DOI:
10.1039/C7RA10127A
(Paper)
RSC Adv., 2017,
7, 50044-50055
Microwave-assisted one-pot synthesis in water of carbonylpyrazolo[3,4-b]pyridine derivatives catalyzed by InCl3 and sonochemical assisted condensation with aldehydes to obtain new chalcone derivatives containing the pyrazolopyridinic moiety†
Received
11th September 2017
, Accepted 16th October 2017
First published on 27th October 2017
Abstract
Pyrazolo[3,4-b]pyridine derivatives have been synthesized via one-pot condensation of 3-methyl-1-phenyl-1H-pyrazolo-5-amine (1), formaldehyde (as paraformaldehyde) (2) and β-diketones (3) under microwave irradiation in aqueous media catalyzed by InCl3. This process has been found to be useful in the preparation of new N-fused heterocycle products in good to excellent yields. Further treatment of pyrazolopyridines (4a and 4e) with aldehyde aromatics (5a–l) afforded chalcone analogs.
Introduction
Over the years it has been found in the literature that nitrogen-containing heterocycles have shown wide spectrums of biological activities for accessing pharmaceutical and medicinal products. Recent studies have shown that greater than 90% of molecules currently under analysis by pharmaceutical companies include nitrogen heterocycles, where pyrrole, imidazole, pyrazole, pyrimidine, pyridine, and derivatives constitute the most important family of these compounds.1 Fused pyridine systems such as pyrazolopyridines and their related derivatives have been identified as a potentially interesting scaffold for biologically active compounds because of their structural analogy to purine bases, an important constituent of DNA and RNA nucleosides.2–5 The pyrazolopyridines, which comprise five isomers (I–V); [3,4-b], [3,4-c], [4,3-c], [4,3-b] and [1,5-a] (Fig. 1).
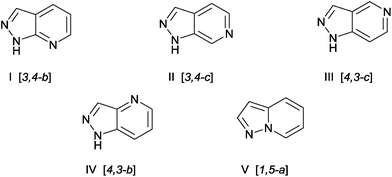 |
| | Fig. 1 Pyrazolopyridine Isomers. | |
In particular pyrazolo[3,4-b]pyridines (I) has aroused great interest in recent years due to a wide variety of biological activities associated with it. Analgesic, anti-microbial, antipyretic, anxiolytic, antagonists stereoselective of A1 receptors in humans, vasodilating, anti-chagasic, respiration stimulating, inhibitors of phosphodiesterase 4B (PDBE4B), bronchodilating, and of this system have been reported in the literature (Fig. 2).6–14
 |
| | Fig. 2 Examples of pyrazolo[3,4-b]pyridines biologically actives. | |
Due to their attractive pharmacological properties, pyrazolo[3,4-b]pyridines have attracted the attention of chemists who have researched ways to obtain the desired properties through different synthetic strategies. The most common reactions include: (a) condensation of pyrazole-5-amine derivatives and activated carbonyl groups;15–17 (b) reaction of 5-aminopyrazoles, 1,3-dicarbonyl derivatives or equivalents and aldehydes through of Hantzsch-type reaction;18–20 (c) through Povarov-type [4 + 2], cycloaddition between 5-aminopyrazoles, aromatic aldehydes, and cycloalkanones in acetic acid;21 and (d) Friedländer condensation of 5-aminopyrazole-4-carbaldehydes with α-methylene ketones such as acetone or acetophenones with potassium hydroxide as basic catalyst.22–24
The methods described in the literature presents several disadvantages such as the use of toxic solvents for the environment, long reaction times, several steps of synthesis, these contradict the current trends of green chemistry,25 as a result of the importance that presents these heterocycles in the medicinal field, different researchers have designed environmentally friendly and efficient synthetic routes, for example have used water as solvent under microwave irradiation (MWI) using catalysts organic or inorganic.26,27 Recently, InCl3 has emerged as useful Lewis acid catalyst in organic synthesis due to its compatibility with both organic and aqueous media in comparison with frequently used Lewis acids such as BF3, TiCl4, and AlCl3 react violently with water and cannot be used under aqueous conditions. The fact that its non-toxic nature enables the chemists to handle its reactions very easily and its lower heterophilicity makes it an ideal catalyst or reagent for the C–C bond formation reactions. In this context, over the past few years, InCl3 has received increasing attention as a novel type of water tolerant Lewis acid catalyst for organic synthesis with high chemo-, regio-, and stereoselectivity.
For example, InCl3 has been utilized in Diels–Alder,28 Friedel–Crafts,29,30 Michael-type,31–34 Mannich type,35,36 Mukaiyama-aldol,37,38 von Pechmann reaction,39 Hosomi–Sakurai allylation reactions,40,41 neat or in water under mild conditions. As InCl3 has these unique properties compared to other Lewis acids which include stability and recoverability from water, we are interested to apply it to other carbon–carbon bond formation reactions in water.
In general, the synthetic methodologies described in the literature are based on the starting materials. Starting from the variety of α,β-unsaturated synthons can build diverse pyrazolo[3,4-b]pyridine core. Herein, we wish to report a simple method for the synthesis of pyrazolo[3,4-b]pyridines derivatives via one-pot three-component reaction between 5-aminopyrazoles derivatives 1, paraformaldehyde 2 and β-diketones 3 catalyzed by InCl3 in aqueous media. Low environmental impact strategy, based on the construction of pyridine ring onto pyrazole combining reuse of catalyst, water and microwave irradiation.
We report the use of this nucleus of pyrazolo[3,4-b]pyridines for structural diversification from carbonyl carbon coupled aromatic aldehydes generating analogs of chalcones. Comparison yields and reaction times are reported by using a conventional method and use ultrasound for the obtaining of new derivatives. The target molecules were obtained in good to excellent isolated yields.
Results and discussion
Initially, held the synthesis of compound 1; prepared from (2E)-3-aminobut-2-enenitrile 7 and 1-phenyl hydrazine 8 was synthesized by conventional methods according to the literature (Scheme 1).42
 |
| | Scheme 1 Synthesis of starting material 1 needed for synthesis of pyrazolo[3,4-b]pyridine derivatives 4a–i. | |
The three-component coupling reaction of 1, paraformaldehyde 2 and indandione 3e under MWI in water was studied comprehensively as a representative example for obtaining pyrazolo[3,4-b]pyridines. For comparison was studied the effect of three different reaction conditions: reflux using AcOH, reflux using water and InCl3 and the use of water and InCl3 with MWI (Scheme 2 and Table 1).
 |
| | Scheme 2 Reaction conditions to obtain pyrazolo[3,4-b]pyridines 4a–i. | |
Table 1 Comparison of yields pyrazolo[3,4-b]pyridines (4a–i)
| Compound |
m/z |
Mp (°C) |
Conventional method |
Microwave irradiation method |
| Time (h)/yield (%) |
Time (min)/yield (%) |
| AcOH |
H2O/InCl3 |
H2O/InCl3 |
| 4a |
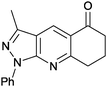 |
277 |
128–130 |
4/60 |
16/33 |
15/75 |
| 123–124 (ref. 45) |
| 4b |
 |
305 |
152–154 |
4/62 |
4/75 |
15/85 |
| 165–166 (ref. 45) |
| 164.5–166 (ref. 43) |
| 4c |
 |
339 |
211–213 |
9/48 |
7/52 |
10/80 |
| 4d |
 |
263 |
215–217 |
8/66 |
7/60 |
15/82 |
| 4e |
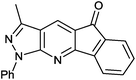 |
311 |
246–248 |
1/44 |
5/72 |
10/91 |
| 4f |
 |
265 |
98–100 |
6/50 |
10/65 |
17/90 |
| 96 (ref. 46) |
| 4g |
 |
227 |
276–278 |
4/47 |
8/50 |
12/67 |
| 4h |
 |
293 |
300–302 |
3/52 |
7/60 |
15/95 |
| 4i |
 |
309 |
>300 |
3/44 |
8/45 |
20/91 |
Previously, Dzvinchuk et al.43 synthesized 4a in 87% yield from the reaction of 1,3-cyclohexanedione 3a, 1 and 4-(dimethylamino)-benzaldehyde in boiling acetic acid for 2 h. In this reaction N,N-dimethylaniline was obtained as a byproduct. Zheng et al.44 reported the synthesis of 4b in 15% yield from a two-step reaction. Recently Sumesh et al.,45 reported the synthesis of 4a and 4b in 98% yield a one-step using L-proline, and water for 2 h.
In order to understand how the working temperature and the presence of different amounts of catalyst influence the performance and reaction time under MWI conditions was studied the behavior of compound 4e which facilitated establish the optimal reaction conditions (Table 2).
Table 2 Temperature screening for the synthesis of 3-methyl-1-phenylindeno[2,3-e]pyrazolo[3,4-b]pyridin-5(1H)-one 4e in MWI at 300 W. Reactions are performed on a 1 mmol scale of the reactants under MWI

|
| Entry |
Catalyst (mol%) |
Reaction temp. °C |
Reaction time (min) |
Isolated yield (%) |
| 1 |
0 |
80 |
45 |
0 |
| 2 |
5 |
100 |
30 |
23 |
| 3 |
10 |
120 |
10 |
80 |
| 4 |
15 |
150 |
10 |
90 |
| 5 |
20 |
150 |
15 |
90 |
| 6 |
25 |
150 |
10 |
87 |
As shown in Table 2, the expected product was obtained in good yield in water under MWI when is working with a temperature of 150 °C; InCl3 at 15% mol. It is also observed that by inducing the reaction at the same temperature (150 °C), and increasing the amount of catalyst (20% mol), the yield does not change. With these results in hand, different pyrazolo[3,4-b]pyridine derivatives 4a–i were prepared using various β-diketones 3a–i (Table 1).
The possibility to recover and recycle InCl3 also offers another significant advantage. Because InCl3 is soluble in reaction medium and the products are insoluble in water, the recovered filtrate containing the catalyst could be recycled. Studies using amine (1), formaldehyde (2), and diketone (3e) as model substrates showed that the recovered filtrate could be successively recycled in subsequent reactions without any significant decrease of yield of 4e. A marginal loss of the yield was observed in first two runs (91% and 89%), while in third and fourth run the yield dropped to 75% and 65%, respectively.
With the optimized protocol in hand, the scope of this domino process was then assessed through the variation of β-diketones 3a–i (Table 1). As a general trend, this reaction is tolerant to a large variety of β-diketones (linear, cyclical, heterocycles). Diversely adducts could be prepared in good yields (up to 95%), demonstrating the versatility of this one-pot process. This variety results very attractively for the establishment of structure–activity relationships after biological evaluation.
InCl3 in water under microwave radiation turns out to be advantageous for the synthesis of these compounds as shown in the Table 1. In conventional method, the yield of all the products is lower as compared to the yield obtained by synthesis by MWI technique. Microwave irradiation method facilitates the polarization of the reacting molecule causing reactions to occur at higher rate.
A reasonable mechanism for the formation of product 4a is outlined in Scheme 3. The formation of 4a is expected to proceed via initial condensation of aldehyde 2 with β-diketones 3a to give an intermediate [9], it may be proposed that the InCl3 catalyst facilitates the formation the intermediate [9] by increasing the electrophilicity of the carbonyl group of the aldehyde which further undergoes Michael addition with 1 to give an open-chain intermediate [10], which is subsequently cyclized, dehydrated and dehydrogenated to afford the aromatized product 4a (Fig. 3).
 |
| | Scheme 3 Plausible mechanism for the formation of pyrazolo[3,4-b]pyridine derivate 4a. | |
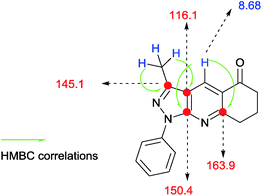 |
| | Fig. 3 Structural determination of compound 4a. | |
However, the compound 4g does not fit this mechanism. Derivatives of Meldrum's acid (1,3-dioxane-4,6-dione) are excellent precursors for the production of a variety of ketones, propadienones, iminopropadienones, and other reactive molecules by flash vacuum thermolysis (FVT),47 via a pseudo-retro-Diels–Alder reaction, eliminating acetone and carbon dioxide. Mechanistically the formation of the product (4g) can be visualized by initial Knoevenagel condensation of aldehyde 2 and 6,6-dimethyl-1,3,5-trioxane-2,4-dione (3h) resulting in adduct 11, followed by a Michael type nucleophilic addition of C-5 of the pyrazole ring (1) to the enone intermediate [11]; in the next step loss of acetone and CO2 and subsequent cyclodehydration to furnish the desired compound (Scheme 4).
 |
| | Scheme 4 Plausible mechanism for the formation of pyrazolo[3,4-b]pyridine 4g. | |
Ketones engage in many organic reactions. The most important reactions follow from the susceptibility of the carbonyl carbon toward nucleophilic addition and the tendency for the enolates to add to electrophiles, most reactions of carbonyl compounds take place by one of four general mechanisms: nucleophilic addition (12–17), nucleophilic acyl substitution, alpha substitution (18), and carbonyl condensation (6a–v) (Scheme 5).
 |
| | Scheme 5 Synthetic versatility of carbonyl carbon. | |
In order to create structural diversity from the carbonyl carbon available, we synthesize chalcones derivatives using pyrazolopyridine 4f or 4a and aldehydes aromatic 5a–l in a typical condensation of Claisen–Schmidt in ethanol with basic catalysis. In this reaction, we evaluate the advantages of sonochemical method over conventional method as is shows in Table 3.
Table 3 Synthesis of heterocyclic analogues of chalcones
A comparison of the results obtained under ultrasonic irradiations with those of conventional stirring in ethanol (Table 3) revealed that the reaction under ultrasonic irradiation proceeds in much lower reaction times and excellent yields. The rate acceleration using ultrasonic irradiations may be due to cavitation phenomena, in which the energy being transmitted more efficiently to the substrates.48–50
The yields of compound 6 were excellent. It is worth noting that the electronic nature of the substituents affects only to a lesser extent the yields of the products and the reaction proceeds quite well with both electron-withdrawing and electron-releasing substituents on chalcones analogues.
Compounds of the series 4 and 6 were easily characterized by 1H-NMR, 13C-NMR, IR and mass spectra. The FT-IR spectra of synthesized pyrazolopyridines derivatives 4 and 6 showed bands at stretching frequencies in the range of 1584–1598 cm−1 and 1488–1502 cm−1, which are characteristic of (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and (–C
N) and (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) groups. 1H-NMR spectra were similar between them in each set, and were characterized by the presence of three groups of signals (aromatics protons, protons near heteroatoms and aliphatic protons). Each series was identified a signal around 8 ppm, typical for γ-unsubstituted pyridine ring shown as in Fig. 3 and 4.
C) groups. 1H-NMR spectra were similar between them in each set, and were characterized by the presence of three groups of signals (aromatics protons, protons near heteroatoms and aliphatic protons). Each series was identified a signal around 8 ppm, typical for γ-unsubstituted pyridine ring shown as in Fig. 3 and 4.
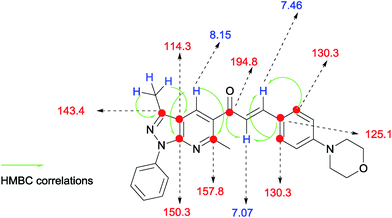 |
| | Fig. 4 HMBC-assisted NMR-based structural determination of compound 6p. | |
Experimental
The experiments were performed in a discover microwave apparatus (CEM Corporation, Matthews, NC, USA); the ultrasonic irradiation was performed by using a Branson ultrasonic cleaner bath, model 1510, 115 v, 1.9 L with mechanical timer (60 min with continuous hold) and heater switch, 47 kHz. All the products were characterized by spectral data (IR, MS, 1H-NMR, 13C-NMR). 1H and 13C NMR spectra (400 MHz for proton and 100 MHz for carbon) were recorded on an AM-400 spectrometer (Bruker, Rheinstetten, Germany), using CDCl3, DMSO-d6 and CD3OD as solvents. Tetramethylsilane (TMS) was used as an internal standard. Chemical shifts (δ) and J values are reported in ppm and Hz, respectively, relative to the solvent peak CDCl3 at 7.24 ppm for protons and 77 ppm for carbon atoms; DMSO-d6 2.5 ppm for protons and 39.7 ppm for carbon atoms, and CD3OD with 3.35 and 4.78 ppm for protons and 49.3 ppm for carbon. Signals are designated as follows: s, singlet; d, doublet; q quartet; t, triplet; m, multiplet. IR spectra (KBr pellets, 500–4000 cm−1) were recorded on a NEXUS 670 FT-IR spectrophotometer (Thermo Nicolet, Madison, WI, USA). High-resolution mass spectrometry ESI-MS and ESI-MS/MS analyses were conducted in a high-resolution hybrid quadrupole (Q) and orthogonal time-of-flight (TOF) mass spectrometer (Waters/Micromass Q-TOF micro, Manchester, UK) with a constant nebulizer temperature of 100 °C. Melting points (uncorrected) were measured on a Electrothermal IA9100 melting point apparatus (Stone, Staffs, UK). Reaction progress was monitored by means of thin-layer chromatography using silica gel 60 (Merck, Darmstadt, Germany). All reagents were purchased from either Merck or Sigma Aldrich (St. Louis, MO, USA) and used without further purification. Final purification of all products for analysis was carried out by recrystallization.
General procedure for the preparation of pyrazolopyridine 4a–j
Conventional method (reflux). (a) A solution of 5-aminopyrazole (1, 1 mmol), p-formaldehyde (2, 1 mmol) and β-diketone (3, 1 mmol) in acetic acid (15 mL)was heated at 80 °C (oil bath) for 4–11 h. Then, the reaction mixture was filtered hot and the resulting solid products were washed with ethanol, dried in air and recrystallized from ethanol.(b) A mixture of 5-aminopyrazole (1, 1 mmol), p-formaldehyde (2, 1 mmol) and β-diketone (3, 1 mmol) was placed in a 50 mL round-bottomed flask; 10 mL of water and InCl3 (15% mol) were added. The mixture was stirred under reflux for 4–16 h. The progress of the reaction was monitored by TLC using petroleum ether/ethylacetate (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as eluent. The reaction mixture was allowed to cool to room temperature. The precipitate formed was collected by filtration at pump washed with a mixture hexane/ethanol.
40) as eluent. The reaction mixture was allowed to cool to room temperature. The precipitate formed was collected by filtration at pump washed with a mixture hexane/ethanol.
Synthesis assisted by microwave
A mixture of 5-aminopyrazole (1, 1 mmol), p-formaldehyde (2, 1 mmol), β-diketone (3, 1 mmol), InCl3 (15% mol), and water (3 mL) were subjected to MWI (maximum power 300 W during 10–20 min at a controlled temperature of 483 K) using a focused microwave reactor (CEM Discover, Matthews, NC, USA). The aqueous phase extracted with EtOAc (2 × 10 mL). The combined organic layers was dried over Na2SO4 and evaporated; to give the corresponding derivatives 4a–j.
3-Methyl-1-phenyl-7,8-dihydrociclohexa[2,3-e]pyrazolo[3,4-b]pyridin-5(6H)-one (4a). Yield: 75% (207.9 mg); orange crystals; mp 128–130 °C; IR (KBr, cm−1): 3063, 2949, 2883, 1680, 1593, 1505, 1419, 1260, 1183, 1015, 904, 778, 748, 667, 559, 530; 1H NMR (400 MHz, DMSO-d6): δH = 2.11 (q, J = 6.0 Hz, 2H), 2.57 (s, 3H, CH3), 2.68 (t, J = 6.5 Hz, 1H), 3.15 (t, J = 6.5 Hz, 2H), 7.31 (t, J = 7.4 Hz, 1H), 7.53 (t, J = 8.0 Hz, 2H), 8.23 (d, J = 8.7 Hz, 2H), 8.68 (s, 1H); 13CNMR (100 MHz DMSO-d6) δC: 12.1 (CH3), 21.3 (CH2), 33.0 (CH2), 38.2 (CH2), 116.1 (C), 120.1 (2× CH), 122.9 (C), 125.7 (CH), 129.1 (2× CH), 130.3 (CH), 138.7 (C), 145.1 (C), 150.4 (C), 163.9 (C), 196.5 (C); HRMS (ESI, m/z): calcd for C17H15N3OK [M + K]+ 316.0858 found 316.4317.
3,7,7-Trimethyl-1-phenyl-7,8-dihydrociclohexa[2,3-e]pyrazolo[3,4-b]pyridin-5(6H)-one (4b). Yield: 85% (259.5 mg); yellow solid; mp 152–154 °C; IR (KBr, cm−1): 3064, 2954, 2929, 2869, 1678, 1591, 1507, 1374, 1244, 1119, 1023, 936, 757, 672, 556, 511; 1H NMR (400 MHz, DMSO-d6) δH: 1.02 (s, 6H), 2.58 (m, 5H), 3.07 (s, 2H), 7.30 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 7.7 Hz, 2H), 8.24 (d, J = 8.2 Hz, 2H), 8.68 (s, 1H); 13CNMR (100 MHz, DMSO-d6) δC: 12.2 (CH3), 27.7 (CH3), 32.5 (CH3), 46.6 (CH2), 51.5 (CH2), 99.5 (C), 116.1 (C), 120.3 (2× CH), 122.0 (C), 125.9 (CH), 129.2 (2× CH), 130.2 (CH), 138.8 (C), 145.4 (C), 151.0 (C), 162.8 (C), 196.7 (C); HRMS (ESI, m/z): calcd for C19H19N3O [M]+ 305.1528 found 305.2056.
3-Methyl-1-phenylnaftalen[2,3-e]pyrazolo[3,4-b]pyridin-5,10-dione (4c). Yield: 80% (271.4 mg); brown solid; mp 211–213 °C; IR (KBr, cm−1): 3476, 3052, 2921, 1683, 1665, 1505, 1290, 1276, 1114, 1094, 1027, 971, 785, 756, 661, 592; 1H NMR (400 MHz, DMSO-d6): δH = 2.58 (s, 3H, CH3), 7.32 (t, J = 7.2 Hz, 1H), 8.22 (d, J = 8.3 Hz, 2H), 8.24 (s, 1H); 13CNMR (100 MHz, DMSO-d6) δC: 12.0 (CH3), 118.5 (C), 120.0 (2× CH), 124.6 (C), 126.0 (CH), 126.5 (CH), 126.8 (CH), 128.1 (CH), 129.0 (2× CH), 131.0 (CH), 132.5 (C), 133.4 (C), 134.4 (C), 138.2 (C), 145.4 (C), 147.5 (C), 150.2 (C), 180.4 (C), 181.3 (C); HRMS (ESI, m/z): calcd for C21H13N3O2Na [M + Na]+ 362.0905 found 362.2862.
3-Methyl-1-phenyl-6,7-dihydrociclopenta[2,3-e]pyrazolo[3,4-b]pyridin-5(1H)-one (4d). Yield: 82% (215.9 mg); yellow solid, mp 215–217 °C; IR (KBr, cm−1): 3392, 3036, 2919, 1735, 1630, 1592, 1493, 1423, 1292, 1241, 1153, 1021, 754, 664, 615, 528; 1H NMR (400 MHz, DMSO-d6): δH = 2.59 (s, 3H, CH3), 7.35 (t, J = 7.4 Hz, 1H), 7.57 (t, J = 7.9 Hz, 2H), 8.21 (d, J = 7.7 Hz, 2H), 8.54 (s, 1H); 13CNMR (100 MHz, DMSO-d6) δC: 12.2 (CH3), 28.5 (CH2), 36.1 (CH2), 116.9 (C), 120.5 (2× CH), 125.0 (C), 126.0 (CH), 127.2 (2× CH), 129.2 (CH), 138.6 (C), 145.8 (C), 152.9 (C), 174.6 (C), 203.4 (C); HRMS (ESI, m/z): calcd for C16H13N3ONa [M + Na]+ 286.0956 found 286.0114.
3-Methyl-1-phenylindeno[2,3-e]pyrazolo[3,4-b]pyridin-5(1H)-one (4e). Yield: 91% (283.3 mg); yellow solid; mp 246–248 °C; IR (KBr, cm−1): 3390, 3054, 2923, 1716, 1613, 1589, 1502, 1337, 1290, 1138, 1028, 809, 781, 727, 636, 553; 1H NMR (400 MHz, DMSO-d6): δH = 2.61 (s, 3H, CH3), 7.38 (t, J = 7.4 Hz, 1H), 7.95 (d, J = 7.2 Hz, 1H), 8.28 (d, J = 7.7 Hz, 2H), 8.47 (s, 1H); 13CNMR (100 MHz, DMSO-d6) δC: 11.3 (CH3), 116.0 (C), 120.5 (2× CH), 120.7 (CH), 122.6 (C), 122.9 (CH), 125.7 (CH), 126.4 (CH), 128.5 (2× CH), 131.3 (CH), 134.8 (CH), 136.3 (C), 138.3 (C), 141.8 (C), 145.5 (C), 152.2 (C), 163.5 (C), 188.9 (C); HRMS (ESI, m/z): calcd for C20H13N3OK [M + K]+ 350.4350 found 349.4350.
1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-il)etanone (4f). Yield: 90% (238.7 mg); yellow solid; mp 100–102 °C; IR (KBr, cm−1): 3065, 2960, 2924, 2853, 1679, 1611, 1592, 1503, 1436, 1236, 1119, 1026, 944, 755, 692, 585; 1H NMR (400 MHz, DMSO-d6): δH = 2.63 (s, 3H, CH3), 2.67 (s, 3H, CH3), 2.77 (s, 3H, CH3), 7.31 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 8.0 Hz, 2H), 8.25 (d, J = 7.7 Hz, 2H), 8.87 (s, 1H); 13CNMR (100 MHz, DMSO-d6) δC: 12.2 (CH3), 25.8 (CH3), 29.4 (CH3), 114.4 (C), 120.1 (2× CH), 125.7 (CH), 127.2 (C) 129.1 (2× CH), 133.3 (CH), 138.9 (C), 144.3 (C), 149.5 (C), 158.5 (C), 199.6 (C); HRMS (ESI, m/z): calcd for C16H16N3O [M + H]+ 266.1293 found 266.2751.
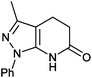
3-Methyl-1-phenyl-4,5-dihydro-1H-pyrazol[3,4-b]pyridine (4g). Yield: 67% (152.2 mg); green crystals; mp 276–278 °C; IR (KBr, cm−1): 3063, 2938, 2917, 2860, 1595, 1570, 1503, 1435, 1385, 1144, 1123, 1062, 950, 911, 785, 712, 690; 1H NMR (400 MHz, DMSO-d6): δH = 1.95 (s, 3H), 3.16 (d, J = 3.7 Hz, 2H), 3.57 (d, J = 15.8 Hz, 1H), 4.26 (t, J = 14.3 Hz, 2H), 7.28 (t, J = 6.9 Hz, 1H), 7.49 (t, J = 7.5 Hz, 2H), 7.95 (d, J = 8.53 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δC: 12.1 (CH3), 47.5 (CH2), 63.0 (CH2), 104.3 (C), 119.8 (2× CH), 125.6 (CH), 129.3 (2× CH), 135.8 (C), 139.2 (C), 144.8 (C), 145.0 (C); HRMS (ESI, m/z): calcd for C15H13N5O2 [M + 2H]+ 229.1215 found 229.1577.
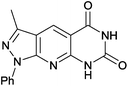
3-Methyl-1-phenyl-1H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H,8H)-dione (4h). Yield: 95% (278.6 mg); yellow solid; mp 300–302 °C; IR (KBr, cm−1): 3169, 3050, 2822, 1716, 1618, 1592, 1493, 1418, 1381, 1242, 1166, 1030, 851, 796, 761, 575; 1H NMR (400 MHz, DMSO-d6): δH 2.24 (s, 3H), 7.51 (m, 4H), 7.71 (s, 1H), 8.11 (s, 1H), 12.14 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δC: 12.3 (CH3), 103.9 (C), 114.3 (C), 120.3 (2× CH), 125.9 (CH), 129.2 (2× CH), 132.8 (C), 138.8 (CH), 145.4 (C), 150.7 (C), 151.1 (C), 161.1 (C), 162.4 (C); HRMS (ESI, m/z): calcd for C15H13N5O2 [M + 2H]+ 295.1069 found 295.1305.
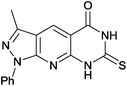
3-Methyl-1-phenyl-7-sulfanylidene-1,6,7,8-tetrahydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidin-5-one (4i). Yield: 91% (281.5 mg); red solid; mp > 300 °C; IR (KBr, cm−1): 3322, 3108, 3045, 2917, 1641, 1600, 1496, 1415, 1378, 1337, 1152, 1094, 920, 781, 698, 677, 527; 1H NMR (400 MHz, DMSO-d6): δH 2.60 (s, 3H), 7.31 (t, J = 8.0 Hz, 1H), 7.51 (t, J = 8.0 Hz, 2H), 8.23 (d, J = 8.0 Hz, 2H), 8.82 (s, 1H), 11.46 (s, 1H), 11.77 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 12.7 (CH3), 104.3 (C), 106.7 (C), 124.6 (2× CH), 128.3 (CH), 129.7 (2× CH), 136.8 (CH), 143.8 (CH), 151.1 (C), 153.6 (C), 162.0 (C), 177.1 (C); calcd for C30H23N10O2S2 [2M + H]+ 619.1447 found 619.1970.
General procedure for the synthesis of chalcones 6a–i
A mixture of pyrazolo[3,4-b]pyridine (4a or 4f 1 mmol), appropriate aromatic aldehyde (1 mmol), KOH (1 mmol) and ethanol (2 mL), was sonicated for 5–20 min in the water bath of an ultrasonic cleaner bath. The progress of the reaction was monitored by TLC using dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (9
ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as eluent. The reaction mixture was cooled in ice-water bath. The formed precipitate was filtered, washed with mixture water
1 v/v) as eluent. The reaction mixture was cooled in ice-water bath. The formed precipitate was filtered, washed with mixture water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and purified by recrystallization with mixture ethanol
1) and purified by recrystallization with mixture ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (1
hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the target compounds in high yields of 50–98%.
1) to give the target compounds in high yields of 50–98%.
(E)-6-((Benzo[d][1,3]dioxol-5-yl)methylene)-7,8-dihydro-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]quinolin-5(6H)-one (6a). Yield: 50% (204.7 mg); yellow solid; mp 221–223 °C; IR (KBr, cm−1): 3065, 2897, 1658, 1592, 1488, 1317, 1239, 1036, 923, 816, 747; 1H NMR (400 MHz, CD3Cl); δH 2.67 (s, 3H), 3.24 (s, 4H), 6.02 (s, 2H), 6.88 (d, J = 8.0 Hz, 1H), 7.00 (m, 2H), 7.29 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.86 (s, 1H), 8.28 (d, J = 8.0 Hz, 2H), 8.81 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 26.3 (CH2), 32.7 (CH2), 101.4 (CH2), 108.5 (CH), 109.8 (CH), 117.0 (C), 120.9 (2× CH), 123.8 (C), 125.3 (CH), 125.9 (CH), 129.0 (2× CH), 129.6 (CH), 131.3 (C), 133.0 (CH), 137.6 (C), 139.1 (C), 145.1 (C), 147.8 (C), 148.3 (C), 151.0 (C), 162.5 (C), 187.0 (C); HRMS (ESI, m/z): calcd for C25H19N3O3Na [M + Na]+ 432.1324 found 432.1360.
(E)-6-(4-Morpholinobenzylidene)-7,8-dihydro-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]quinolin-5(6H)-one (6b). Yield: 65% (292.9 mg); yellow solid; mp 175–177 °C IR (KBr, cm−1): 3062, 2955, 2920, 2894, 2851, 1664, 1589, 1508, 1421, 1378, 1184, 1114, 923; 1H NMR (400 MHz, CD3Cl); δH 2.45 (s, 3H), 3.03 (s, 8H) 3.63–3.66 (m, 4H), 6.53 (s, 1H), 6.68 (d, J = 8.0 Hz, 2H), 7.04–7.09 (m, 1H), 7.23–7.31 (m, 4H), 7.68 (s, 1H), 8.08 (d, J = 7.8 Hz, 2H), 8.58 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 26.3 (CH2), 32.7 (CH2), 48.1 (CH2), 66.6 (CH2), 114.4 (2× CH), 116.9 (CH), 120.8 (CH), 124.0 (C), 125.8 (CH), 126.4 (C), 129.0 (2× CH), 131.1 (CH), 131.6 (C), 131.9 (2× CH), 137.9 (CH), 139.1 (C), 145.0 (C), 151.0 (C), 151.4 (C), 162.5 (C), 187.0 (C); HRMS (ESI, m/z): calcd for C28H26N4O2Na [M + Na]+ 473.1948 found 473.1105.
(E)-7,8-Dihydro-3-methyl-6-((1-methyl-1H-imidazol-2-yl)methylene)-1-phenyl-1H-pyrazolo[3,4-b]quinolin-5(6H)-one (6c). Yield: 72% (265.9 mg); yellow solid; mp 210–212 °C; IR (KBr, cm−1): 3097, 3062, 2952, 2920, 2877, 1676, 1612, 1592, 1505, 1415, 1256, 1210, 1111, 1016, 889, 750; 1H NMR (400 MHz, CD3Cl) 2.62 (s, 3H), 3.26 (t, J = 6.6 Hz, 1H), 3.74 (m, 5H), 6.95 (s, 1H), 7.22 (d, J = 6.6 Hz, 2H), 7.45 (t, J = 7.3 Hz, 1H), 7.60 (s, 1H), 7.80 (s, 1H), 8.24 (d, J = 8.0 Hz, 2H), 8.74 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 25.7 (CH2), 32.3 (CH2), 33.2 (CH3), 116.9 (C), 119.2 (CH), 120.8 (CH), 120.9 (2× H), 122.9 (CH), 123.5 (C), 125.8 (C), 125.9 (CH), 129.0 (2× CH), 130.2 (CH), 131.2 (CH), 136.1 (C), 139.0 (C), 143.9 (C), 145.0 (C), 151.0 (C), 163.3 (C), 187.0 (C); HRMS (ESI, m/z): calcd for C22H19N5O [M + K]+ 408.1221 found 408.1104.
(E)-7,8-Dihydro-3-methyl-1-phenyl-6-((pyridin-3-yl)methylene)-1H-pyrazolo[3,4-b]quinolin-5(6H)-one (6d). Yield: 52% (190.5 mg); beige solid; mp 165–167 °C; IR (KBr, cm−1): 3050, 2937, 2917, 2842, 1951, 1872, 1751, 1658, 1615, 1514, 1482, 1418, 1328, 1268, 1195, 1097, 1016, 758; 1H NMR (400 MHz, CD3Cl) 2.61 (s, 3H), 3.14–3.21 (m, 4H), 7.23 (t, J = 8.0 Hz, 1H), 7.30–7.33 (m, 1H), 7.44 (t, J = 7.8 Hz, 2H), 7.70 (d, J = 8.0 Hz, 1H), 7.59 (s, 1H), 7.80 (s, 1H), 8.22 (d, J = 8.0 Hz, 2H), 8.53 (s, 1H); 8.67 (s, 1H); 8.74 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.8 (CH3), 26.5 (CH2), 32.9 (CH2), 117.4 (C), 121.2 (2× CH), 123.6 (CH), 126.3 (CH), 129.3 (2× CH), 131.8 (CH), 133.7 (CH), 136.8 (CH), 137.0 (C), 139.3 (C), 145.4 (C), 149.7 (CH), 150.9 (CH), 151.4 (C), 162.7 (C), 186.8 (C); HRMS (ESI, m/z): calcd for C23H18N4ONa [M + Na]+ 389.1373 found 389.1219.
4-((E)-(7,8-Dihydro-3-methyl-5-oxo-1-phenyl-1H-pyrazolo[3,4-b]quinolin-6(5H)-ylidene)methyl)benzonitrile (6e). Yield: 72% (281.1 mg); brown solid; mp 123–125 °C; IR (KBr, cm−1): 3062, 2949, 2920, 2851, 2231, 1942, 1867, 1667, 1595, 1508, 1485, 1328, 1268, 1204, 1120, 1022, 854, 755; 1H NMR (400 MHz, CD3Cl) 2.65 (s, 3H), 3.13–3.20 (m, 4H), 7.28 (t, J = 8.0 Hz, 1H), 7.47–7.52 (m, 4H), 7.70 (d, J = 8.0 Hz, 2H), 7.84 (s, 1H), 8.26 (d, J = 8.0 Hz, 2H), 8.78 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.7 (CH3), 26.5 (CH2), 32.8 (CH2), 112.3 (C), 117.4 (C), 118.7 (C), 121.1 (C), 121.1 (2× CH), 123.5 (C), 126.3 (CH), 129.3 (2× CH), 130.5 (2× CH), 131.8 (CH), 132.5 (2× CH), 135.1 (CH), 137.4 (C), 139.2 (C), 140.4 (C), 145.4 (C), 151.3 (C), 162.5 (C), 186.7 (C); HRMS (ESI, m/z): calcd for C25H18N4O [M + H]+ 391.1559 found 390.9908.
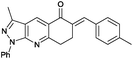
(E)-6-Benzylidene-7,8-dihydro-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]quinolin-5(6H)-one (6f). Yield: 80% (292.3 mg); white solid; mp 189–191 °C; IR (KBr, cm−1): 3062, 3045, 2966, 2940, 2920, 1661, 1609, 1583, 1511, 1485, 1418, 1268, 1201, 1016, 941, 790, 764; 1H NMR (400 MHz, CD3Cl) 2.48 (s, 3H), 3.03 (s, 4H), 7.05–7.10 (m, 1H), 7.15–732 (m, 6H), 7.74 (s, 1H), 8.08 (d, J = 8.0 Hz, 2H), 8.61 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 26.1 (CH2), 32.7 (CH2), 117.0 (C), 120.8 (2× CH), 123.6 (CH), 125.9 (CH), 128.5 (2× CH), 128.8 (CH), 129.0 (2× CH), 129.9 (2× CH), 131.3 (CH), 134.5 (C), 135.5 (C), 137.5 (CH), 139.1 (C), 145.0 (C), 151.0 (C), 162.5 (C), 187.0 (C); HRMS (ESI, m/z): calcd for C24H19N3ONa [M + Na]+ 388.1426 found 388.1700.
(E)-3-Methyl-6-(4-methylbenzylidene)-1-phenyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (6g). Yield: 61% (231.4 mg); white solid; mp 178–180 °C; IR (KBr, cm−1): 3045, 2952, 2923, 1658, 1589, 1418, 1265, 1172, 1016, 813, 753; 1H NMR (400 MHz, CD3Cl); 2.40 (s, 3H), 2.66 (s, 3H), 3.23 (s, 4H), 7.23–7.30 (m, 3H), 7.38 (d, J = 8.0 Hz, 2H), 7.48–7.50 (m, 2H), 7.92 (s, 1H), 8.29 (d, J = 8.0 Hz, 2H), 8.80 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 21.4 (CH3), 26.2 (CH2), 32.7 (CH2), 117.0 (C), 120.8 (2× CH), 123.7 (C), 125.9 (CH), 129.0 (2× CH), 129.2(2× CH), 130.0 (2× CH), 131.2 (CH), 132.6 (C), 133.7 (C), 137.6 (CH), 139.1 (C), 139.1 (C), 145.0 (C), 151.0 (C), 162.5 (C), 187.1 (C); HRMS (ESI, m/z): calcd for C25H22N3O [M + H]+ 380.1763 found 380.1763.
(E)-6-((Furan-2-yl)methylene)-7,8-dihydro-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]quinolin-5(6H)-one (6h). Yield: 83% (294.9 mg); beige solid; mp 180–182 °C; IR (KBr, cm−1): 3140, 3123, 3053, 2955, 2897, 2842, 1742, 1658, 1664, 1609, 1545, 1499, 1317, 1265, 1198, 1068, 961, 880; 1H NMR (400 MHz, CD3Cl) 2.65 (s, 3H), 3.30 (t, J = 6.0 Hz, 2H) 3.40–3.44 (m, 2H), 6.53 (s, 1H), 6.74 (s, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.59 (s, 1H), 7.64 (s, 1H), 8.29 (d, J = 8.0 Hz, 2H), 8.78 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 25.6 (CH2), 32.3 (CH2), 112.3 (CH), 116.9 (C), 117.2 (CH), 120.9 (2× CH), 123.4 (CH), 123.8 (C), 125.9 (CH), 129.0 (2× CH), 130.7 (C), 131.2 (CH), 139.1 (C), 144.7 (CH), 145.0 (C), 150.9 (C), 152.2 (C), 162.8 (C), 186.7 (C); HRMS (ESI, m/z): calcd for C22H17N3O2Na [M + Na]+ 378.1218 found 378.0209.
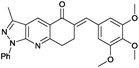
(E)-6-(3,5-Dimethoxybenzylidene)-3-methyl-1-phenyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (6i). Yield: 90% (382.9 mg); yellow Solid; mp 179–181 °C; IR (KBr, cm−1): 3004, 2935, 2833, 1653, 1598, 1519, 1421, 1328, 1253, 1143, 1022, 764; 1H NMR (400 MHz, CD3Cl) 2.90 (s, 3H), 3.50 (s, 4H) 4.17 (s, 6H) 7.16 (d, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.52 (m, 1H), 7.74 (t, J = 8.0 Hz, 2H), 8.13 (s, 1H), 8.53 (d, J = 8.0 Hz, 2H), 9.04 (m, 1H); 13C NMR (100 MHz, CD3Cl); 12.4 (CH3), 26.2 (CH2), 32.6 (CH2), 55.9 (CH3), 110.9 (CH), 113.3 (CH), 116.9 (C), 120.8 (2× CH), 123.4 (CH), 123.7 (C), 125.8 (CH), 128.3 (C), 128.9 (2× CH), 131.1 (CH), 132.7 (C), 137.7 (CH), 139.1 (C), 145.0 (C), 148.7 (C), 149.8 (C), 150.9 (C), 162.4 (C), 186.9 (C); HRMS (ESI, m/z): calcd for C26H24N3O3 [M + H]+ 426.1818 found 426.1818.
(E)-3-Methyl-1-phenyl-6-(3,4,5-trimethoxybenzylidene)-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (6j). Yield: 92% (419.1 mg); yellow solid; mp 177–179 °C; IR (KBr, cm−1): 2935, 2836, 2593, 1661, 1598, 1505, 1323, 1259, 1123, 1007, 834, 747; 1H NMR (400 MHz, CD3Cl) 2.60 (s, 3H), 3.20 (s, 4H) 3.83 (s, 9H) 6.64 (s, 2H), 7.22 (m, 1H), 7.44 (t, J = 7.5 Hz, 2H), 7.81 (s, 1H), 8.23 (d, J = 8.0 Hz, 2H), 8.74 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.4 (CH3), 26.2 (CH2), 32.7 (CH2) 56.2 (CH3), 60.9 (CH3), 107.4 (2× CH), 117.0 (C), 120.8 (2× CH), 123.6 (C), 125.9 (CH), 129.0 (2× CH), 130.9 (C), 131.2 (CH), 133.8 (C), 137.7 (CH), 138.9 (C), 139.0 (C), 145.0 (C), 151.0 (C), 153.1 (C), 162.4 (C), 186.9 (C); (ESI, m/z): calcd for C27H25N3O4K [M + K]+ 494.1482 found 494.1456.
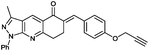
(E)-3-Methyl-1-phenyl-6-(4-(prop-2-yn-1-yloxy)benzylidene)-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (6k). Yield: 72% (302.0 mg); white solid; mp 176–178 °C; IR (KBr, cm−1): 3288, 3241, 2932, 1658, 1505, 1412, 1276, 1218, 1178, 1019, 842, 764; 1H NMR (400 MHz, CD3Cl) 2.25 (s, 1H), 2.67 (s, 3H), 3.24 (s, 4H) 4.73 (s, 2H) 7.04 (d, J = 8.0 Hz, 2H), 7.28 (t, J = 7.3 Hz, 1H), 7.52 (m, 4H), 7.90 (s, 1H), 8.29 (d, J = 8.0 Hz, 2H), 8.80 (m, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 26.2 (CH2), 32.7 (CH2), 55.8 (CH2), 75.9 (CH), 78.1 (C), 114.9 (2× CH), 117.0 (C), 120.9 (2× CH), 123.8 (C), 125.9 (CH), 128.9 (C), 129.0 (2× CH), 131.2 (2× CH), 131.8 (CH), 132.9 (C), 137.2 (CH), 139.1 (C), 145.0 (C), 151.0 (C), 158.0 (C), 162.5 (C), 187.0 (C); (ESI, m/z): calcd for C27H21N3O2K [M + K]+ 458.1271 found 458.1261.
tert-Butyl-(E)-4-(4-((3-methyl-5-oxo-1-phenyl-1,5,7,8-tetrahydro-6H-pyrazolo[3,4-b]quinolin-6-ylidene)methyl)phenyl)piperazine-1-carboxylate (6l). Yield: 67% (326.5 mg); brown solid; mp 108–110 °C; IR (KBr, cm−1): 3065, 2958, 2877, 1681, 1592, 1517, 1421, 1265, 1175, 1106, 906, 750; 1H NMR (400 MHz, CD3Cl); 1.44 (s, 9H), 2.16 (m, 2H), 2.58 (s, 3H), 2.68 (t, J = 6.0 Hz, 2H), 7.19 (m, 6H), 3.54 (bs, 2H), 6.87 (d, J = 8.0 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.45 (t, J = 8.0 Hz, 3H), 8.23 (m, 3H), 8.66 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.5 (CH3), 21.9 (CH2), 26.3 (CH2), 28.4 (CH3), 32.7 (CH2), 33.7 (CH2), 38.8 (CH2), 48.0 (CH2), 80.0 (C), 115.1 (CH), 116.7 (C), 120.8 (2× CH), 123.0 (C), 124.0 (C), 125.8 (CH), 125.9 (CH), 126.5 (C), 129.0 (2× CH), 130.4 (CH), 131.1 (CH), 131.6 (C), 131.9 (CH), 137.9 (CH), 139.2 (C), 145.1 (C), 151.2 (C), 154.6 (C), 163.8 (C), 187.0 (C); HRMS (ESI, m/z): calcd for C33H36N5O3 [M + H]+ 550.2818 found 550.2818.
(E)-6-((5-(4-Chlorophenyl)furan-2-yl)methylene)-3-methyl-1-phenyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (6m). Yield: 98% (456.6 mg); yellow solid; mp 170–172 °C; IR (KBr, cm−1): 3065, 2949, 1664, 1589, 1421, 1320, 1239, 1201, 1091, 1036, 912, 787; 1H NMR (400 MHz, CD3Cl) 2.65 (s, 3H), 3.35 (t, J = 6.1 Hz, 2H), 3.46 (m, 2H) 6.77 (s, 1H), 6.82 (s, 1H), 7.28 (t, J = 7.2 Hz, 1H), 7.38 (d, J = 8.0 Hz, 2H), 7.50 (t, J = 7.3 Hz, 2H), 7.63 (d, J = 8.0 Hz, 3H), 8.30 (d, J = 8.0 Hz, 2H), 8.77 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 12.5 (CH3), 25.8 (CH2), 108.3 (CH), 117.0 (C), 119.8 (CH), 120.9 (2× CH), 123.1 (CH), 123.8 (C), 125.4 (2× CH), 125.9 (CH), 128.3 (C), 129.0 (2× CH), 129.1(2× CH), 130.6 (C), 131.1 (CH), 134.2 (C), 139.2 (C), 145.0 (C), 151.0 (C), 152.0 (C), 155.0 (C), 162.7 (C), 186.4 (C); HRMS (ESI, m/z): calcd for C28H20ClN3O2 [M]+ 465.1244 found 465.1226.
(E)-3-Methyl-1-phenyl-6-(quinolin-3-ylmethylene)-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (6n). Yield: 87% (362.3 mg); yellow solid; mp 211–213 °C; IR (KBr, cm−1): 3053, 2961, 2917, 1667, 1600, 1514, 1253, 1114, 1022, 747; 1H NMR (400 MHz, CD3Cl) 2.68 (s, 3H), 3.30 (s, 4H), 7.29 (t, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.59 (t, J = 8.0 Hz, 1H), 7.76 (t, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 8.04 (s, 1H), 8.12 (d, J = 8.0 Hz, 1H), 8.21 (s, 1H), 8.28 (d, J = 8.0 Hz, 2H), 8.84 (s, 1H), 9.01 (s, 1H); 13C NMR (100 MHz, CD3Cl); 12.8 (CH3), 26.7 (CH2), 32.9 (CH2), 117.4 (C), 121.2 (2× CH), 123.7 (C), 126.3 (CH), 127.6 (CH), 127.7 (C), 128.4 (CH), 129.0 (C), 129.3 (2× CH), 129.6 (CH), 130.7 (CH), 131.8 (CH), 133.9 (CH), 136.8 (CH), 136.9 (C), 139.3 (C), 145.4 (C), 147.8 (C), 151.4 (C), 151.6 (CH), 162.6 (C), 186.8 (C); HRMS (ESI, m/z): calcd for C27H20N4OK [M + K]+ 455.1274 found 455.1271.
(E)-3-(Benzo[d][1,3]dioxol-5-yl)-1-(3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)prop-2-en-1-one (6o). Yield: 63% (250.3 mg); yellow solid; mp 186–188 °C; IR (KBr, cm−1): 3082, 2990, 2894, 2787, 1655, 1589, 1577, 1444, 1308, 1184, 1033, 984, 754; 1H NMR (CDCl3, 400 MHz) δH: 2.63 (s, 3H), 2.81 (s, 3H), 6.01 (s, 2H), 6.82 (d, J = 8.0 Hz, 1H), 7.12–7.04 (m, 3H), 7.28 (t, J = 7.4 Hz, 1H), 7.52–7.45 (m, 3H), 8.16 (s, 1H), 8.32 (d, J = 7.5 Hz, 2H). 13C NMR (CDCl3, 100 MHz) δC: 12.4 (CH3), 24.7 (CH3), 101.6 (CH2), 106.5 (CH), 108.5 (CH), 114.2 (C), 120.6 (2× CH), 123.9 (CH), 125.3 (CH), 125.5 (CH), 128.7 (C), 128.7 (C), 128.9 (2× CH), 129.8 (CH), 139.2 (C), 143.3 (C), 145.7 (CH), 148.4 (C), 150.1 (C), 150.1 (C), 158.0 (C), 193.8 (C); HRMS (ESI, m/z): calcd for C24H19N3O3Na [M + Na]+ 420.1324 found 420.0670.
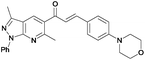
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(4-morpholinophenyl)prop-2-en-1-one (6p). Yield: 82% (359.6 mg); yellow solid; mp 230–232 °C; IR (KBr, cm−1): 3069, 2961, 2921, 2892, 2849, 1953, 1885, 1737, 1668, 1600, 1508, 1380, 1303, 1180, 1123, 923, 761; 1H NMR (CDCl3, 400 MHz) δH: 2.65 (s, 3H), 2.81 (s, 3H, CH3), 3.27 (t, J = 4.6 Hz, 4H), 3.86 (t, J = 4.5 Hz, 4H), 6.89 (d, J = 8.3 Hz, 2H), 7.07 (d, J = 15.9 Hz, 1H), 7.29 (t, J = 7.4 Hz, 1H), 7.46 (d, J = 16.1 Hz, 1H), 7.53–7.50 (m, 4H), 8.15 (s, 1H), 8.33 (d, J = 8.3 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δC: 12.5 (CH3), 24.6 (CH3), 47.8 (2× CH2), 66.5 (2× CH2), 114.2 (C), 114.5 (2× CH), 120.7 (2× CH), 122.9 (CH), 125.0 (C), 125.6 (CH), 129.0 (2× CH), 129.3 (C), 129.7 (CH), 130.2 (2× CH), 139.4 (C), 143.3 (C), 146.5 (CH), 150.2 (C), 152.9 (C), 157.8 (C), 194.7 (C); HRMS (ESI, m/z): calcd for C27H26N4O2Na [M + Na]+ 461.1953 found 461.8987.
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(1-methyl-1H imidazol-2-yl)prop-2-en-1-one (6q). Yield: 93% (332.4 mg); yellow solid; mp 150–152 °C; IR (KBr, cm−1): 3107, 2955, 2923, 2847, 1685, 1602, 1555, 1504, 1376, 1300, 1241, 1175, 1124, 1033, 972, 830, 756; 1H NMR (CDCl3, 400 MHz) δH: 2.86 (s, 3H), 3.12 (s, 3H), 4.02 (s, 3H), 7.51–7.43 (m, 3H), 7.72 (t, J = 7.5 Hz, 2H), 7.85 (d, J = 15.1 Hz, 1H), 8.05 (d, J = 15.1 Hz, 1H), 8.51 (d, J = 8.0 Hz, 2H), 8.67 (s, 1H); 13C NMR (CDCl3, 100 MHz) δC: 12.5 (CH3), 25.5 (CH3), 33.0 (CH3), 114.4 (C), 120.8 (2× CH), 124.13 (CH), 125.7 (CH), 125.9 (CH), 127.7 (CH), 128.0 (C), 128.9 (2× CH), 130.6 (CH), 131.1 (CH), 139.2 (C), 143.5 (C), 143.9 (C), 150.2 (C), 159.4 (C), 191.3 (C); HRMS (ESI, m/z): calcd for C21H19N5OK [M + K]+ 396.1227 found 396.9227.
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(4-methoxyphenyl)prop-2-en-1-one (6r). Yield: 72% (276.1 mg); yellow solid; mp 134–136 °C; IR (KBr, cm−1): 3163, 3069, 3001, 2915, 2841, 1962, 1805, 1739, 1654, 1503, 1420, 1300, 1251, 1169, 1029, 983, 820, 758; 1H NMR (CDCl3, 400 MHz) δH: 2.64 (s, 3H), 2.82 (s, 3H), 3.83 (s, 3H), 6.92 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 15.8 Hz, 1H), 7.28 (t, J = 7.5 Hz, 1H), 7.55–7.48 (m, 5H), 8.16 (s, 1H), 8.32 (d, J = 7.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δC: 12.8 (CH3), 25.0 (CH3), 55.6 (CH3), 114.5 (C), 114.7 (2× CH), 121.0 (2× CH), 124.2 (CH), 125.9 (CH), 127.3 (C), 129.2 (2× CH), 130.1 (CH), 130.6 (2× CH), 139.6 (C), 143.7 (C), 146.3 (CH), 150.5 (C), 158.2 (C), 162.2 (C), 194.6 (C); HRMS (ESI, m/z): calcd for C24H21N3O2Na [M + Na]+ 406.1531 found 406.1426.
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (6s). Yield: 91% (403.6 mg); yellow solid; mp 180–182 °C; IR (KBr, cm−1): 3062, 2998, 2929, 2836, 1954, 1875, 1760, 1681, 1595, 1508, 1418, 1300, 1239, 1120, 1004, 822, 755; 1H NMR (CDCl3, 400 MHz) δH: 3.09 (s, 3H), 3.25 (s, 3H), 4.34 (s, 9H), 7.55 (d, J = 15.9 Hz, 1H), 7.73 (t, J = 7.3 Hz, 1H), 7.85 (d, J = 15.9 Hz, 1H), 7.98–7.92 (m, 4H), 8.60 (s, 1H), 8.76 (d, J = 8.3 Hz, 2H);·13C NMR (CDCl3, 100 MHz) δC: 12.5 (CH3), 24.7 (CH3), 56.2 (2× CH3), 61.0 (CH3), 105.7 (2× CH), 114.3 (C), 120.8 (2× CH), 125.7 (CH), 125.8 (CH), 128.8 (C), 129.0 (2× CH), 129.7 (C), 129.9 (CH), 139.3 (C), 140.8 (C), 143.4 (C), 146.5 (CH), 150.3 (C), 153.5 (2× C), 157.8 (C), 194.7 (C); HRMS (ESI, m/z): calcd for C26H25N3O4Na [M + Na]+ 466.1743 found 465.9858.
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(quinolin-3-yl)prop-2-en-1-one (6t). Yield: 87% (351.9 mg); brown solid; mp 195–197 °C; IR (KBr, cm−1): 3058, 2918, 2850, 1874, 1793, 1737, 1602, 1511, 1384, 1251, 1126, 1031, 979, 906, 866, 756; 1H NMR (CDCl3, 400 MHz) δH: 2.66 (s, 3H), 2.87 (s, 3H), 7.29 (t, J = 7.4 Hz, 1H), 7.53–7.46 (m, 3H), 7.59 (t, J = 7.4 Hz, 1H), 7.79–7.73 (m, 2H), 7.86 (d, J = 8.3 Hz, 1H), 8.12 (d, J = 8.5 Hz, 1H), 8.33–8.27 (m, 4H), 9.17 (s, 1H); 13C NMR (CDCl3, 100 MHz) δC: 12.6 (CH3), 25.0 (CH3), 114.3 (C), 120.8 (2× CH), 125.8 (CH), 127.30 (C), 127.4 (C), 127.6 (CH), 128.2 (CH), 128.4 (C), 129.0 (2× CH), 129.5 (CH), 130.4 (CH), 130.9 (2× CH), 136.2 (CH), 139.2 (C), 142.2 (CH), 143.6 (C), 148.8 (C), 149.3 (CH), 150.3 (C), 158.5 (C), 193.1 (C); HRMS (ESI, m/z): calcd for C26H21N4O [M + H]+ 405.1715 found 405.0341.
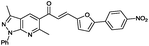
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(5-(4-nitrophenyl)furan-2-yl)prop-2-en-1-one (6u). Yield: 90% (418.0 mg); yellow solid; mp > 300 °C; IR (KBr, cm−1): 2982, 2929, 1729, 1684, 1592. 1H NMR (CDCl3, 400 MHz) δH: 2.67 (s, 3H, CH3), 2.86 (s, 3H), 6.87 (d, J = 3.4 Hz, 1H), 6.99 (d, J = 3.4 Hz, 1H), 7.32–7.26 (m, 2H), 7.41 (d, J = 15.6 Hz, 1H), 7.52 (t, J = 7.5 Hz, 2H), 7.88 (d, J = 8.8 Hz, 2H), 8.34–8.25 (m, 6H). 13C NMR (CDCl3, 100 MHz) δC: 12.5 (CH3), 24.9 (CH3), 111.6 (CH), 114.3 (C), 118.8 (CH), 120.7 (2× CH), 124.4 (2× CH), 124.7 (2× CH), 125.7 (CH), 125.8 (CH), 128.5 (C), 129.0 (2× CH), 130.1 (CH), 130.7 (CH), 135.1 (CH), 139.2 (C), 143.5 (C), 147.1 (C), 150.3 (C), 152.3 (C), 153.9 (C), 158.2 (C), 193.0 (C); HRMS (ESI, m/z): calcd for C27H21N4O4 [M + H]+ 465.1563 found 465.1836.
(E)-1-(3,6-Dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-3-(4-nitrophenyl)prop-2-en-1-one (6v). Yield: 75% (298.8 mg); orange solid; mp > 300 °C; IR (KBr, cm−1): 3063, 2983, 1725, 1626, 1586; 1H NMR (CDCl3, 400 MHz) δH: 2.66 (s, 3H), 2.86 (s, 3H), 7.30 (t, J = 7.3 Hz, 1H), 7.38 (d, J = 15.8 Hz, 1H), 7.52 (t, J = 7.7 Hz, 2H), 7.64 (d, J = 15.8 Hz, 1H), 7.76 (d, J = 8.3 Hz, 2H), 8.32–8.26 (m, 5H);·13C NMR (CDCl3, 100 MHz) δC: 12.5 (CH3), 25.1 (CH3), 114.3 (C), 120.8 (CH ×2), 124.2 (CH ×2), 125.9 (CH), 127.8 (C), 129.0 (2× CH), 129.1 (2× CH), 129.2 (CH), 130.5 (CH), 139.2 (C), 140.5 (C), 142.2 (CH), 143.6 (C), 148.7 (C), 150.3 (C), 158.6 (C), 192.6 (C). Calcd for C23H18N4O3K [M + K]+ 437.1016 found 437.1286.
Conclusions
In summary, we developed an efficient three-component reaction of 3-methyl-1-phenyl-1H-pyrazolo-5-amine, paraformaldehyde and β-diketones under MWI in aqueous media catalyzed by InCl3. Compared with previous methods, this new protocol has the advantages of simple operation, higher yields, low cost and is an environmentally benign procedure. Synthetic versatility showed for carbonyl group allows generating great structural variety, which facilitates the obtaining of a greater number of compounds with biological activity on different therapeutic targets depending on the group that originates. In the preparation of chalcones employment of sonication reduced times reaction from hours to minutes. These new compounds present a privileged core from a biological point of view.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This research was supported by a FONDECYT project number 1150712, PIEI QUIMBIO, project, Utalca. E. P. and MG thanks the CONICYT beca No. 63140046, for financial support. J. T., K. F. and J. Q. thanks to Universidad del Atlántico and Universidad del Valle.
Notes and references
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311–335 CrossRef CAS PubMed.
- L. Commeiras, S. C. Woodcock, J. E. Baldwin, R. M. Adlington, A. R. Cowley and P. J. Wilkinson, Tetrahedron, 2004, 60, 933–938 CrossRef CAS.
- C. Samar, J. Fayçel and K. Jameleddine, Tetrahedron Lett., 2011, 52, 3648–3650 CrossRef CAS.
- C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868 CrossRef CAS PubMed.
- C. Chen, T. R. Webb, J. R. Mccarthy, T. J. Moran, K. M. Wilcoxen and C. Huang, WO1997029109 A1, 1997.
- M. A. Gaston, L. R. S. Dias, A. C. C. Freitas, A. L. P. Miranda and E. J. Barreiro, Pharm. Acta Helv., 1996, 71, 213–219 CrossRef CAS PubMed.
- F. E. Goda, A. A.-M. Abdel-Aziz and O. A. Attef, Bioorg. Med. Chem., 2004, 12, 1845–1852 CrossRef CAS PubMed.
- T. Tuccinardi, A. T. Zizzari, C. Brullo, S. Daniele, F. Musumeci, S. Schenone, M. L. Trincavelli, C. Martini, A. Martinelli, G. Giorgi and M. Botta, Org. Biomol. Chem., 2011, 9, 4448–4455 CAS.
- J.-P. Stasch, E. M. Becker, C. Alonso-Alija, H. Apeler, K. Dembowsky, A. Feurer, R. Gerzer, T. Minuth, E. Perzborn, U. Pleiß, H. Schröder, W. Schroeder, E. Stahl, W. Steinke, A. Straub and M. Schramm, Nature, 2001, 410, 212–215 CrossRef CAS PubMed.
- A. Straub, J. Benet-Buckholz, R. Fröde, A. Kern, C. Kohlsdorfer, P. Schmitt, T. Schwarz, H.-M. Siefert and J.-P. Stasch, Bioorg. Med. Chem., 2002, 10, 1711–1717 CrossRef CAS PubMed.
- L. R. S. Dias, M. B. Santos, S. de Albuquerque, H. C. Castro, A. M. T. de Souza, A. C. C. Freitas, M. A. V. DiVaio, L. M. Cabral and C. R. Rodrigues, Bioorg. Med. Chem., 2007, 15, 211–219 CrossRef CAS PubMed.
- C. J. Mitchell, S. P. Ballantine, D. M. Coe, C. M. Cook, C. J. Delves, M. D. Dowle, C. D. Edlin, J. N. Hamblin, S. Holman, M. R. Johnson, P. S. Jones, S. E. Keeling, M. Kranz, M. Lindvall, F. S. Lucas, M. Neu, Y. E. Solanke, D. O. Somers, N. A. Trivedi and J. O. Wiseman, Bioorg. Med. Chem. Lett., 2010, 20, 5803–5806 CrossRef CAS PubMed.
- T. M. Bare, C. D. McLaren, J. B. Campbell, J. W. Firor, J. F. Resch, C. P. Walters, A. I. Salama, B. A. Meiners and J. B. Patel, J. Med. Chem., 1989, 32, 2561–2573 CrossRef CAS PubMed.
- J. B. Patel, B. A. Meiners, A. I. Salama, J. B. Malick, D. C. U'Prichard, R. E. Giles, M. E. Goldberg and T. M. Bare, Pharmacol., Biochem. Behav., 1988, 29, 775–779 CrossRef CAS.
- A. Ghaedi, G. R. Bardajee, A. Mirshokrayi, M. Mahdavi, A. Shafiee and T. Akbarzadeh, RSC Adv., 2015, 5, 89652–89658 RSC.
- C. Bülow and K. Haas, Ber. Dtsch. Chem. Ges., 1910, 43, 1975–1984 CrossRef.
- C. Simon, T. Constantieux and J. Rodriguez, Eur. J. Org. Chem., 2004, 2004, 4957–4980 CrossRef.
- F. Shi, Y. Zhang, S.-J. Tu, D.-X. Zhou, C.-M. Li, Q.-Q. Shao and L.-J. Cao, Chin. J. Chem., 2008, 26, 1262–1266 CrossRef CAS.
- J. Quiroga, D. Cobo, B. Insuasty, R. Abonía, S. Cruz, M. Nogueras and J. Cobo, J. Heterocycl. Chem., 2008, 45, 155–159 CrossRef CAS.
- C.-L. Shi, D.-Q. Shi, S. H. Kim, Z.-B. Huang, S.-J. Ji and M. Ji, Tetrahedron, 2008, 64, 2425–2432 CrossRef CAS.
- B. Jiang, Y.-P. Liu and S.-J. Tu, Eur. J. Org. Chem., 2011, 2011, 3026–3035 CrossRef.
- E. J. Barreiro, C. A. Camara, H. Verli, L. Brazil-Más, N. G. Castro, W. M. Cintra, Y. Aracava, C. R. Rodrigues and C. A. M. Fraga, J. Med. Chem., 2003, 46, 1144–1152 CrossRef CAS PubMed.
- M. N. Jachak, A. B. Avhale, C. D. Tantak, R. B. Toche, C. Reidlinger and W. Stadlbauer, J. Heterocycl. Chem., 2005, 42, 1311–1319 CrossRef CAS.
- D. Yang, K. Jiang, J. Li and F. Xu, Tetrahedron, 2007, 63, 7654–7658 CrossRef CAS.
- J. H. Clark, Green Chem., 2006, 8, 17–21 RSC.
- F. Shi, D. Zhou, S. Tu, C. Li, L. Cao and Q. Shao, J. Heterocycl. Chem., 2008, 45, 1305–1310 CrossRef CAS.
- J. M. Khurana, A. Chaudhary, B. Nand and A. Lumb, Tetrahedron Lett., 2012, 53, 3018–3022 CrossRef CAS.
- G. Babu and P. T. Perumal, Tetrahedron Lett., 1998, 39, 3225–3228 CrossRef CAS.
- T. Miyai, Y. Onishi and A. Baba, Tetrahedron Lett., 1998, 39, 6291–6294 CrossRef CAS.
- T. Miyai, Y. Onishi and A. Baba, Tetrahedron, 1999, 55, 1017–1026 CrossRef CAS.
- J. Yadav, S. Abraham, B. Subba Reddy and G. Sabitha, Tetrahedron Lett., 2001, 42, 8063–8065 CrossRef CAS.
- J. S. Yadav, S. Abraham, B. V. S. Reddy and G. Sabitha, Synthesis, 2001, 2001, 2165–2169 CrossRef.
- T.-P. Loh and L.-L. Wei, Synlett, 1998, 1998, 975–976 CrossRef.
- T.-P. Loh and L.-L. Wei, Tetrahedron, 1998, 54, 7615–7624 CrossRef CAS.
- T.-P. Loh and S.-L. Chen, Org. Lett., 2002, 4, 3647–3650 CrossRef CAS PubMed.
- T.-P. Loh and L.-L. Wei, Tetrahedron Lett., 1998, 39, 323–326 CrossRef CAS.
- S. Kobayashi, T. Busujima and S. Nagayama, Tetrahedron Lett., 1998, 39, 1579–1582 CrossRef CAS.
- T.-P. Loh, J.-M. Huang, S.-H. Goh and J. J. Vittal, Org. Lett., 2000, 2, 1291–1294 CrossRef CAS PubMed.
- D. Subhas Bose, A. P. Rudradas and M. Hari Babu, Tetrahedron Lett., 2002, 43, 9195–9197 CrossRef CAS.
- G. K. Friestad, C. S. Korapala and H. Ding, J. Org. Chem., 2006, 71, 281–289 CrossRef CAS PubMed.
- Y. Onishi, T. Ito, M. Yasuda and A. Baba, Eur. J. Org. Chem., 2002, 2002, 1578–1581 CrossRef.
- J. Quiroga, J. Portilla, R. Abonía, B. Insuasty, M. Nogueras and J. Cobo, Tetrahedron Lett., 2008, 49, 5943–5945 CrossRef CAS.
- I. B. Dzvinchuk, N. A. Tolmachova, A. N. Chernega and M. O. Lozinskii, Chem. Heterocycl. Compd., 2009, 45, 194–200 CrossRef CAS.
- A. Zheng, W. Zhang and J. Pan, Synth. Commun., 2006, 36, 1549–1556 CrossRef CAS.
- R. V. Sumesh, M. Muthu, A. I. Almansour, R. Suresh Kumar, N. Arumugam, S. Athimoolam, E. A. Jeya Yasmi Prabha and R. R. Kumar, ACS Comb. Sci., 2016, 18, 262–270 CrossRef CAS PubMed.
- J. Häufel and E. Breitmaier, Angew. Chem., Int. Ed. Engl., 1974, 13, 604 CrossRef.
- A. E.-A. M. Gaber and H. McNab, Synthesis, 2001, 2001, 2059–2074 CrossRef.
- R. B. N. Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180–196 RSC.
- J. P. Lorimer and T. J. Mason, Chem. Soc. Rev., 1987, 16, 239–274 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10127a |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Karoll Ferrer-Pertuzbc,
Jorge Trilleras
a,
Karoll Ferrer-Pertuzbc,
Jorge Trilleras c,
Jairo Quiroga
c,
Jairo Quiroga d and
Margarita Gutiérrez
d and
Margarita Gutiérrez *a
*a










![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and (–C
N) and (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) groups. 1H-NMR spectra were similar between them in each set, and were characterized by the presence of three groups of signals (aromatics protons, protons near heteroatoms and aliphatic protons). Each series was identified a signal around 8 ppm, typical for γ-unsubstituted pyridine ring shown as in Fig. 3 and 4.
C) groups. 1H-NMR spectra were similar between them in each set, and were characterized by the presence of three groups of signals (aromatics protons, protons near heteroatoms and aliphatic protons). Each series was identified a signal around 8 ppm, typical for γ-unsubstituted pyridine ring shown as in Fig. 3 and 4.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as eluent. The reaction mixture was allowed to cool to room temperature. The precipitate formed was collected by filtration at pump washed with a mixture hexane/ethanol.
40) as eluent. The reaction mixture was allowed to cool to room temperature. The precipitate formed was collected by filtration at pump washed with a mixture hexane/ethanol.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (9
ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as eluent. The reaction mixture was cooled in ice-water bath. The formed precipitate was filtered, washed with mixture water
1 v/v) as eluent. The reaction mixture was cooled in ice-water bath. The formed precipitate was filtered, washed with mixture water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and purified by recrystallization with mixture ethanol
1) and purified by recrystallization with mixture ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (1
hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the target compounds in high yields of 50–98%.
1) to give the target compounds in high yields of 50–98%.