 Hz, 2H), 7.78–7.72 (m, 1H), 7.59 (d, J = 8.2 Hz, 2H), 7.37 (s, 1H), 4.84 (d, J = 5.5 Hz, 2H), 4.06 (s, 1H), 1.08 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 159.90, 158.94, 144.21, 139.90, 138.89, 135.05, 132.28, 128.26, 128.17, 124.24, 123.70, 118.75, 109.73, 62.76, 42.89, 22.03. HRMS: m/z calcd for C19H20N5 [M + H]+ 318.1713; found 318.1719. Melting point 257–260 °C.
A2 N2-Isopropyl-N4-(2-(methylsulfonyl)benzyl)quinazoline-2,4-diamine. Brown solid; yield, 49%; Rf = 0.42 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.9, 1.3 Hz, 1H), 7.71 (dd, J = 7.6, 0.8 Hz, 1H), 7.64–7.56 (m, 2H), 7.55–7.47 (m, 2H), 7.39 (d, J = 8.0 Hz, 1H), 7.14–7.08 (m, 1H), 5.15 (d, J = 5.6 Hz, 2H), 4.29 (m, 1H), 3.18 (s, 3H), 1.27 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.11, 156.10, 146.37, 137.59, 137.55, 133.57, 133.36, 129.09, 129.02, 127.57, 122.05, 122.00, 120.69, 109.96, 43.48, 42.45, 41.53, 21.67. HRMS: m/z calcd for C19H23N4O2S [M + H]+ 371.1536; found 371.1540. Melting point 171–173 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.9, 1.3 Hz, 1H), 7.71 (dd, J = 7.6, 0.8 Hz, 1H), 7.64–7.56 (m, 2H), 7.55–7.47 (m, 2H), 7.39 (d, J = 8.0 Hz, 1H), 7.14–7.08 (m, 1H), 5.15 (d, J = 5.6 Hz, 2H), 4.29 (m, 1H), 3.18 (s, 3H), 1.27 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.11, 156.10, 146.37, 137.59, 137.55, 133.57, 133.36, 129.09, 129.02, 127.57, 122.05, 122.00, 120.69, 109.96, 43.48, 42.45, 41.53, 21.67. HRMS: m/z calcd for C19H23N4O2S [M + H]+ 371.1536; found 371.1540. Melting point 171–173 °C.
A3 N2-Isopropyl-N4-(4-(methylsulfonyl)benzyl)quinazoline-2,4-diamine. Brown solid; yield, 53%; Rf = 0.39 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 6.9 Hz, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.50–7.47 (m, 2H), 7.46 (d, J = 7.2 Hz 1H), 7.32 (d, J = 8.3 Hz, 1H), 7.07 (t, J = 7.6 Hz, 1H), 4.85 (s, 2H), 4.07 (m, 1H), 2.97 (s, 3H), 1.109 (d, J = 6.4 Hz 6H). 13C NMR (100 MHz, CDCl3) δ 160.52, 155.15, 151.64, 145.21, 139.02, 134.17, 128.10, 127.42, 122.94, 122.67, 119.93, 110.00, 44.37, 44.11, 43.14, 22.25. HRMS: m/z calcd for C19H23N4O2S [M + H]+ 371.1536; found 371.1539. Melting point 175–177 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 6.9 Hz, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.50–7.47 (m, 2H), 7.46 (d, J = 7.2 Hz 1H), 7.32 (d, J = 8.3 Hz, 1H), 7.07 (t, J = 7.6 Hz, 1H), 4.85 (s, 2H), 4.07 (m, 1H), 2.97 (s, 3H), 1.109 (d, J = 6.4 Hz 6H). 13C NMR (100 MHz, CDCl3) δ 160.52, 155.15, 151.64, 145.21, 139.02, 134.17, 128.10, 127.42, 122.94, 122.67, 119.93, 110.00, 44.37, 44.11, 43.14, 22.25. HRMS: m/z calcd for C19H23N4O2S [M + H]+ 371.1536; found 371.1539. Melting point 175–177 °C.
A4 N2-Isopropyl-N4-(2-(trifluoromethyl)benzyl)quinazoline-2,4-diamine. Yellow solid; yield, 26%; Rf = 0.29 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, DMSO-d6) δ 10.01 (s, 1H), 8.35 (s, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 10.0 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.33 (s, 1H), 4.95 (d, J = 4.4 Hz, 2H), 4.01–3.83 (m, 1H), 1.02 (dd, J = 22.3, 15.7 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 163.46, 162.50, 160.05, 150.15, 138.99, 136.68, 136.03, 132.66, 131.96, 127.73, 127.34, 126.61, 126.15, 125.85, 125.80, 123.17, 42.54, 41.04, 21.93. HRMS: m/z calcd for C19H20F3N4 [M + H]+ 361.1635; found 361.1639. Melting point 231–233 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, DMSO-d6) δ 10.01 (s, 1H), 8.35 (s, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 10.0 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.33 (s, 1H), 4.95 (d, J = 4.4 Hz, 2H), 4.01–3.83 (m, 1H), 1.02 (dd, J = 22.3, 15.7 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 163.46, 162.50, 160.05, 150.15, 138.99, 136.68, 136.03, 132.66, 131.96, 127.73, 127.34, 126.61, 126.15, 125.85, 125.80, 123.17, 42.54, 41.04, 21.93. HRMS: m/z calcd for C19H20F3N4 [M + H]+ 361.1635; found 361.1639. Melting point 231–233 °C.
A5 N2-Isopropyl-N4-(4-(trifluoromethyl)benzyl)quinazoline-2,4-diamine. Yellow solid; yield, 19%; Rf = 0.47 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.0 Hz, 1H), 7.52 (t, J = 5.6 Hz, 2H), 7.48 (dd, J = 6.9, 1.2 Hz, 1H), 7.42 (d, J = 4.5 Hz, 2H), 7.41 (s, 1H), 7.07–7.00 (m, 1H), 6.62 (s, 1H), 4.82 (d, J = 5.0 Hz, 3H), 4.17 (m, 1H), 1.14 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.21, 158.84, 151.97, 143.19, 133.03, 129.75, 127.92, 125.65, 125.29, 122.92, 121.17, 121.01, 110.72, 44.59, 42.88, 23.26. HRMS: m/z calcd for C19H20F3N4 [M + H]+ 361.1635; found 361.1639. Melting point 69–71 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.0 Hz, 1H), 7.52 (t, J = 5.6 Hz, 2H), 7.48 (dd, J = 6.9, 1.2 Hz, 1H), 7.42 (d, J = 4.5 Hz, 2H), 7.41 (s, 1H), 7.07–7.00 (m, 1H), 6.62 (s, 1H), 4.82 (d, J = 5.0 Hz, 3H), 4.17 (m, 1H), 1.14 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.21, 158.84, 151.97, 143.19, 133.03, 129.75, 127.92, 125.65, 125.29, 122.92, 121.17, 121.01, 110.72, 44.59, 42.88, 23.26. HRMS: m/z calcd for C19H20F3N4 [M + H]+ 361.1635; found 361.1639. Melting point 69–71 °C.
A6 N4-(2-Fluorobenzyl)-N2-isopropylquinazoline-2,4-diamine. Yellow oil; yield, 89%; Rf = 0.32 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 5.6 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.36 (t, J = 7.1 Hz, 2H), 7.19 (d, J = 7.8 Hz, 1H), 7.17 (dd, J = 4.7, 2.8 Hz, 1H), 7.05–7.00 (m, 1H), 6.98 (d, J = 10.0 Hz, 1H), 4.86 (d, J = 3.5 Hz, 2H), 4.14 (ddd, J = 18.0, 12.2, 5.3 Hz, 3H), 1.15 (d, J = 6.7 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 163.65, 162.04, 160.65, 160.43, 159.59, 152.91, 134.92, 129.74, 129.39, 124.56, 124.32, 124.11, 115.55, 109.62, 43.83, 40.46, 22.46. HRMS: m/z calcd for C18H20N4 [M + H]+ 311.1667; found 311.1669.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 5.6 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.36 (t, J = 7.1 Hz, 2H), 7.19 (d, J = 7.8 Hz, 1H), 7.17 (dd, J = 4.7, 2.8 Hz, 1H), 7.05–7.00 (m, 1H), 6.98 (d, J = 10.0 Hz, 1H), 4.86 (d, J = 3.5 Hz, 2H), 4.14 (ddd, J = 18.0, 12.2, 5.3 Hz, 3H), 1.15 (d, J = 6.7 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 163.65, 162.04, 160.65, 160.43, 159.59, 152.91, 134.92, 129.74, 129.39, 124.56, 124.32, 124.11, 115.55, 109.62, 43.83, 40.46, 22.46. HRMS: m/z calcd for C18H20N4 [M + H]+ 311.1667; found 311.1669.
A7 N4-(4-Fluorobenzyl)-N2-isopropylquinazoline-2,4-diamine. Yellow solid; yield, 83%; Rf = 0.29 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 9.71 (s, NH), 8.49 (s, NH), 7.47 (t, J = 7.7 Hz, 1H), 7.37 (dd, J = 8.3, 5.5 Hz, 2H), 7.25 (d, J = 5.7 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 6.87 (t, J = 8.6 Hz, 2H), 4.80 (s, 2H), 4.17 (m, 1H), 1.18 (d, J = 6.5 Hz, 5H). 13C NMR (100 MHz, CDCl3) δ 163.51, 161.07, 160.22, 134.50, 133.60, 129.77, 129.69, 123.62, 115.66, 115.45, 109.87, 63.88, 44.75, 43.69, 22.72. HRMS: m/z calcd for C18H20N4 [M + H]+ 311.1667; found 311.1669. Melting point 240–243 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 9.71 (s, NH), 8.49 (s, NH), 7.47 (t, J = 7.7 Hz, 1H), 7.37 (dd, J = 8.3, 5.5 Hz, 2H), 7.25 (d, J = 5.7 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 6.87 (t, J = 8.6 Hz, 2H), 4.80 (s, 2H), 4.17 (m, 1H), 1.18 (d, J = 6.5 Hz, 5H). 13C NMR (100 MHz, CDCl3) δ 163.51, 161.07, 160.22, 134.50, 133.60, 129.77, 129.69, 123.62, 115.66, 115.45, 109.87, 63.88, 44.75, 43.69, 22.72. HRMS: m/z calcd for C18H20N4 [M + H]+ 311.1667; found 311.1669. Melting point 240–243 °C.
A8 N4-(2-Chlorobenzyl)-N2-isopropylquinazoline-2,4-diamine. Yellow solid; yield, 39%; Rf = 0.31 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.19–8.13 (m, 1H), 7.58 (td, J = 7.2, 3.2 Hz, 1H), 7.42 (d, J = 7.0 Hz, 1H), 7.37–7.27 (m, 3H), 7.21–7.15 (m, 2H), 4.90 (d, J = 2.3 Hz, 2H), 4.13 (m, 1H), 1.14 (d, J = 6.5 Hz 6H). 13C NMR (100 MHz, CDCl3) δ 160.37, 152.21, 138.98, 135.00, 134.60, 133.02, 129.48, 128.69, 128.56, 126.88, 124.39, 123.67, 116.89, 109.49, 43.79, 43.03, 21.97. HRMS: m/z calcd for C18H20ClN4 [M + H]+ 327.1371; found 327.1374. Melting point 209–212 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.19–8.13 (m, 1H), 7.58 (td, J = 7.2, 3.2 Hz, 1H), 7.42 (d, J = 7.0 Hz, 1H), 7.37–7.27 (m, 3H), 7.21–7.15 (m, 2H), 4.90 (d, J = 2.3 Hz, 2H), 4.13 (m, 1H), 1.14 (d, J = 6.5 Hz 6H). 13C NMR (100 MHz, CDCl3) δ 160.37, 152.21, 138.98, 135.00, 134.60, 133.02, 129.48, 128.69, 128.56, 126.88, 124.39, 123.67, 116.89, 109.49, 43.79, 43.03, 21.97. HRMS: m/z calcd for C18H20ClN4 [M + H]+ 327.1371; found 327.1374. Melting point 209–212 °C.
A9 N4-(4-Chlorobenzyl)-N2-isopropylquinazoline-2,4-diamine. Yellow oil; yield, 94%; Rf = 0.26 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 7.7 Hz, 1H), 7.49 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 8.3 Hz, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 6.21–6.09 (m, 2H), 4.76 (s, 2H), 4.14 (dt, J = 11.4, 5.0 Hz, 1H), 1.19 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 172.91, 160.45, 152.77, 139.19, 136.22, 134.97, 133.35, 129.25, 128.74, 124.31, 116.95, 109.75, 44.85, 43.89, 22.51. HRMS: m/z calcd for C18H20ClN4 [M + H]+ 327.1371; found 327.1373.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 7.7 Hz, 1H), 7.49 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 8.3 Hz, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 6.21–6.09 (m, 2H), 4.76 (s, 2H), 4.14 (dt, J = 11.4, 5.0 Hz, 1H), 1.19 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 172.91, 160.45, 152.77, 139.19, 136.22, 134.97, 133.35, 129.25, 128.74, 124.31, 116.95, 109.75, 44.85, 43.89, 22.51. HRMS: m/z calcd for C18H20ClN4 [M + H]+ 327.1371; found 327.1373.
A10 N2-Isopropyl-N4-(2-methoxybenzyl)quinazoline-2,4-diamine. White solid; yield, 70%; Rf = 0.31 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 7.4 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.28 (d, J = 7.2 Hz 1H), 7.23 (d, J = 7.8 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 6.87 (d, J = 6.6 Hz, 1H), 6.85 (d, J = 6.6 Hz, 1H), 4.84 (d, J = 5.0 Hz, 2H), 4.23 (m, 6.3 Hz, 1H), 3.88 (s, 3H), 1.25 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.09, 157.57, 134.60, 129.89, 129.42, 129.18, 125.18, 123.71, 122.98, 120.91, 120.76, 110.75, 110.58, 109.71, 55.59, 43.78, 41.41, 22.62. HRMS: m/z calcd for C19H23N4O [M + H]+ 323.1866; found 323.1870. Melting point 138–140 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 7.4 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.28 (d, J = 7.2 Hz 1H), 7.23 (d, J = 7.8 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 6.87 (d, J = 6.6 Hz, 1H), 6.85 (d, J = 6.6 Hz, 1H), 4.84 (d, J = 5.0 Hz, 2H), 4.23 (m, 6.3 Hz, 1H), 3.88 (s, 3H), 1.25 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.09, 157.57, 134.60, 129.89, 129.42, 129.18, 125.18, 123.71, 122.98, 120.91, 120.76, 110.75, 110.58, 109.71, 55.59, 43.78, 41.41, 22.62. HRMS: m/z calcd for C19H23N4O [M + H]+ 323.1866; found 323.1870. Melting point 138–140 °C.
A11 N2-Isopropyl-N4-(4-methoxybenzyl)quinazoline-2,4-diamine. Yellow solid; yield, 73%; Rf = 0.31 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.29 (s, 1H), 7.45 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 8.6 Hz, 2H), 7.27 (d, J = 10.2 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 6.77 (d, J = 8.4 Hz, 2H), 4.78 (d, J = 4.2 Hz, 2H), 4.24 (m, 1H), 3.73 (s, 3H), 1.24 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.18, 160.11, 159.21, 134.63, 129.80, 129.56, 129.51, 123.97, 114.26, 114.21, 114.08, 110.01, 55.41, 44.98, 43.81, 22.72. HRMS: m/z calcd for C19H23N4O [M + H]+ 323.1866; found 323.1869. Melting point 208–210 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.29 (s, 1H), 7.45 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 8.6 Hz, 2H), 7.27 (d, J = 10.2 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 6.77 (d, J = 8.4 Hz, 2H), 4.78 (d, J = 4.2 Hz, 2H), 4.24 (m, 1H), 3.73 (s, 3H), 1.24 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.18, 160.11, 159.21, 134.63, 129.80, 129.56, 129.51, 123.97, 114.26, 114.21, 114.08, 110.01, 55.41, 44.98, 43.81, 22.72. HRMS: m/z calcd for C19H23N4O [M + H]+ 323.1866; found 323.1869. Melting point 208–210 °C.
A12 N4-(3,4-Dimethoxybenzyl)-N2-isopropylquinazoline-2,4-diamine. Yellow solid; yield, 15%; Rf = 0.39 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H), 7.44 (t, J = 7.7 Hz, 1H), 7.27 (d, J = 10.7 Hz, 1H), 7.11 (t, J = 7.6 Hz, 1H), 7.04 (d, J = 1.3 Hz, 1H), 6.95 (dd, J = 8.2, 1.4 Hz, 1H), 6.74 (d, J = 8.2 Hz, 1H), 4.77 (s, 2H), 4.25 (m, 1H), 3.81 (s, 3H), 3.80 (s, 3H), 1.24 (d, J = 6.4 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.19, 154.16, 149.26, 148.68, 148.65, 134.36, 130.48, 130.02, 123.87, 123.56, 120.57, 111.85, 111.31, 110.13, 56.08, 56.05, 45.27, 43.66, 22.75. HRMS: m/z calcd for C20H25N4O2 [M + H]+ 353.1972; found 353.1976. Melting point 192–194 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H), 7.44 (t, J = 7.7 Hz, 1H), 7.27 (d, J = 10.7 Hz, 1H), 7.11 (t, J = 7.6 Hz, 1H), 7.04 (d, J = 1.3 Hz, 1H), 6.95 (dd, J = 8.2, 1.4 Hz, 1H), 6.74 (d, J = 8.2 Hz, 1H), 4.77 (s, 2H), 4.25 (m, 1H), 3.81 (s, 3H), 3.80 (s, 3H), 1.24 (d, J = 6.4 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.19, 154.16, 149.26, 148.68, 148.65, 134.36, 130.48, 130.02, 123.87, 123.56, 120.57, 111.85, 111.31, 110.13, 56.08, 56.05, 45.27, 43.66, 22.75. HRMS: m/z calcd for C20H25N4O2 [M + H]+ 353.1972; found 353.1976. Melting point 192–194 °C.
A13 N2-Isopropyl-N4-((6-(trifluoromethyl)pyridin-3-yl)methyl)quinazoline-2,4-diamine. Yellow solid; yield, 23%; Rf = 0.39 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.78 (s, 1H), 8.08 (d, J = 6.3 Hz, 1H), 8.07 (s, 1H), 7.79 (d, J = 8.1 Hz, 1H), 7.70 (t, J = 7.7 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.33 (t, J = 7.7 Hz, 1H), 4.95 (s, 2H), 4.12 (dq, J = 12.5, 6.3 Hz, 1H), 1.17 (d, J = 6.4 Hz, 6H). 13C NMR (101 MHz, CD3OD) δ 162.08, 155.98, 150.39, 147.70, 144.62, 139.76, 138.25, 135.77, 124.75, 124.37, 121.72, 121.70, 120.28, 111.44, 44.59, 43.34, 22.71. HRMS: m/z calcd for C19H20F3N4 [M + H]+ 362.1587; found 362.1590. Melting point 202–205 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.78 (s, 1H), 8.08 (d, J = 6.3 Hz, 1H), 8.07 (s, 1H), 7.79 (d, J = 8.1 Hz, 1H), 7.70 (t, J = 7.7 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.33 (t, J = 7.7 Hz, 1H), 4.95 (s, 2H), 4.12 (dq, J = 12.5, 6.3 Hz, 1H), 1.17 (d, J = 6.4 Hz, 6H). 13C NMR (101 MHz, CD3OD) δ 162.08, 155.98, 150.39, 147.70, 144.62, 139.76, 138.25, 135.77, 124.75, 124.37, 121.72, 121.70, 120.28, 111.44, 44.59, 43.34, 22.71. HRMS: m/z calcd for C19H20F3N4 [M + H]+ 362.1587; found 362.1590. Melting point 202–205 °C.
B1 N4-(4-Cyanobenzyl)-N2-isopropylthieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 61%; Rf = 0.31 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.63–7.58 (m, 2H), 7.56 (d, J = 5.3 Hz, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.11 (d, J = 5.3 Hz, 1H), 5.43 (s, NH), 4.96 (s, NH), 4.84 (d, J = 5.8 Hz, 2H), 4.07 (m, 1H), 1.16 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.09, 160.16, 157.48, 144.78, 132.52, 131.18, 128.13, 123.67, 118.88, 111.28, 106.04, 44.43, 43.23, 23.13. HRMS: m/z calcd for C17H17N5S [M + H]+ 324.1277; found 324.1281.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.63–7.58 (m, 2H), 7.56 (d, J = 5.3 Hz, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.11 (d, J = 5.3 Hz, 1H), 5.43 (s, NH), 4.96 (s, NH), 4.84 (d, J = 5.8 Hz, 2H), 4.07 (m, 1H), 1.16 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.09, 160.16, 157.48, 144.78, 132.52, 131.18, 128.13, 123.67, 118.88, 111.28, 106.04, 44.43, 43.23, 23.13. HRMS: m/z calcd for C17H17N5S [M + H]+ 324.1277; found 324.1281.
B2 N2-Isopropyl-N4-(2-(methylsulfonyl)benzyl)thieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 38%; Rf = 0.53 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.03–7.99 (m, 1H), 7.72 (d, J = 7.5 Hz, 1H), 7.56 (t, J = 7.4 Hz, 1H), 7.50 (d, J = 5.3 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.04 (d, J = 5.3 Hz, 1H), 5.88 (s, 1H), 5.10 (d, J = 6.3 Hz, 2H), 4.70 (d, J = 8.0 Hz, 1H), 4.17 (dq, J = 13.0, 6.5 Hz, 1H), 3.14 (s, 3H), 1.20 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.70, 160.53, 157.18, 138.64, 138.40, 134.08, 131.84, 130.89, 129.79, 128.40, 123.72, 106.24, 45.10, 42.99, 42.05, 23.21. HRMS: m/z calcd for C17H20N2NaO2S2 [M + Na]+ 399.0920; found 399.0925.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.03–7.99 (m, 1H), 7.72 (d, J = 7.5 Hz, 1H), 7.56 (t, J = 7.4 Hz, 1H), 7.50 (d, J = 5.3 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.04 (d, J = 5.3 Hz, 1H), 5.88 (s, 1H), 5.10 (d, J = 6.3 Hz, 2H), 4.70 (d, J = 8.0 Hz, 1H), 4.17 (dq, J = 13.0, 6.5 Hz, 1H), 3.14 (s, 3H), 1.20 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.70, 160.53, 157.18, 138.64, 138.40, 134.08, 131.84, 130.89, 129.79, 128.40, 123.72, 106.24, 45.10, 42.99, 42.05, 23.21. HRMS: m/z calcd for C17H20N2NaO2S2 [M + Na]+ 399.0920; found 399.0925.
B3 N2-Isopropyl-N4-(4-(methylsulfonyl)benzyl)thieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 31%; Rf = 0.56 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.3 Hz, 2H), 7.52 (d, J = 5.3 Hz, 1H), 7.49 (d, J = 8.2 Hz, 2H), 7.07 (d, J = 5.3 Hz, 1H), 5.82 (s, 1H), 4.82 (d, J = 5.9 Hz, 2H), 4.75 (d, J = 7.9 Hz, 1H), 4.05 (dq, J = 13.1, 6.5 Hz, 1H), 3.00 (s, 3H), 1.12 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.70, 160.46, 157.44, 146.04, 139.18, 130.98, 128.27, 127.59, 123.78, 106.06, 44.61, 44.08, 43.05, 23.12. HRMS: m/z calcd for C17H20N2NaO2S2 [M + Na]+ 399.0920; found 399.0924.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.3 Hz, 2H), 7.52 (d, J = 5.3 Hz, 1H), 7.49 (d, J = 8.2 Hz, 2H), 7.07 (d, J = 5.3 Hz, 1H), 5.82 (s, 1H), 4.82 (d, J = 5.9 Hz, 2H), 4.75 (d, J = 7.9 Hz, 1H), 4.05 (dq, J = 13.1, 6.5 Hz, 1H), 3.00 (s, 3H), 1.12 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.70, 160.46, 157.44, 146.04, 139.18, 130.98, 128.27, 127.59, 123.78, 106.06, 44.61, 44.08, 43.05, 23.12. HRMS: m/z calcd for C17H20N2NaO2S2 [M + Na]+ 399.0920; found 399.0924.
B4 N2-Isopropyl-N4-(2-(trifluoromethyl)benzyl)thieno[3,2-d]pyrimidine-2,4-diamine. Yellow solid; yield, 39%; Rf = 0.33 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 7.77 (d, J = 5.4 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.52 (d, J = 4.3 Hz, 2H), 7.38 (dt, J = 8.1, 4.1 Hz, 1H), 7.03 (d, J = 5.4 Hz, 1H), 4.95 (s, 2H), 4.00–3.89 (m, 1H), 1.04 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CD3OD) δ 161.85, 161.69, 159.04, 139.67, 133.28, 133.27, 132.88, 128.90, 127.95, 126.81, 123.49, 107.34, 43.87, 41.71, 23.02. HRMS: m/z calcd for C17H18F3N4S [M + H]+ 367.1199; found 367.1203. Melting point 135–137 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 7.77 (d, J = 5.4 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.52 (d, J = 4.3 Hz, 2H), 7.38 (dt, J = 8.1, 4.1 Hz, 1H), 7.03 (d, J = 5.4 Hz, 1H), 4.95 (s, 2H), 4.00–3.89 (m, 1H), 1.04 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CD3OD) δ 161.85, 161.69, 159.04, 139.67, 133.28, 133.27, 132.88, 128.90, 127.95, 126.81, 123.49, 107.34, 43.87, 41.71, 23.02. HRMS: m/z calcd for C17H18F3N4S [M + H]+ 367.1199; found 367.1203. Melting point 135–137 °C.
B5 N2-Isopropyl-N4-(4-(trifluoromethyl)benzyl)thieno[3,2-d]pyrimidine-2,4-diamine. Brown solid; yield, 47%; Rf = 0.31 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.2 Hz, 2H), 7.50 (d, J = 5.3 Hz, 1H), 7.43 (d, J = 8.1 Hz, 2H), 7.07 (d, J = 5.3 Hz, 1H), 5.97 (s, 1H), 5.14 (s, 1H), 4.81 (d, J = 5.6 Hz, 2H), 4.09 (dq, J = 13.2, 6.5 Hz, 1H), 1.15 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.49, 160.45, 157.54, 143.25, 130.91, 129.96, 127.89, 125.69, 123.81, 122.91, 105.96, 44.39, 43.17, 23.16. HRMS: m/z calcd for C17H18F3N4NaS [M + Na]+ 389.1018; found 389.1016. Melting point 62–64 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.2 Hz, 2H), 7.50 (d, J = 5.3 Hz, 1H), 7.43 (d, J = 8.1 Hz, 2H), 7.07 (d, J = 5.3 Hz, 1H), 5.97 (s, 1H), 5.14 (s, 1H), 4.81 (d, J = 5.6 Hz, 2H), 4.09 (dq, J = 13.2, 6.5 Hz, 1H), 1.15 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.49, 160.45, 157.54, 143.25, 130.91, 129.96, 127.89, 125.69, 123.81, 122.91, 105.96, 44.39, 43.17, 23.16. HRMS: m/z calcd for C17H18F3N4NaS [M + Na]+ 389.1018; found 389.1016. Melting point 62–64 °C.
B6 N4-(2-Fluorobenzyl)-N2-isopropylthieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 49%; Rf = 0.51 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 5.3 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.23 (m, 1H), 7.10–7.01 (m, 3H), 5.54 (s, NH), 5.16 (s, NH), 4.83 (d, J = 5.3 Hz, 2H), 4.16 (td, J = 13.0, 6.5 Hz, 1H), 1.20 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 162.35, 159.96, 157.55, 130.98, 130.01, 129.21, 125.82, 124.28, 123.25, 115.56, 115.35, 106.21, 43.19, 38.67, 23.14. HRMS: m/z calcd for C16H18FN4S [M + H]+ 317.1231; found 317.1234.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 5.3 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.23 (m, 1H), 7.10–7.01 (m, 3H), 5.54 (s, NH), 5.16 (s, NH), 4.83 (d, J = 5.3 Hz, 2H), 4.16 (td, J = 13.0, 6.5 Hz, 1H), 1.20 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 162.35, 159.96, 157.55, 130.98, 130.01, 129.21, 125.82, 124.28, 123.25, 115.56, 115.35, 106.21, 43.19, 38.67, 23.14. HRMS: m/z calcd for C16H18FN4S [M + H]+ 317.1231; found 317.1234.
B7 N4-(4-Fluorobenzyl)-N2-isopropylthieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 58%; Rf = 0.56 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.48 (dd, J = 5.3, 0.6 Hz, 1H), 7.29 (dd, J = 8.2, 5.5 Hz, 2H), 7.04 (d, J = 5.3 Hz, 1H), 6.96 (t, J = 8.3 Hz, 2H), 5.94 (s, 1H), 5.06 (s, 1H), 4.71 (d, J = 4.6 Hz, 2H), 4.13 (td, J = 13.4, 6.5 Hz, 1H), 1.17 (d, J = 6.4 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 163.24, 160.80, 159.34, 157.42, 134.63, 131.31, 129.40, 129.32, 122.55, 115.30, 106.33, 43.95, 43.12, 22.97. HRMS: m/z calcd for C16H18FN4S [M + H]+ 317.1231; found 317.1234.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.48 (dd, J = 5.3, 0.6 Hz, 1H), 7.29 (dd, J = 8.2, 5.5 Hz, 2H), 7.04 (d, J = 5.3 Hz, 1H), 6.96 (t, J = 8.3 Hz, 2H), 5.94 (s, 1H), 5.06 (s, 1H), 4.71 (d, J = 4.6 Hz, 2H), 4.13 (td, J = 13.4, 6.5 Hz, 1H), 1.17 (d, J = 6.4 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 163.24, 160.80, 159.34, 157.42, 134.63, 131.31, 129.40, 129.32, 122.55, 115.30, 106.33, 43.95, 43.12, 22.97. HRMS: m/z calcd for C16H18FN4S [M + H]+ 317.1231; found 317.1234.
B8 N4-(2-Chlorobenzyl)-N2-isopropylthieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 28%; Rf = 0.33 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 5.3 Hz, 1H), 7.39 (dd, J = 5.0, 4.2 Hz, 1H), 7.34 (dd, J = 5.8, 3.5 Hz, 1H), 7.19–7.17 (m, 1H), 7.16 (d, J = 5.1 Hz, 1H), 7.06 (d, J = 5.3 Hz, 1H), 4.84 (d, J = 5.9 Hz, 2H), 4.13 (dq, J = 19.5, 6.5 Hz, 1H), 1.16 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.61, 160.09, 157.43, 136.19, 133.37, 130.89, 129.56, 129.45, 128.60, 126.89, 123.30, 106.14, 43.05, 42.46, 23.06. HRMS: m/z calcd for C16H18ClN4S [M + H]+ 333.0935; found 333.0937.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 5.3 Hz, 1H), 7.39 (dd, J = 5.0, 4.2 Hz, 1H), 7.34 (dd, J = 5.8, 3.5 Hz, 1H), 7.19–7.17 (m, 1H), 7.16 (d, J = 5.1 Hz, 1H), 7.06 (d, J = 5.3 Hz, 1H), 4.84 (d, J = 5.9 Hz, 2H), 4.13 (dq, J = 19.5, 6.5 Hz, 1H), 1.16 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.61, 160.09, 157.43, 136.19, 133.37, 130.89, 129.56, 129.45, 128.60, 126.89, 123.30, 106.14, 43.05, 42.46, 23.06. HRMS: m/z calcd for C16H18ClN4S [M + H]+ 333.0935; found 333.0937.
B9 N4-(4-Chlorobenzyl)-N2-isopropylthieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 50%; Rf = 0.38 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 5.3 Hz, 1H), 7.32–7.24 (m, 4H), 7.09 (d, J = 5.3 Hz, 1H), 5.65 (b, 1H), 5.32 (b, 1H), 4.74 (d, J = 5.6 Hz, 2H), 4.14 (dq, J = 13.1, 6.5 Hz, 1H), 1.20 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.77, 160.61, 157.46, 137.62, 133.07, 130.67, 129.07, 128.72, 123.86, 106.00, 44.02, 43.03, 23.18. HRMS: m/z calcd for C16H18ClN4S [M + H]+ 333.0935; found 333.0938.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 5.3 Hz, 1H), 7.32–7.24 (m, 4H), 7.09 (d, J = 5.3 Hz, 1H), 5.65 (b, 1H), 5.32 (b, 1H), 4.74 (d, J = 5.6 Hz, 2H), 4.14 (dq, J = 13.1, 6.5 Hz, 1H), 1.20 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.77, 160.61, 157.46, 137.62, 133.07, 130.67, 129.07, 128.72, 123.86, 106.00, 44.02, 43.03, 23.18. HRMS: m/z calcd for C16H18ClN4S [M + H]+ 333.0935; found 333.0938.
B10 N2-Isopropyl-N4-(2-methoxybenzyl)thieno[3,2-d]pyrimidine-2,4-diamine. Yellow solid; yield, 37%; Rf = 0.31 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 5.3 Hz, 1H), 7.34 (dd, J = 7.4, 1.3 Hz, 1H), 7.28–7.22 (m, 1H), 7.07 (d, J = 5.3 Hz, 1H), 6.91 (d, J = 7.4 Hz, 1H), 6.88 (d, J = 7.9 Hz, 1H), 5.42 (s, 1H), 4.82 (d, J = 7.8 Hz, 1H), 4.78 (d, J = 5.8 Hz, 2H), 4.22 (qd, J = 13.0, 6.5 Hz, 1H), 3.86 (s, 3H), 1.23 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.31, 160.61, 157.71, 157.67, 130.25, 129.75, 128.80, 126.80, 123.81, 120.64, 110.49, 106.25, 55.41, 43.02, 40.55, 23.23. HRMS: m/z calcd for C17H21N4OS [M + H]+ 329.1431; found 329.1434. Melting point 60–62 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 5.3 Hz, 1H), 7.34 (dd, J = 7.4, 1.3 Hz, 1H), 7.28–7.22 (m, 1H), 7.07 (d, J = 5.3 Hz, 1H), 6.91 (d, J = 7.4 Hz, 1H), 6.88 (d, J = 7.9 Hz, 1H), 5.42 (s, 1H), 4.82 (d, J = 7.8 Hz, 1H), 4.78 (d, J = 5.8 Hz, 2H), 4.22 (qd, J = 13.0, 6.5 Hz, 1H), 3.86 (s, 3H), 1.23 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.31, 160.61, 157.71, 157.67, 130.25, 129.75, 128.80, 126.80, 123.81, 120.64, 110.49, 106.25, 55.41, 43.02, 40.55, 23.23. HRMS: m/z calcd for C17H21N4OS [M + H]+ 329.1431; found 329.1434. Melting point 60–62 °C.
B11 N2-Isopropyl-N4-(4-methoxybenzyl)thieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 51%; Rf = 0.22 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 5.3 Hz, 1H), 7.29 (d, J = 8.5 Hz, 2H), 7.09 (d, J = 5.3 Hz, 1H), 6.86 (d, J = 8.6 Hz, 2H), 5.17 (s, 1H), 4.77 (d, J = 7.8 Hz, 1H), 4.70 (d, J = 5.5 Hz, 2H), 4.20 (m, 1H), 3.79 (s, 3H), 1.22 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.71, 159.18, 157.54, 131.00, 130.45, 129.29, 123.97, 114.20, 106.10, 55.41, 44.42, 43.08, 23.27. HRMS: m/z calcd for C17H21N4OS [M + H]+ 329.1431; found 329.1432.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 5.3 Hz, 1H), 7.29 (d, J = 8.5 Hz, 2H), 7.09 (d, J = 5.3 Hz, 1H), 6.86 (d, J = 8.6 Hz, 2H), 5.17 (s, 1H), 4.77 (d, J = 7.8 Hz, 1H), 4.70 (d, J = 5.5 Hz, 2H), 4.20 (m, 1H), 3.79 (s, 3H), 1.22 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.71, 159.18, 157.54, 131.00, 130.45, 129.29, 123.97, 114.20, 106.10, 55.41, 44.42, 43.08, 23.27. HRMS: m/z calcd for C17H21N4OS [M + H]+ 329.1431; found 329.1432.
B12 N4-(3,4-Dimethoxybenzyl)-N2-isopropylthieno[3,2-d]pyrimidine-2,4-diamine. Yellow solid; yield, 51%; Rf = 0.24 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 5.3 Hz, 1H), 7.10 (d, J = 5.3 Hz, 1H), 6.92 (m, 2H), 6.86–6.81 (m, 1H), 5.05 (s, 1H), 4.83 (s, 1H), 4.71 (d, J = 5.4 Hz, 2H), 4.21 (td, J = 13.1, 6.5 Hz, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 1.24 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.33, 160.52, 157.58, 149.40, 148.77, 131.41, 130.66, 123.88, 120.37, 111.54, 111.52, 106.15, 56.14, 56.07, 44.95, 43.18, 23.28. HRMS: m/z calcd for C18H23N4O2S [M + H]+ 359.1536; found 359.1542. Melting point 134–136 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 5.3 Hz, 1H), 7.10 (d, J = 5.3 Hz, 1H), 6.92 (m, 2H), 6.86–6.81 (m, 1H), 5.05 (s, 1H), 4.83 (s, 1H), 4.71 (d, J = 5.4 Hz, 2H), 4.21 (td, J = 13.1, 6.5 Hz, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 1.24 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 161.33, 160.52, 157.58, 149.40, 148.77, 131.41, 130.66, 123.88, 120.37, 111.54, 111.52, 106.15, 56.14, 56.07, 44.95, 43.18, 23.28. HRMS: m/z calcd for C18H23N4O2S [M + H]+ 359.1536; found 359.1542. Melting point 134–136 °C.
B13 N2-Isopropyl-N4-((6-(trifluoromethyl)pyridin-3-yl)methyl)thieno[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 46%; Rf = 0.29 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 7.85 (d, J = 7.1 Hz, 1H), 7.60 (d, J = 8.1 Hz, 1H), 7.53 (d, J = 5.3 Hz, 1H), 7.08 (d, J = 5.3 Hz, 1H), 5.69 (s, 1H), 4.84 (d, J = 5.7 Hz, 2H), 4.77 (d, J = 7.6 Hz, 1H), 4.05 (m, 1H), 1.14 (d, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 161.85, 160.44, 157.36, 149.41, 147.19, 138.42, 136.49, 131.14, 123.86, 123.03, 120.39, 106.02, 43.16, 41.96, 23.13. HRMS: m/z calcd for C16H16F3N5NaS [M + Na]+ 390.0971; found 390.0973.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 7.85 (d, J = 7.1 Hz, 1H), 7.60 (d, J = 8.1 Hz, 1H), 7.53 (d, J = 5.3 Hz, 1H), 7.08 (d, J = 5.3 Hz, 1H), 5.69 (s, 1H), 4.84 (d, J = 5.7 Hz, 2H), 4.77 (d, J = 7.6 Hz, 1H), 4.05 (m, 1H), 1.14 (d, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 161.85, 160.44, 157.36, 149.41, 147.19, 138.42, 136.49, 131.14, 123.86, 123.03, 120.39, 106.02, 43.16, 41.96, 23.13. HRMS: m/z calcd for C16H16F3N5NaS [M + Na]+ 390.0971; found 390.0973.
C1 N4-(4-Cyanobenzyl)-N2-isopropylpyrido[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 62%; Rf = 0.56 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 3.4 Hz, 1H), 7.68 (d, J = 7.5 Hz, 1H), 7.57 (d, J = 7.9 Hz, 2H), 7.43 (d, J = 7.0 Hz, 2H), 7.40–7.30 (m, 1H), 5.19 (s, 1H), 4.80 (d, J = 5.9 Hz, 2H), 4.17 (m, 1H), 1.18 (d, J = 5.8 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 159.91, 158.77, 146.89, 144.35, 142.89, 132.24, 128.75, 127.98, 127.71, 118.75, 110.90, 72.69, 43.85, 42.83, 23.01. HRMS: m/z calcd for C18H19N6 [M + H]+ 319.1666; found 319.1669.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 3.4 Hz, 1H), 7.68 (d, J = 7.5 Hz, 1H), 7.57 (d, J = 7.9 Hz, 2H), 7.43 (d, J = 7.0 Hz, 2H), 7.40–7.30 (m, 1H), 5.19 (s, 1H), 4.80 (d, J = 5.9 Hz, 2H), 4.17 (m, 1H), 1.18 (d, J = 5.8 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 159.91, 158.77, 146.89, 144.35, 142.89, 132.24, 128.75, 127.98, 127.71, 118.75, 110.90, 72.69, 43.85, 42.83, 23.01. HRMS: m/z calcd for C18H19N6 [M + H]+ 319.1666; found 319.1669.
C2 N2-Isopropyl-N4-(2-(methylsulfonyl)benzyl)pyrido[3,2-d]pyrimidine-2,4-diamine. White solid; yield, 71%; Rf = 0.49 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 4.2 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H), 7.75 (s, NH), 7.72 (d, J = 7.7 Hz, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.59 (t, J = 7.5 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.41 (dd, J = 8.5, 4.2 Hz, 1H), 5.17 (d, J = 6.5 Hz, 2H), 4.91 (s, NH), 4.28–4.18 (m, 1H), 3.17 (s, 3H), 1.24 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.03, 158.90, 146.86, 143.35, 138.75, 138.43, 134.14, 132.35, 131.63, 130.08, 128.57, 127.86, 72.93, 45.27, 43.08, 42.06, 23.32. HRMS: m/z calcd for C18H22N5O2S [M + H]+ 372.1489; found 372.1495. Melting point 161–162 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 4.2 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H), 7.75 (s, NH), 7.72 (d, J = 7.7 Hz, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.59 (t, J = 7.5 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.41 (dd, J = 8.5, 4.2 Hz, 1H), 5.17 (d, J = 6.5 Hz, 2H), 4.91 (s, NH), 4.28–4.18 (m, 1H), 3.17 (s, 3H), 1.24 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.03, 158.90, 146.86, 143.35, 138.75, 138.43, 134.14, 132.35, 131.63, 130.08, 128.57, 127.86, 72.93, 45.27, 43.08, 42.06, 23.32. HRMS: m/z calcd for C18H22N5O2S [M + H]+ 372.1489; found 372.1495. Melting point 161–162 °C.
C3 N2-Isopropyl-N4-(4-(methylsulfonyl)benzyl)pyrido[3,2-d]pyrimidine-2,4-diamine. Yellow oil; yield, 62%; Rf = 0.50 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.32–8.28 (m, 1H), 7.90 (d, J = 8.3 Hz, 2H), 7.70 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 8.1 Hz, 2H), 7.44 (dd, J = 8.5, 4.2 Hz, 1H), 4.87 (d, J = 6.2 Hz, 2H), 4.18 (dt, J = 13.4, 6.7 Hz, 1H), 3.03 (s, 3H), 1.21 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.00, 158.51, 145.20, 143.20, 139.61, 132.14, 128.36, 127.92, 127.78, 127.71, 72.80, 44.57, 43.91, 43.02, 23.06. HRMS: m/z calcd for C18H22N5O2S [M + H]+ 372.1489; found 372.1491.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.32–8.28 (m, 1H), 7.90 (d, J = 8.3 Hz, 2H), 7.70 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 8.1 Hz, 2H), 7.44 (dd, J = 8.5, 4.2 Hz, 1H), 4.87 (d, J = 6.2 Hz, 2H), 4.18 (dt, J = 13.4, 6.7 Hz, 1H), 3.03 (s, 3H), 1.21 (d, J = 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 160.00, 158.51, 145.20, 143.20, 139.61, 132.14, 128.36, 127.92, 127.78, 127.71, 72.80, 44.57, 43.91, 43.02, 23.06. HRMS: m/z calcd for C18H22N5O2S [M + H]+ 372.1489; found 372.1491.
C5 N2-Isopropyl-N4-(4-(trifluoromethyl)benzyl)pyrido[3,2-d]pyrimidine-2,4-diamine. Yellow solid; yield, 91%; Rf = 0.55 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 4.2 Hz, 1H), 7.72 (d, J = 8.5 Hz, 1H), 7.60 (d, J = 8.1 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.45 (dd, J = 8.5, 4.2 Hz, 1H), 4.85 (d, J = 6.1 Hz, 2H), 4.21 (dq, J = 13.4, 6.7 Hz, 1H), 1.23 (d, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 160.00, 158.03, 143.44, 142.43, 130.35, 128.06, 127.98, 127.88, 125.57, 122.78, 72.81, 44.08, 43.19, 22.99. HRMS: m/z calcd for C18H19F3N5 [M + H]+ 362.1587; found 362.1595. Melting point 92–94 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 4.2 Hz, 1H), 7.72 (d, J = 8.5 Hz, 1H), 7.60 (d, J = 8.1 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.45 (dd, J = 8.5, 4.2 Hz, 1H), 4.85 (d, J = 6.1 Hz, 2H), 4.21 (dq, J = 13.4, 6.7 Hz, 1H), 1.23 (d, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 160.00, 158.03, 143.44, 142.43, 130.35, 128.06, 127.98, 127.88, 125.57, 122.78, 72.81, 44.08, 43.19, 22.99. HRMS: m/z calcd for C18H19F3N5 [M + H]+ 362.1587; found 362.1595. Melting point 92–94 °C.
C13 N2-Isopropyl-N4-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyrido[3,2-d]pyrimidine-2,4-diamine. Yellow solid; yield, 45%; Rf = 0.54 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.77 (s, 1H), 8.28 (dd, J = 4.2, 1.1 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.63 (d, J = 8.1 Hz, 1H), 7.43 (dd, J = 8.5, 4.2 Hz, 1H), 4.94 (s, 1H), 4.85 (d, J = 6.2 Hz, 2H), 4.17 (d, J = 6.3 Hz, 1H), 1.20 (d, J = 6.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 160.09, 158.83, 149.61, 147.54, 147.20, 147.10, 143.21, 137.97, 136.52, 132.68, 127.99, 123.05, 120.47, 43.08, 41.71, 23.19. HRMS: m/z calcd for C17H18F3N6 [M + H]+ 363.1540; found 363.1546. Melting point 111–113 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CDCl3) δ 8.77 (s, 1H), 8.28 (dd, J = 4.2, 1.1 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.63 (d, J = 8.1 Hz, 1H), 7.43 (dd, J = 8.5, 4.2 Hz, 1H), 4.94 (s, 1H), 4.85 (d, J = 6.2 Hz, 2H), 4.17 (d, J = 6.3 Hz, 1H), 1.20 (d, J = 6.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 160.09, 158.83, 149.61, 147.54, 147.20, 147.10, 143.21, 137.97, 136.52, 132.68, 127.99, 123.05, 120.47, 43.08, 41.71, 23.19. HRMS: m/z calcd for C17H18F3N6 [M + H]+ 363.1540; found 363.1546. Melting point 111–113 °C.
D1 N4-(4-Cyanobenzyl)-N2-isopropylpyrido[2,3-d]pyrimidine-2,4-diamine. Yellow oil; yield, 24%; Rf = 0.32 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.53 (dd, J = 4.5, 1.6 Hz, 1H), 8.27 (d, J = 7.7 Hz, 1H), 7.59 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.2 Hz, 2H), 7.00 (dd, J = 8.0, 4.6 Hz, 1H), 4.77 (s, 2H), 4.11 (s, 1H), 3.28–3.26 (m, 1H), 1.12 (d, J = 23.6 Hz, 6H). 13C NMR (101 MHz, CD3OD) δ 162.49, 162.34, 161.84, 155.60, 146.66, 133.54, 133.28, 129.18, 119.76, 117.46, 111.56, 107.13, 45.26, 43.64, 23.06. HRMS: m/z calcd for C18H18N6Na [M + Na]+ 341.1485; found 341.1489.
1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.53 (dd, J = 4.5, 1.6 Hz, 1H), 8.27 (d, J = 7.7 Hz, 1H), 7.59 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.2 Hz, 2H), 7.00 (dd, J = 8.0, 4.6 Hz, 1H), 4.77 (s, 2H), 4.11 (s, 1H), 3.28–3.26 (m, 1H), 1.12 (d, J = 23.6 Hz, 6H). 13C NMR (101 MHz, CD3OD) δ 162.49, 162.34, 161.84, 155.60, 146.66, 133.54, 133.28, 129.18, 119.76, 117.46, 111.56, 107.13, 45.26, 43.64, 23.06. HRMS: m/z calcd for C18H18N6Na [M + Na]+ 341.1485; found 341.1489.
D2 N2-Isopropyl-N4-(2-(methylsulfonyl)benzyl)pyrido[2,3-d]pyrimidine-2,4-diamine. Yellow solid; yield, 6%; Rf = 0.40 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.64–8.60 (m, 1H), 8.34 (d, J = 7.5 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.63 (d, J = 5.5 Hz, 2H), 7.51 (dd, J = 10.9, 5.5 Hz, 1H), 7.10 (dd, J = 7.9, 4.6 Hz, 1H), 5.22 (s, 2H), 4.18 (s, 1H), 3.31 (s, 3H), 1.12 (s, 6H). 13C NMR (101 MHz, CD3OD) δ 162.64, 155.76, 151.44, 139.95, 139.49, 135.12, 133.48, 130.85, 130.57, 130.28, 130.08, 128.96, 117.56, 44.66, 30.82, 30.31, 22.99. HRMS: m/z calcd for C18H21NaN5O2S [M + Na]+ 394.1308; found 394.1313.
1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.64–8.60 (m, 1H), 8.34 (d, J = 7.5 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.63 (d, J = 5.5 Hz, 2H), 7.51 (dd, J = 10.9, 5.5 Hz, 1H), 7.10 (dd, J = 7.9, 4.6 Hz, 1H), 5.22 (s, 2H), 4.18 (s, 1H), 3.31 (s, 3H), 1.12 (s, 6H). 13C NMR (101 MHz, CD3OD) δ 162.64, 155.76, 151.44, 139.95, 139.49, 135.12, 133.48, 130.85, 130.57, 130.28, 130.08, 128.96, 117.56, 44.66, 30.82, 30.31, 22.99. HRMS: m/z calcd for C18H21NaN5O2S [M + Na]+ 394.1308; found 394.1313.
D3 N2-Isopropyl-N4-(4-(methylsulfonyl)benzyl)pyrido[2,3-d]pyrimidine-2,4-diamine. Yellow solid; yield, 20%; Rf = 0.36 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.56 (dd, J = 4.6, 1.6 Hz, 1H), 8.30 (d, J = 7.7 Hz, 1H), 7.84 (d, J = 8.3 Hz, 2H), 7.57 (d, J = 8.3 Hz, 2H), 7.02 (dd, J = 8.0, 4.6 Hz, 1H), 4.82 (s, 2H), 3.30–3.27 (m, 1H), 3.04 (s, 3H), 1.14 (d, J = 34.1 Hz, 6H). 13C NMR (101 MHz, CD3OD) δ 162.53, 162.33, 161.90, 155.62, 147.41, 140.48, 133.56, 129.21, 128.51, 117.47, 107.15, 45.16, 44.44, 43.64, 23.07. HRMS: m/z calcd for C18H21NaN5O2S [M + Na]+ 394.1308; found 394.1311. Melting point 162–164 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.56 (dd, J = 4.6, 1.6 Hz, 1H), 8.30 (d, J = 7.7 Hz, 1H), 7.84 (d, J = 8.3 Hz, 2H), 7.57 (d, J = 8.3 Hz, 2H), 7.02 (dd, J = 8.0, 4.6 Hz, 1H), 4.82 (s, 2H), 3.30–3.27 (m, 1H), 3.04 (s, 3H), 1.14 (d, J = 34.1 Hz, 6H). 13C NMR (101 MHz, CD3OD) δ 162.53, 162.33, 161.90, 155.62, 147.41, 140.48, 133.56, 129.21, 128.51, 117.47, 107.15, 45.16, 44.44, 43.64, 23.07. HRMS: m/z calcd for C18H21NaN5O2S [M + Na]+ 394.1308; found 394.1311. Melting point 162–164 °C.
D5 N2-Isopropyl-N4-(4-(trifluoromethyl)benzyl)pyrido[2,3-d]pyrimidine-2,4-diamine. Yellow solid; yield, 74%; Rf = 0.47 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, DMSO-d6) δ 8.72–8.44 (m, 1H), 8.44 (d, J = 42.8 Hz, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.52 (d, J = 7.9 Hz, 2H), 7.05 (d, J = 35.0 Hz, 1H), 4.74 (d, J = 4.3 Hz, 2H), 4.14–3.88 (m, 1H), 1.01 (dd, J = 23.4, 16.7 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 166.89, 160.53, 144.22, 143.15, 131.44, 127.87, 127.60, 127.29, 125.65, 125.10, 122.95, 64.96, 62.77, 22.37. HRMS: m/z calcd for C18H18F3N5Na [M + Na]+ 384.1407; found 384.1410. Melting point 107–110 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, DMSO-d6) δ 8.72–8.44 (m, 1H), 8.44 (d, J = 42.8 Hz, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.52 (d, J = 7.9 Hz, 2H), 7.05 (d, J = 35.0 Hz, 1H), 4.74 (d, J = 4.3 Hz, 2H), 4.14–3.88 (m, 1H), 1.01 (dd, J = 23.4, 16.7 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 166.89, 160.53, 144.22, 143.15, 131.44, 127.87, 127.60, 127.29, 125.65, 125.10, 122.95, 64.96, 62.77, 22.37. HRMS: m/z calcd for C18H18F3N5Na [M + Na]+ 384.1407; found 384.1410. Melting point 107–110 °C.
D13 N2-Isopropyl-N4-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyrido[2,3-d]pyrimidine-2,4-diamine. Yellow solid; yield, 7%; Rf = 0.26 (9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.76 (s, 1H), 8.64 (s, 1H), 8.39 (s, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.17 (s, 1H), 4.90 (d, J = 2.4 Hz, 2H), 4.18 (d, J = 8.2 Hz, 1H), 1.15 (s, 6H). 13C NMR (101 MHz, CD3OD) δ 162.36, 155.77, 150.41, 147.57, 140.24, 138.24, 133.92, 130.83, 127.14, 124.43, 121.67, 121.64, 119.00, 107.13, 64.33, 43.99, 43.20, 22.95. HRMS: m/z calcd for C17H17F3N6Na [M + Na]+ 385.1359; found 385.1366. Melting point 108–110 °C.
1 dichloromethane to methanol); 1H NMR (400 MHz, CD3OD) δ 8.76 (s, 1H), 8.64 (s, 1H), 8.39 (s, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.17 (s, 1H), 4.90 (d, J = 2.4 Hz, 2H), 4.18 (d, J = 8.2 Hz, 1H), 1.15 (s, 6H). 13C NMR (101 MHz, CD3OD) δ 162.36, 155.77, 150.41, 147.57, 140.24, 138.24, 133.92, 130.83, 127.14, 124.43, 121.67, 121.64, 119.00, 107.13, 64.33, 43.99, 43.20, 22.95. HRMS: m/z calcd for C17H17F3N6Na [M + Na]+ 385.1359; found 385.1366. Melting point 108–110 °C.
Antibacterial activities assay
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 into 5 mL of broth media. 400 μL of diluted culture was added to an agar plate with a diameter of 120 mm and was coated uniformly, allowed to dry for 15 min before use. After that, nine sterile filter disks with a diameter of 6 mm were added and 5 μL of 5 mg mL−1 test compound dissolved in DMSO were dropwise added to each filter disk. Bacterial plates were incubated overnight. The assays were performed in triplicate and zones of inhibition were measured in millimeters.
1000 into 5 mL of broth media. 400 μL of diluted culture was added to an agar plate with a diameter of 120 mm and was coated uniformly, allowed to dry for 15 min before use. After that, nine sterile filter disks with a diameter of 6 mm were added and 5 μL of 5 mg mL−1 test compound dissolved in DMSO were dropwise added to each filter disk. Bacterial plates were incubated overnight. The assays were performed in triplicate and zones of inhibition were measured in millimeters.DMPK properties assays
The DMPK properties data described above were measured through a high through-put platform kindly provided by AstraZeneca U.K. The methods of the five assays, including log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4, aqueous solubility, plasma protein binding, microsome and hepatocyte clearance measurements has been reported previously (Basarab et al., 2014 and Doyle et al., 2016) and are described briefly as below34,35.
D7.4, aqueous solubility, plasma protein binding, microsome and hepatocyte clearance measurements has been reported previously (Basarab et al., 2014 and Doyle et al., 2016) and are described briefly as below34,35.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) D7.4 Determination assay. The partition coefficient (log
D7.4 Determination assay. The partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D) was measured by shake flask method, using 10 mM phosphate buffer at pH 7.4 and n-octanol. The samples were allowed to reach equilibrium by shaking for 1 hour at 1200 rpm, and sample analysis was done by LC/UV, with MS for mass confirmation.
D) was measured by shake flask method, using 10 mM phosphate buffer at pH 7.4 and n-octanol. The samples were allowed to reach equilibrium by shaking for 1 hour at 1200 rpm, and sample analysis was done by LC/UV, with MS for mass confirmation.Conflicts of interest
Compounds described in this manuscript are included in a UK patent application filed by the Liverpool School of Tropical Medicine and the University of Liverpool (application No. 1700814.5, filed 17 January 2017). The authors declare that they have no other competing interests.Acknowledgements
The authors want to thank the DMPK group (led by Peter Webborn) in AstraZeneca U.K. for providing the in vitro measurement of DMPK properties, including log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4, aqueous solubility, human plasma protein binding, mouse microsome clearance and rat hepatocytes clearance of hit and lead molecules. Majority of the synthetic and biological research work was carried out in the Liverpool-GDUT Joint Laboratory for Drug Discovery located in Guangdong University of Technolgy (GDUT). Financial support was provided by the Guangzhou Municipal Science and technology project for major project of industry-university-research cooperation and collaborative innovation (project No. 2016201604030025), Liverpool-GDUT Drug Discovery Initiative (project No. CA131122SWGDUT), the Department of Science and Technology of Guangdong province (project No. 2017A050501034), the Department of Education of Guangdong Province (project No. 2013JDXM27) and Guangzhou Science and Technology Plan (project No. 201604030020 and No. 2017A050501034). Compounds descripted in this manuscript are included in an UK Patent Application No. 1700814.5.
D7.4, aqueous solubility, human plasma protein binding, mouse microsome clearance and rat hepatocytes clearance of hit and lead molecules. Majority of the synthetic and biological research work was carried out in the Liverpool-GDUT Joint Laboratory for Drug Discovery located in Guangdong University of Technolgy (GDUT). Financial support was provided by the Guangzhou Municipal Science and technology project for major project of industry-university-research cooperation and collaborative innovation (project No. 2016201604030025), Liverpool-GDUT Drug Discovery Initiative (project No. CA131122SWGDUT), the Department of Science and Technology of Guangdong province (project No. 2017A050501034), the Department of Education of Guangdong Province (project No. 2013JDXM27) and Guangzhou Science and Technology Plan (project No. 201604030020 and No. 2017A050501034). Compounds descripted in this manuscript are included in an UK Patent Application No. 1700814.5.
References
- C. Walsh, Nat. Rev. Microbiol., 2003, 1, 65–70 CrossRef CAS PubMed.
- L. Feng, M. M. Maddox, M. Z. Alam, L. S. Tsutsumi, G. Narula, D. F. Bruhn, X. Wu, S. Sandhaus, R. B. Lee, C. J. Simmons, Y. C. Tse-Dinh, J. G. Hurdle, R. E. Lee and D. Sun, J. Med. Chem., 2014, 57, 8398–8420 CrossRef CAS PubMed.
- L. G. M. Bode, J. A. J. W. Kluytmans, H. F. L. Wertheim, D. Bogaers, C. M. J. E. Vandenbroucke-Grauls, R. Roosendaal, A. Troelstra, A. T. A. Box, A. Voss, I. van der Tweel, A. van Belkum, H. A. Verbrugh and M. C. Vos, N. Engl. J. Med., 2010, 362, 9–17 CrossRef CAS PubMed.
- H. Mohammad, P. V. Reddy, D. Monteleone, A. S. Mayhoub, M. Cushman and M. N. Seleem, Eur. J. Med. Chem., 2015, 94, 306–316 CrossRef CAS PubMed.
- S. M. Lehar, T. Pillow, M. Xu, L. Staben, K. K. Kajihara, R. Vandlen, L. DePalatis, H. Raab, W. L. Hazenbos, J. H. Morisaki, J. Kim, S. Park, M. Darwish, B. C. Lee, H. Hernandez, K. M. Loyet, P. Lupardus, R. Fong, D. Yan, C. Chalouni, E. Luis, Y. Khalfin, E. Plise, J. Cheong, J. P. Lyssikatos, M. Strandh, K. Koefoed, P. S. Andersen, J. A. Flygare, M. Wah Tan, E. J. Brown and S. Mariathasan, Nature, 2015, 527, 323–328 CrossRef CAS PubMed.
- S. R. Martinez, D. M. Rocca, V. Aiassa and M. C. Becerra, RSC Adv., 2016, 6, 101023–101028 RSC.
- H. F. Chambers and F. R. Deleo, Nat. Rev. Microbiol., 2009, 7, 629–641 CrossRef CAS PubMed.
- B. A. Diep, S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh and F. Perdreau-Remington, Lancet, 2006, 367, 731–739 CrossRef CAS.
- L. C. Chan, A. Gilbert, L. Basuino, T. M. da Costa, S. M. Hamilton, K. R. Dos Santos, H. F. Chambers and S. S. Chatterjee, Antimicrob. Agents Chemother., 2016, 60, 3934–3941 CrossRef CAS PubMed.
- R. J. Guidos, B. Spellberg, M. Blaser, H. W. Boucher, J. S. Bradley, B. I. Eisenstein, D. Gerding, R. Lynfield, L. B. Reller, J. Rex, D. Schwartz, E. Septimus, F. C. Tenover, D. N. Gilbert and IDSA, Clin. Infect. Dis., 2011, 52, S397–S428 CrossRef PubMed.
- J. P. Surivet, C. Zumbrunn, G. Rueedi, D. Bur, T. Bruyere, H. Locher, D. Ritz, P. Seiler, C. Kohl, E. A. Ertel, P. Hess, J. C. Gauvin, A. Mirre, V. Kaegi, M. Dos Santos, S. Kraemer, M. Gaertner, J. Delers, M. Enderlin-Paput, M. Weiss, R. Sube, H. Hadana, W. Keck and C. Hubschwerlen, J. Med. Chem., 2015, 58, 927–942 CrossRef CAS PubMed.
- R. J. Gordon and F. D. Lowy, Clin. Infect. Dis., 2008, 46(suppl. 5), S350–S359 CrossRef CAS PubMed.
- C. Vuong, A. J. Yeh, G. Y. Cheung and M. Otto, Expert Opin. Invest. Drugs, 2016, 25, 73–93 CrossRef CAS PubMed.
- C. M. Macal, M. J. North, N. Collier, V. M. Dukic, D. T. Wegener, M. Z. David, R. S. Daum, P. Schumm, J. A. Evans, J. R. Wilder, L. G. Miller, S. J. Eells and D. S. Lauderdale, J. Transl. Med., 2014, 12, 124–135 CrossRef PubMed.
- L. M. Weigel, D. B. Clewell, S. R. Gill, N. C. Clark, L. K. McDougal, S. E. Flannagan, J. F. Kolonay, J. Shetty, G. E. Killgore and F. C. Tenover, Science, 2003, 302, 1569–1571 CrossRef CAS PubMed.
- M. K. Hayden, K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn and R. A. Weinstein, J. Clin. Microbiol., 2005, 43, 5285–5287 CrossRef CAS PubMed.
- G. Morales, J. J. Picazo, E. Baos, F. J. Candel, A. Arribi, B. Pelaez, R. Andrade, M. A. de la Torre, J. Fereres and M. Sanchez-Garcia, Clin. Infect. Dis., 2010, 50, 821–825 CrossRef CAS PubMed.
- L. C. Chan, L. Basuino, B. Diep, S. Hamilton, S. S. Chatterjee and H. F. Chambers, Antimicrob. Agents Chemother., 2015, 59, 2960–2963 CrossRef CAS PubMed.
- W. H. Organization, WHO publishes list of bacteria for which new antibiotics are urgently needed, http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ Search PubMed.
- D. Wang and F. Gao, Chem. Cent. J., 2013, 7, 95–109 CrossRef PubMed.
- E. Jafari, M. R. Khajouei, F. Hassanzadeh, G. H. Hakimelahi and G. A. Khodarahmi, Res. Pharm. Sci., 2016, 11, 1–14 Search PubMed.
- Y. L. Gao, Q. Z. Xiong, R. An and X. P. Bao, Chin. J. Org. Chem., 2011, 31, 1529–1537 CAS.
- P. M. Bedi, V. Kumar and M. P. Mahajan, Bioorg. Med. Chem. Lett., 2004, 14, 5211–5213 CrossRef CAS PubMed.
- P. M. Chandrika, T. Yakaiah, G. Gayatri, K. P. Kumar, B. Narsaiah, U. S. N. Murthy and A. R. R. Rao, Eur. J. Med. Chem., 2010, 45, 78–84 CrossRef PubMed.
- K. S. Van Horn, W. N. Burda, R. Fleeman, L. N. Shaw and R. Manetsch, J. Med. Chem., 2014, 57, 3075–3093 CrossRef CAS PubMed.
- D. Gonzalez Cabrera, C. Le Manach, F. Douelle, Y. Younis, T. S. Feng, T. Paquet, A. T. Nchinda, L. J. Street, D. Taylor, C. de Kock, L. Wiesner, S. Duffy, K. L. White, K. M. Zabiulla, Y. Sambandan, S. Bashyam, D. Waterson, M. J. Witty, S. A. Charman, V. M. Avery, S. Wittlin and K. Chibale, J. Med. Chem., 2014, 57, 1014–1022 CrossRef CAS PubMed.
- M. T. Kenji Yoshida, J. Chem. Soc. Pak., 1992, 1, 919–923 Search PubMed.
- K. Kanuma, K. Omodera, M. Nishiguchi, T. Funakoshi, S. Chaki, Y. Nagase, I. Iida, J. Yamaguchi, G. Semple, T. A. Tran and Y. Sekiguchi, Bioorg. Med. Chem., 2006, 14, 3307–3319 CrossRef CAS PubMed.
- P. R. Gilson, C. Tan, K. E. Jarman, K. N. Lowes, J. M. Curtis, W. Nguyen, A. E. Di Rago, H. E. Bullen, B. Prinz, S. Duffy, J. B. Baell, C. A. Hutton, H. Jousset Subroux, B. S. Crabb, V. M. Avery, A. F. Cowman and B. E. Sleebs, J. Med. Chem., 2017, 60, 1171–1188 CrossRef CAS PubMed.
- X. Zhu, K. S. Van Horn, M. M. Barber, S. Yang, M. Z. Wang, R. Manetsch and K. A. Werbovetz, Bioorg. Med. Chem., 2015, 23, 5182–5189 CrossRef CAS PubMed.
- W. Devine, J. L. Woodring, U. Swaminathan, E. Amata, G. Patel, J. Erath, N. E. Roncal, P. J. Lee, S. E. Leed, A. Rodriguez, K. Mensa-Wilmot, R. J. Sciotti and M. P. Pollastri, J. Med. Chem., 2015, 58, 5522–5537 CrossRef CAS PubMed.
- K. L. Johnston, D. A. N. Cook, N. G. Berry, W. David Hong, R. H. Clare, M. Goddard, L. Ford, G. L. Nixon, P. M. O'Neill, S. A. Ward and M. J. Taylor, Sci. Adv., 2017, 3, 1551–1560 CrossRef PubMed.
- W. N. Burda, K. B. Fields, J. B. Gill, R. Burt, M. Shepherd, X. P. Zhang and L. N. Shaw, Eur. J. Clin. Microbiol. Infect. Dis., 2012, 31, 327–335 CrossRef CAS PubMed.
- G. S. Basarab, P. J. Hill, C. E. Garner, K. Hull, O. Green, B. A. Sherer, P. B. Dangel, J. I. Manchester, S. Bist, S. Hauck, F. Zhou, M. Uria-Nickelsen, R. Illingworth, R. Alm, M. Rooney and A. E. Eakin, J. Med. Chem., 2014, 57, 6060–6082 CrossRef CAS PubMed.
- K. Doyle, H. Lonn, H. Kack, A. Van de Poel, S. Swallow, P. Gardiner, S. Connolly, J. Root, C. Wikell, G. Dahl, K. Stenvall and P. Johannesson, J. Med. Chem., 2016, 59, 9457–9472 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |