Open Access Article
Open Access ArticleCopolymerization modification of poly(vinyltriethoxysilane) membranes for ethanol recovery by pervaporation
Wei Jiaa,
Wei Suna,
Chunjie Xiab,
Xianxue Yanga,
Zhongqi Caoa and
Weidong Zhang *a
*a
aState Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Membrane Science and Technology, Beijing University of Chemical Technology, Beijing 100029, People's Republic of China. E-mail: zhangwd@mail.buct.edu.cn
bDepartment of Civil & Environmental Engineering, Southern Illinois University Carbondale, 1230 Lincoln Dr, Carbondale, IL 62901, USA
First published on 27th November 2017
Abstract
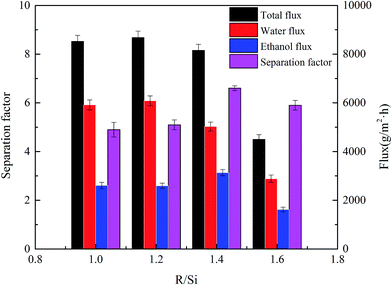
In this study, a copolymerization modification method is used to modify the rigid structure of a high-flux poly(vinyltriethoxysilane) (PVTES) membrane, which was prepared from VTES monomers in our previous work, to further enhance its separation factor. A value of R/Si, which could characterize the density of organic groups and the chain flexibility in membrane materials, is introduced here to guide the selection of modification chemicals. Modifiers with different polymerization degree but the same flexible chain unit (DMDES, HSO and PDMS) are chosen to be copolymerized with PVTES. Analysis results show that the PVTES-HSO membrane possesses lower thickness, higher amount of hydrophobic groups, and an inner structure with greater chain flexibility and lower crystallinity, leading to the best pervaporation (PV) performance among these modified membranes, which is much better than the original PVTES membrane. Furthermore, PVTES-HSO membranes with different R/Si values are prepared to optimize their PV performance. And the optimal PVTES-HSO membrane (R/Si = 1.4) shows the best performance with the separation factor of 6.6 and flux up to 8160 g m−2 h−1 when separating 9 wt% ethanol aqueous solution at 35 °C.
Introduction
In recent years, fuel prepared from renewable bioethanol has been promoted as an environmentally benign source of energy for the next generation. Pervaporation (PV), as an environmentally-friendly and energy-saving separation technology, is a potential process for in situ recovery of bioethanol to reduce the inhibitory effect of high alcohol concentration and thereafter to enhance the fermentation efficiency.1–4 The key element of this integrated process is the pervaporation membrane with high flux and separation factor. In recent years, a great deal of pervaporation membrane materials have been developed, among which silicone-based polymers are widely employed in the separation of organics from aqueous solutions.5 And for the separation of dilute ethanol aqueous solution, polydimethylsiloxane (PDMS) membrane is the most well-studied representative silicone-based polymeric membrane.6–19 Generally, the reported separation factors of polymer-supported PDMS membranes for the ethanol/water system have ranged from 4.4 to 10.8 with an average of about 7–8.20 However, due to its large molecular weight, the thickness of PDMS membrane is usually large, which causes low flux. Beaumelle et al.21 have indicated that the total flux of unmodified PDMS membranes only ranges from 1 to 1000 g m−2 h−1 for the ethanol/water separation by PV. This low flux of PDMS membrane significantly limits its industrial application. Therefore, it is necessary to develop a new thin membrane with a comparable separation factor for ethanol recovery.Currently, membrane fabrication methods like interfacial polymerization, plasma polymerization, atom transfer radical polymerization (ATRP), dip-coating and also other methods have been applied to the preparation of ultrathin pervaporation membranes.22–25 It can be concluded that the dramatic decrease of selective layer thickness has been achieved mostly because small molecules are used instead of polymers, like PDMS, as film forming monomers in most of these methods. Therefore, in our previous study, Zhang et al.26 have reported the preparation of thin high-flux poly(vinyltriethoxysilane) (PVTES) membranes with vinyltriethoxysilane (VTES) monomers for ethanol recovery, which possessed a flux over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g m−2 h−1 and a separation factor of 5 with the active layer thickness of ∼20 nm. Although the flux of PVTES membrane achieves a breakthrough, the separation factor is a little low due to its rigid inner structure, which may easily lead to some free volume cavity and structural defects in membrane structure. So the PVTES membrane performance is expected to be improved through modification by lowering its rigidity.
000 g m−2 h−1 and a separation factor of 5 with the active layer thickness of ∼20 nm. Although the flux of PVTES membrane achieves a breakthrough, the separation factor is a little low due to its rigid inner structure, which may easily lead to some free volume cavity and structural defects in membrane structure. So the PVTES membrane performance is expected to be improved through modification by lowering its rigidity.
Copolymerization modification is a commonly used method to adjust membrane inner structure via copolymerization of additives and the original membrane materials to change the functional groups and chain structures, which definitely affects the PV performance of membranes. Poly(1-trimethylsilyl-1-propyne) (PTMSP) is a typical silicon-based membrane with rigid inner structure, which leads to its relatively low selectivity for ethanol/water separation and low stability in the PV process.27 Therefore, Nagase et al.27 once modified PTMSP with the more flexible PDMS by copolymerization, experimental results showed that the selectivity depended on the PDMS content of the copolymer, and the separation factor and permeation rate assumed the maximum values at 12 mol% PDMS content. At that point, the separation factor and permeation rate were 28.3 and 2.45 × 10−3 g m m−2 h−1, respectively, compared with 11.2 and 1.15 × 10−3 g m m−2 h−1 for pure PTMSP membrane. Such a high permselectivity for ethanol might be due to a delicate alteration of membrane structure, which was induced by the introduction of a short flexible PDMS side chain into a rigid PTMSP backbone. However, the experimental result also showed that excessively high content of PDMS would lead to a decrease in the separation factor, because the large amount of flexible PDMS chain may easily over swelled in PV process. So a proper flexibility of membrane inner structure is needed to obtain a higher separation factor. Thus, for our research, the PVTES membrane with rigid backbone needs to be modified by introducing a proper amount of flexible segments to adjust membrane inner structure and then to enhance the separation factor.
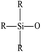
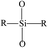
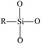
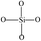
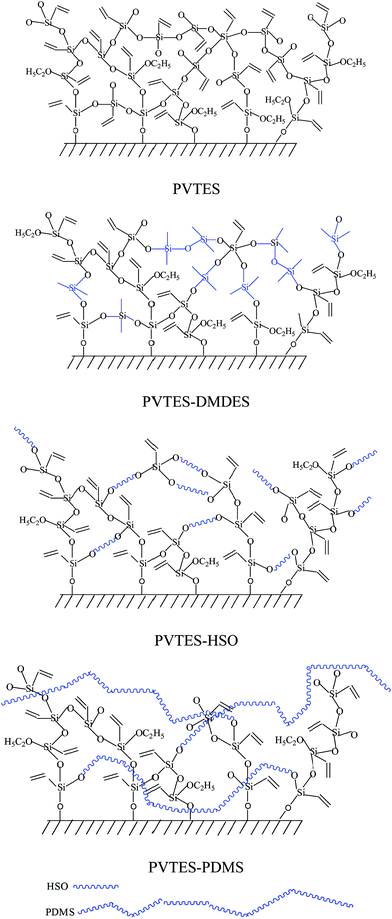
In order to characterize the flexibility of modified copolymers, we introduce a value as R/Si.28 It is defined as the average number of organic groups (R) attached directly to one silicon atom for the organic silicon resin. With the value R/Si increasing, the density of organic groups and segmental mobility of polymers increase. Generally, silicone-based polymer consists of four elementary units, and their R/Si values are shown in Table 1. For the PVTES membrane only consisting of T units (R/Si = 1), which shows higher rigidity, its flexibility can be enhanced by introducing appropriate amount of D units to adjust R/Si value between 1 and 2. Dimethyldiethoxysilane (DMDES) and its polymers with –CH3 groups are the most commonly used hydrophobic silicon resins consisting of D units. They have a good affinity with ethanol, and –CH3 can make a better flexibility for the material because of its smaller steric hindrance than the other organic groups.
So, in this work, the modifiers with different polymerization degree but the same flexible chain unit, which are DMDES, the oligomer of hydroxy silicone oil (HSO) and PDMS, are employed to be copolymerized with PVTES via hydrolytic condensation reaction, marked as PVTES-X, to obtain ultrathin membranes with higher separation factor. The morphology, membrane thickness, surface hydrophobic property, inner crystallization property and thermal stability of PVTES-X and PVTES membranes are investigated and compared by means of scanning electron microscope (SEM), atomic force microscope (AFM), contact angle (CA) measurement, Fourier transform infrared (FTIR), X-ray diffraction (XRD) and thermogravimetric (TGA) analysis. Furthermore, the pervaporation performances of the PVTES-X and PVTES membranes for separating dilute ethanol aqueous solution are also compared to select the best modifier. And then the chosen PVTES-HSO membranes with different R/Si values are prepared to further optimize their PV performance. Finally effects of operation conditions (feed temperature and feed concentration) on the PV performance of the optimal PVTES-HSO membrane are also systematically investigated.
Experimental
Materials
Poly(vinylidene fluoride) (PVDF) microfiltration membranes with an average pore size of 0.22 μm and thickness of 0.1 mm are purchased from Beihua Liming Co., Ltd. (Beijing, China). Dimethyldiethoxysilane (DMDES) of analytical grade is obtained from Beijing Hvsco Technology Co., Ltd. (Beijing, China). Hydroxyl silicon oil (HSO, polymerization degree n = 8) is purchased from Beijing Dingye Co., Ltd. (Beijing, China). Vinyltriethoxysilane (VTES, density of 0.904 g ml−1) of analytical grade is obtained from Tianjin Chemical Reagent Institute (Tianjin, China). Polydimethylsiloxane (PDMS, polymerization degree n ≈ 800), ethanol (purity > 99.7%) and n-heptane of analytical grade are supplied by Beijing Chemical Co., Ltd. Dibutyltin dilaurate (DBTL) of chemical grade is purchased from Fuchen Chemical Reagent Factory (Tianjin, China). All the chemicals are used without further purification. Deionized water is used in this study.Membrane preparation
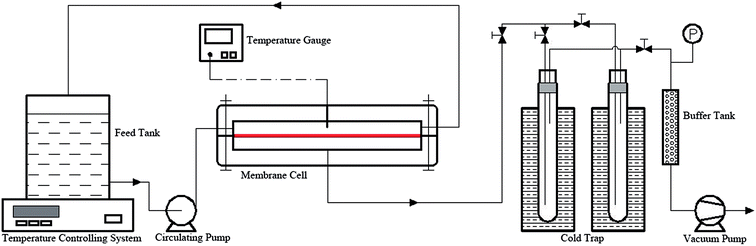
The procedure for the preparation of pure PVTES and PVTES-X composite membranes is described as follows. In order to prevent coating solution from penetrating micro-pores in PVDF membrane, PVDF porous membrane as support layer is first wetted with ethanol, then immersed in water. After about 10 minutes, the PVDF is placed on a clean glass plate and excess water on the surface is wiped off quickly with a filter paper. Meanwhile, the coating solution is prepared to form the active layer of composite membranes. First, VTES and the solvent n-heptane are mixed up with the ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) under stirring to form a homogenous solution at room temperature. The modifier (DMDES, HSO or PDMS) is then added into the above solution with a given R/Si to form organic silicon resin (no modifier is added into the pure PVTES membrane). Finally, the catalyst, dibutyltin dilaurate, is put in with a weight ratio of 0.04 to VTES. After degassing, the solution is uniformly poured over the pre-treated PVDF support. The solvent evaporates at room temperature and the resultant membrane is then cured in a vacuum drying oven at the desired temperature to complete crosslinking.
10 (w/w) under stirring to form a homogenous solution at room temperature. The modifier (DMDES, HSO or PDMS) is then added into the above solution with a given R/Si to form organic silicon resin (no modifier is added into the pure PVTES membrane). Finally, the catalyst, dibutyltin dilaurate, is put in with a weight ratio of 0.04 to VTES. After degassing, the solution is uniformly poured over the pre-treated PVDF support. The solvent evaporates at room temperature and the resultant membrane is then cured in a vacuum drying oven at the desired temperature to complete crosslinking.
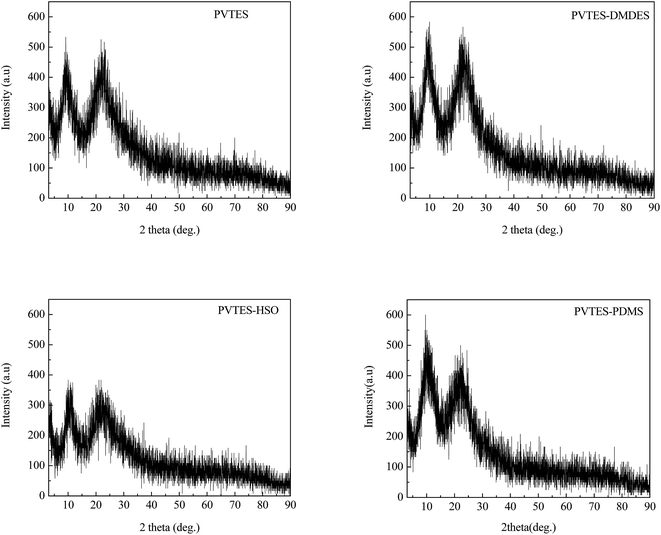
During the membrane formation process, the silanization of organic silicone resins forms a polymer with Si–O–Si bonds.29,30 The hydrolysis and condensation reaction occurs between the Si–OCH2CH3 groups existing in VTES, DMDES, HSO and PDMS, and the same reaction also occurs between Si–OH on the PVDF surface and the Si–OCH2CH3 groups, which enhances the interaction of the selective layer and support layer. The detailed reaction process has been reported in our previous research.26 And the possible structures of these four resultant membranes are illustrated in Fig. 1. Further characterization by FTIR and XRD spectrum will be discussed in the Results and discussions section.
Membrane characterization
The permeation flux (J) is calculated using the following equation:
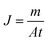 | (1) |
The separation factor (α), which reflects the membrane separation performance, is defined by the equation below:
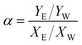 | (2) |
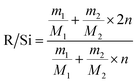 | (3) |
Results and discussions
Characterization of PVTES and PVTES-X membranes
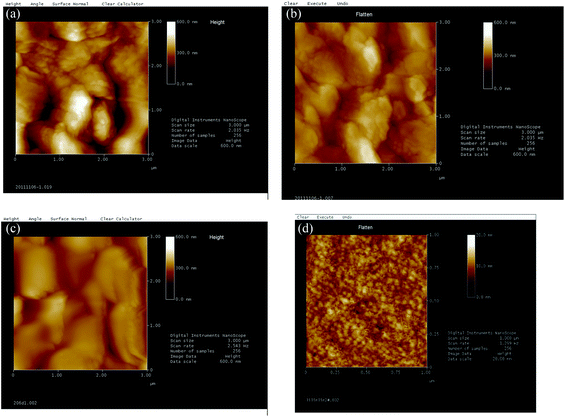 | ||
| Fig. 4 AFM images of PVTES and PVTES-X membranes. (a) PVTES membrane; (b) PVTES-DMDES membrane; (c) PVTES-HSO membrane; (d) PVTES-PDMS membrane. | ||
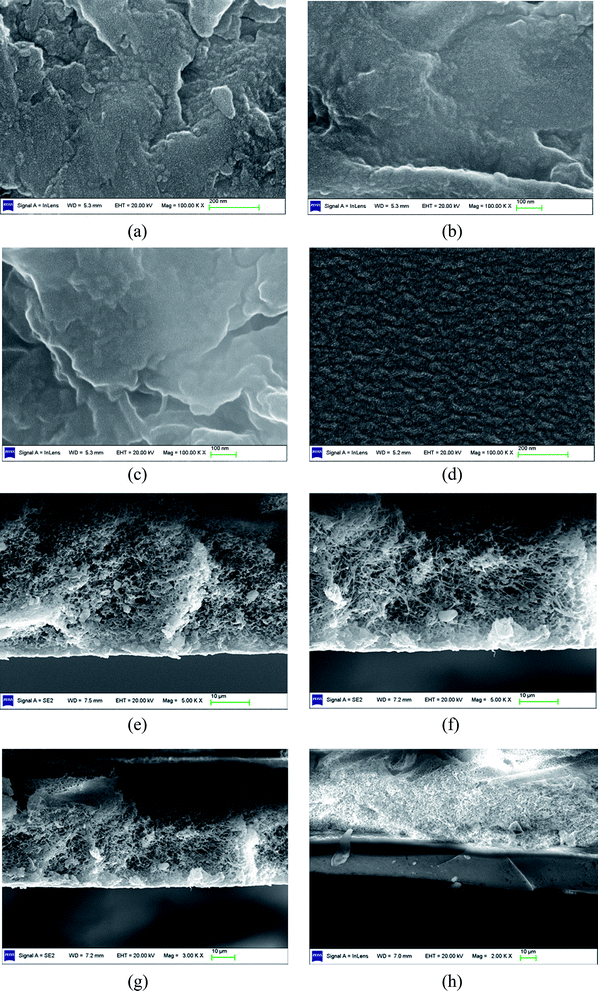
From the cross-sectional SEM images, it can be observed that the selective layers merge well with the supporting membrane for PVTES, PVTES-DMDES and PVTES-HSO membranes. And it is difficult to obtain the exact thicknesses of their selective layers on top of the supporting PVDF membranes, because the interface is not clear. While the thicknesses of the PVTES, PVTES-DMDES and PVTES-HSO membranes can be estimated to far less than 1 μm, which is much smaller than that of PVTES-PDMS membrane (∼10 μm). This probably leads to a higher flux of the PVTES, PVTES-DMDES and PVTES-HSO membranes than that of PVTES-PDMS membrane according to the inversely proportional relationship between the thickness of the selective layer and the flux.33
| Type of membrane | PVTES | PVTES-DMDES | PVTES-HSO | PVTES-PDMS |
|---|---|---|---|---|
| CA (°) | 101.0 | 114.0 | 113.0 | 115.0 |
| Membrane | XEa (wt%) | Ta (°C) | la (μm) | αa | Ja (g m−2 h−1) | Ref. |
|---|---|---|---|---|---|---|
| a XE-feed ethanol concentration, T-temperature, l-membrane thickness, α-separation factor, J-total flux. | ||||||
| PDMS | 5.0 | 40 | 5 | 8.9 | 1600 | 7 |
| 5.0 | 40 | 1–2 | 9.3 | 1140 | 8 | |
| 4.0 | 45 | 5 | 8.5 | 1850 | 9 | |
| 5.0 | 40 | 8 | 8.5 | 1300 | 10 | |
| 8.0 | 42 | 1 | 6.7 | 1440 | 11 | |
| 8.0 | 50 | — | 6.4 | 265 | 12 | |
| 4.0 | 45 | 1 | 5.0 | 1600 | 9 | |
| 3.0 | 50 | 8 | 1.0 | 2800 | 13 | |
| 5.0 | 40 | 10 | 8.8 | ∼240 | 14 | |
| 2.0 | 30 | 50 | 10.0 | ∼102 | 15 | |
| 4.3 | 40 | <10 | 6.3 | 5150 | 16 | |
| 10.0 | 40 | 120 | 7.4 | 53.3 | 17 | |
| 6.4 | 30 | 100 | 10.8 | 25.1 | 18 | |
| 3.0 | 41 | 12.5 ± 2 | 4.6 | 120 | 19 | |
| PVTES | 9.0 | 35 | — | 4.9 | 8523.4 | This work |
| PVTES-DMDES | 9.0 | 35 | — | 5.6 | 6909.3 | This work |
| PVTES-HSO | 9.0 | 35 | — | 6.6 | 8160.1 | This work |
| PVTES-PDMS | 9.0 | 35 | — | 6.3 | 539.8 | This work |
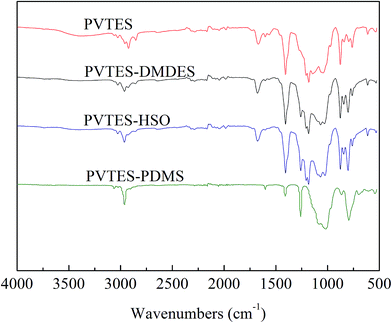
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 absorbance signals. The peak at 1030–1160 cm−1 is characteristic of Si–O–Si stretching and the peak at 1183 cm−1 is characteristic of Si–O–C,34 which indicates that the hydrophobic Si–O–Si backbone has been formed by the hydrolysis and condensation reactions of Si–OCH2CH3 groups, and that the Si–OCH2CH3 groups are not completely reacted, namely, the minority of them still stays in all four membranes. The peak at 1259 cm−1 existing in the FTIR spectra of the PVTES-X membranes is assigned to the –CH3 absorbance signal (the symmetric deformation of Si–CH3),35 which suggests that the materials used to modify PVTES membrane (DMDES, HSO and PDMS) are successfully copolymerized with VTES. In conclusion, compared with PVTES membrane, the PVTES-X membranes contain additional hydrophobic –CH3 groups. This phenomenon illustrates that the hydrophobicity of the PVTES-X membranes is stronger than that of PVTES membrane, which agrees with the CA results, possibly leading to higher sorption selectivity for ethanol/water separation.
CH2 absorbance signals. The peak at 1030–1160 cm−1 is characteristic of Si–O–Si stretching and the peak at 1183 cm−1 is characteristic of Si–O–C,34 which indicates that the hydrophobic Si–O–Si backbone has been formed by the hydrolysis and condensation reactions of Si–OCH2CH3 groups, and that the Si–OCH2CH3 groups are not completely reacted, namely, the minority of them still stays in all four membranes. The peak at 1259 cm−1 existing in the FTIR spectra of the PVTES-X membranes is assigned to the –CH3 absorbance signal (the symmetric deformation of Si–CH3),35 which suggests that the materials used to modify PVTES membrane (DMDES, HSO and PDMS) are successfully copolymerized with VTES. In conclusion, compared with PVTES membrane, the PVTES-X membranes contain additional hydrophobic –CH3 groups. This phenomenon illustrates that the hydrophobicity of the PVTES-X membranes is stronger than that of PVTES membrane, which agrees with the CA results, possibly leading to higher sorption selectivity for ethanol/water separation.
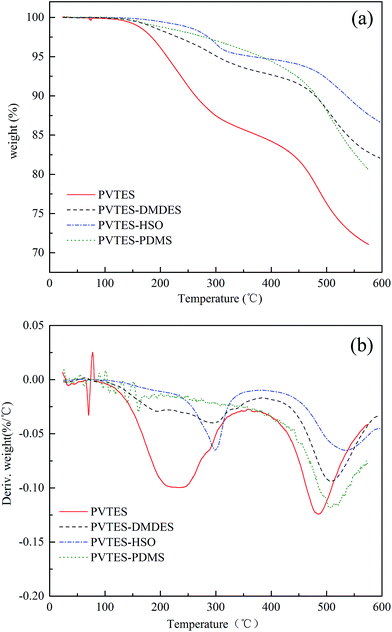 | ||
| Fig. 7 Comparison of TGA and DTG curves for PVTES and PVTES-X polymers. (a) TGA curves of polymers, (b) DTG curves of polymers. | ||
In conclusion, with the lowest degradation degree and highest degradation temperature, PVTES-HSO membrane exhibits more excellent thermal stability than other membranes, showing its homogeneous and stable inner structure.
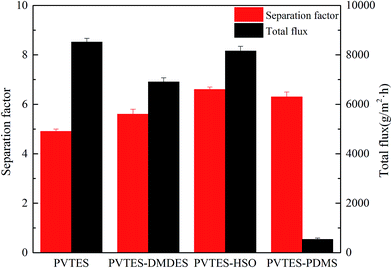 | ||
| Fig. 8 PV performance of PVTES and PVTES-X membranes (R/Si = 1.4; feed concentration: 9 wt%; feed temperature: 35 °C). | ||
Fig. 8 also shows that the total flux of PVTES, PVTES-DMDES and PVTES-HSO membranes is almost equal and is much larger than that of PVTES-PDMS membrane. The flux of different membranes is mainly determined by the membrane thickness. Therefore, similar flux of those three membranes is obtained due to their similar membrane thickness, and PVTES-PDMS membrane exhibits lower flux, resulting from its larger thickness, as shown in Fig. 3. This is because the higher viscosity of PVTES-PDMS coating solution brings about a thicker selective layer than other membranes. In summary, PVTES-HSO membrane possesses a superior pervaporation performance among PVTES and the modified membranes.
Moreover, considering PDMS as the most commonly used polymeric membrane for PV separation of ethanol/water, the performance of these membranes are compared with the published PV data of the PDMS membranes in Fig. 9. It shows that the PV performance of PVTES-PDMS membrane is close to that of PDMS membrane, which means that the nature of PDMS domains in the PVTES-PDMS membrane. The total flux of PVTES, PVTES-DMDES and PVTES-HSO membranes is much higher than that of PDMS membranes and the high flux means a low membrane area requirement for the ethanol recovery per unit weight, which leads to lower capital investment, lower annual cost and a smaller footprint. And the separation factor with high flux of PVTES, PVTES-DMDES and PVTES-HSO membranes clearly transcend the upper limit of PDMS membranes reported in most literatures (Fig. 9), which implies that PVTES, PVTES-DMDES and PVTES-HSO membranes offer significant potential for PV integrated with ethanol fermentation process.
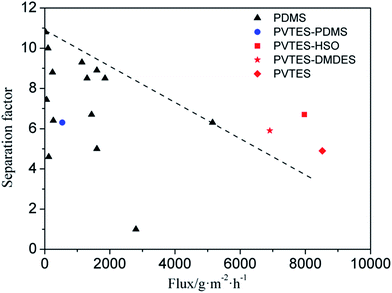 | ||
| Fig. 9 PV performance of different membranes in comparison with the published data of pristine PDMS membranes. The dashed line represents the upper limit of the PDMS membranes. The detailed information of data points is shown in Table 3. | ||
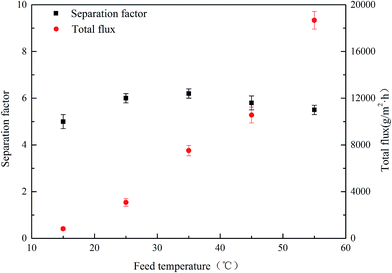 | ||
| Fig. 11 Effect of feed temperature on the PV performance for PVTES-HSO membrane (feed concentration: 9 wt%). | ||
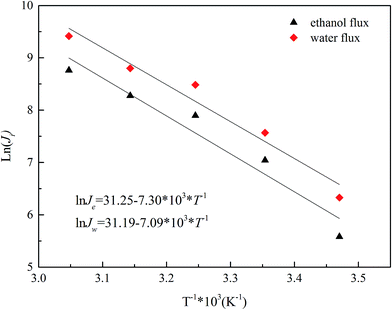
To get a deeper view of the relationship between the temperature and permeation flux, an Arrhenius type equation is applied as follows:42,43
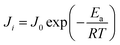 | (4) |
Eqn (5) is obtained, by rearranging eqn (4).
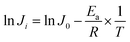 | (5) |
The Ea values for ethanol and water are determined from the slopes of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ji versus 1/T plots (Fig. 12). The apparent activation energy Ea reflects the sensitive behavior of the component permeation through the membrane towards temperature alteration.44 If the apparent activation energy is positive, permeate flux increases with the increasing temperature, which is observed in most pervaporation experiments. And a higher value of Ea for component permeation through membrane implies a more sensitive behaviour towards temperature alteration. From Fig. 12, the calculated apparent activation energy of ethanol is 60.7 kJ mol−1, which is close to that of water (58.9 kJ mol−1). The same phenomenon of the similar apparent activation energy of ethanol and water is observed in other literatures.7,26 Therefore, the separation factor of PVTES-HSO membrane maintains at a fixed value, as shown in Fig. 11.
Ji versus 1/T plots (Fig. 12). The apparent activation energy Ea reflects the sensitive behavior of the component permeation through the membrane towards temperature alteration.44 If the apparent activation energy is positive, permeate flux increases with the increasing temperature, which is observed in most pervaporation experiments. And a higher value of Ea for component permeation through membrane implies a more sensitive behaviour towards temperature alteration. From Fig. 12, the calculated apparent activation energy of ethanol is 60.7 kJ mol−1, which is close to that of water (58.9 kJ mol−1). The same phenomenon of the similar apparent activation energy of ethanol and water is observed in other literatures.7,26 Therefore, the separation factor of PVTES-HSO membrane maintains at a fixed value, as shown in Fig. 11.
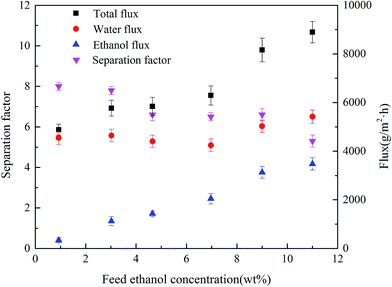 | ||
| Fig. 13 Effect of feed concentration on the PV performance for PVTES-HSO membrane (feed temperature: 35 °C). | ||
Theoretically, the driving force for component i to transport through a membrane is the difference of its partial vapor pressure at the feed side and that at the permeate side and can be written as follows:45
| Driving force = xiγipsati − xipp | (6) |
In this work, the permeate pressure is kept close to zero, and the driving force of the component is therefore only determined by its feed fugacity. As shown in Table 4, for the ethanol/water separation process, a higher ethanol fraction leads to lower activity coefficient, but the corresponding ethanol fugacity increases sharply, resulting in a higher driving force and therefore a higher ethanol flux. And the water fugacity decreases very slightly, which is inconsistent with the small upward trend of water flux. This is mainly because of the coupling effect46 of hydrogen bonds between water and ethanol, leading to the enhancement of water permeation with the increase of ethanol permeation. Another reason is that higher ethanol concentration could lead to higher degree of membrane swelling, which benefits both ethanol and water permeations. These causes make water flux increase slightly with the increase of ethanol flux, which results in the decrease of separation factor and the increase of total flux. Similar phenomena have also been reported by previous researchers.45
| Ethanol concentration (wt%) | Activity coefficient | Feed fugacity (kPa) | ||
|---|---|---|---|---|
| Ethanol | Water | Ethanol | Water | |
| 1 | 4.6388 | 1.0001 | 0.253 | 5.608 |
| 3 | 4.4619 | 1.0003 | 0.738 | 5.565 |
| 5 | 4.2914 | 1.0010 | 1.198 | 5.522 |
| 7 | 4.1271 | 1.0020 | 1.633 | 5.480 |
| 9 | 3.9688 | 1.0033 | 2.045 | 5.438 |
| 11 | 3.8164 | 1.0050 | 2.435 | 5.397 |
Conclusions
In this paper, the modifiers, which are DMDES, HSO and PDMS containing the same flexible chain unit, are applied to modify the rigid PVTES membrane by copolymerization. A value of R/Si is introduced here to guide the selection of modification chemicals, which could characterize the density of organic groups and the flexibility of membrane materials. SEM, AFM, CA, FTIR, XRD and TGA methods are used to characterize the physical and chemical structure of the PVTES-X and PVTES membranes. Analysis results show that among these modified membranes, PVTES-HSO membrane possesses lower thickness, higher amount of hydrophobic groups, and an inner structure with more proper chain flexibility and lower crystallinity, thus lowering the non-continuity nature and the possibility of large structural defects in the ultrathin membranes. These properties result in the best separation performance of PVTES-HSO membrane, which is much better than that of PVTES membrane. Furthermore, PVTES-HSO membranes with different R/Si values are prepared to optimize their PV performance. And when feed concentration is 9 wt% and feed temperature is 35 °C, the separation factor of the optimal PVTES-HSO membrane (R/Si = 1.4) is around 6.6 and total flux is up to 8160 g m−2 h−1. In conclusion, this PVTES-HSO membrane with good PV performance and thermal stability would be promising for the industrial application of PV process.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors gratefully acknowledge the financial support from Research Project of Chinese Ministry of Education (No. v201308), National Natural Science Foundation of China (No. 21476010), as well as the special program of Beijing Municipal Science & Technology Commission (No. Z111109055311095).Notes and references
- A. W. Verkerk, P. V. Male, M. A. G. Vorstman and J. T. F. Keurentjes, J. Membr. Sci., 2001, 193, 227–238 CrossRef CAS.
- T. C. Bowen, R. G. Meier and L. M. Vane, J. Membr. Sci., 2007, 298, 117–125 CrossRef CAS.
- G. Liu, W. Wei, H. Wu, X. L. Dong, M. Jiang and W. Q. Jin, J. Membr. Sci., 2011, 373, 121–129 CrossRef CAS.
- D. J. O'Brien, L. H. Roth and A. J. McAloon, J. Membr. Sci., 2005, 166, 105–111 CrossRef.
- M. Bennett, B. J. Brisdon, R. England and R. W. Field, J. Membr. Sci., 1997, 137, 63 CrossRef CAS.
- P. Peng, B. Shi and Y. Lan, Sep. Sci. Technol., 2011, 46, 420–427 CrossRef CAS.
- W. Wei, S. Xia, G. Liu, X. Dong, W. Jin and N. Xu, J. Membr. Sci., 2011, 375, 334–344 CrossRef CAS.
- Y. Luo, S. Tan, H. Wang, F. Wu, X. Liu, L. Li and Z. Zhang, Chem. Eng. J., 2008, 137, 496–502 CrossRef CAS.
- E. Shi, W. Huang, Z. Xiao, D. Li and M. Tang, J. Appl. Polym. Sci., 2007, 104, 2468–2477 CrossRef CAS.
- L. Li, Z. Xiao, S. Tan, L. Pu and Z. Zhang, J. Membr. Sci., 2004, 243, 177–187 CrossRef CAS.
- W. Zhang, X. Yu and Q. Yuan, Biotechnol. Tech., 1995, 9, 299–304 CrossRef CAS.
- J. Guo, G. Zhang, W. Wu, S. Ji, Z. Qin and Z. Liu, Chem. Eng. J., 2010, 158, 558–565 CrossRef CAS.
- T. Mohammadi, A. Aroujalian and A. Bakhshi, Chem. Eng. Sci., 2005, 60, 1875–1880 CrossRef CAS.
- X. Zhan, J. Li, J. Huang and C. Chen, Appl. Biochem. Biotechnol., 2010, 160, 632–642 CrossRef CAS PubMed.
- W. Zhang, W. Sun, J. Yang and Z. Q. Ren, Appl. Biochem. Biotechnol., 2010, 160, 156–167 CrossRef CAS PubMed.
- F. Xiangli, Y. Chen, W. Jin and N. Xu, Ind. Eng. Chem. Res., 2007, 46, 2224–2230 CrossRef CAS.
- T. Uragami and T. Morikawa, J. Appl. Polym. Sci., 1992, 44, 2009–2018 CrossRef CAS.
- K. Ishihara and K. Matsui, J. Appl. Polym. Sci., 1987, 34, 437–440 CrossRef CAS.
- A. Dobrak, A. Figoli, S. Chovau, F. Galiano, S. Simone, I. F. J. Vankelecom, E. Drioli and B. V. Bruggen, J. Colloid Interface Sci., 2010, 346, 254–264 CrossRef CAS PubMed.
- L. M. Vane, J. Chem. Technol. Biotechnol., 2005, 80, 603 CrossRef CAS.
- D. Beaumelle, M. Marin and H. Gibert, Food Bioprod. Process., 1993, 71, 77 CAS.
- C. H. Yu, I. Kusumawardhana, J. Y. Lai and Y. L. Liu, J. Colloid Interface Sci., 2009, 336, 260–267 CrossRef CAS PubMed.
- Q. An, W. S. Hung, S. C. Lo, Y. H. Li, M. D. Guzman, C. C. Hu, K. R. Lee, Y. C. Jean and J. Y. Lai, Macromolecules, 2012, 45, 3428–3435 CrossRef CAS.
- H. Matsuyama, A. Kariya and M. Teramoto, J. Membr. Sci., 1994, 88, 85–92 CrossRef CAS.
- F. Pan, H. Jia, S. Qiao, Z. Jiang, J. Wang, B. Wang and Y. Zhong, J. Membr. Sci., 2009, 341, 279–285 CrossRef CAS.
- W. Zhang, C. Xia, L. Li, Z. Ren, J. Liu and X. Yang, RSC Adv., 2014, 4, 14592–14596 RSC.
- Y. Nagase, K. Ishihara and K. Matsui, J. Polym. Sci., Part B: Polym. Phys., 1990, 28, 377–386 CrossRef CAS.
- Q. Guo, H. Huang and Q. Wu, Silicone Mater., 2006, 20, 51–52 Search PubMed.
- W. Yoshida, R. P. Castro, J. D. Jou and Y. Cohen, Langmuir, 2001, 17, 5882–5888 CrossRef CAS.
- N. N. Herrera, J. M. Letoffe, J. P. Reymond and E. B. Lami, J. Mater. Chem., 2005, 15, 863–871 RSC.
- S. Feng, Organic silicon resins and their applications, ed. J. Zhang and M. Li, Chemical Industry Press, Beijing, CN, 2004, pp. 160–162 Search PubMed.
- C. E. Goodyer and A. L. Bunge, J. Membr. Sci., 2012, 409–410, 127–136 CrossRef CAS.
- L. Sun, G. L. Baker and M. L. Bruening, Macromolecules, 2005, 38, 2307–2314 CrossRef CAS.
- H. Dong, M. A. Brook and J. D. Brennan, Chem. Mater., 2005, 17, 2807–2816 CrossRef CAS.
- C. Wu, D. Gao, W. Li and M. Jia, Paint Coat. Ind., 2009, 39, 22–25 CAS.
- X. Jiang, Y. Zeng, Y. Shi and G. Chen, J. Funct. Polym., 2001, 14, 133–137 CrossRef CAS.
- G. L. Jadav, V. K. Aswal, H. Bhatt, J. C. Chaudhari and P. S. Singh, J. Membr. Sci., 2012, 415–416, 624–634 CrossRef CAS.
- A. Q. Hou, J. B. Yu and Y. Q. Shi, Eur. Polym. J., 2008, 44, 1696–1700 CrossRef CAS.
- J. L. Hua, Z. Li, J. G. Qin, S. J. Li, C. Ye and Z. H. Lu, React. Funct. Polym., 2007, 67, 25–32 CrossRef CAS.
- S. Li, F. Qin, P. Qin, M. N. Karimb and T. Tan, Green Chem., 2013, 15, 2180 RSC.
- X. Han, L. Wang, J. Li, X. Zhan, J. Chen and J. Yang, J. Appl. Polym. Sci., 2011, 119, 3413 CrossRef CAS.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1993, 79, 101–113 CrossRef CAS.
- X. Feng and R. Y. M. Huang, J. Membr. Sci., 1996, 118, 127–131 CrossRef CAS.
- V. García, E. Pongrácz, E. Muurinen and R. Keiski, J. Membr. Sci., 2009, 326, 92–102 CrossRef.
- N. L. Le, Y. Wang and T. S. Chung, J. Membr. Sci., 2011, 379, 174–183 CrossRef CAS.
- E. Drioli, S. Zhang and A. Basile, J. Membr. Sci., 1993, 81, 43–55 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |