Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diethylenetriamine-assisted in situ synthesis of TiO2 nanoparticles on carbon nanotubes with well-defined structure and enhanced photocatalytic performance†
Hailong Penga,
Xiaoyan Yangb,
Peng Zhang c,
Yiming Zhanga,
Chengwei Liua,
Dan Liu*a and
Jianzhou Gui
c,
Yiming Zhanga,
Chengwei Liua,
Dan Liu*a and
Jianzhou Gui *ac
*ac
aState Key Laboratory of Separation Membranes and Membrane Processes, College of Environment and Chemical Engineering, Tianjin Polytechnic University, Tianjin 300387, China. E-mail: jzgui@hotmail.com; ldan2000@163.com; Tel: +86-022-83955668
bSchool of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, China
cSchool of Material Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, China
First published on 27th October 2017
Abstract
A simple diethylenetriamine (DETA)-assisted solvothermal method is utilized for in situ synthesis of TiO2 nanoparticles on carbon nanotubes (CNTs), fabricating TiO2/CNT composites with well-defined structure and enhanced photocatalytic activity for the degradation of methylene blue (MB). It is found that the DETA plays an important role on the structure, photoelectrochemistry and catalytic performance of the TiO2/CNT composites. Particularly, the TiO2/CNT catalyst obtained in the presence of 0.02 mL DETA exhibits both a low adsorption capacity and high photodegrading activity for MB removal under the UV-light irradiation, proving the uniform and well-dispersed TiO2 particles loaded. Systematic characterization reveals the strong interaction and high electron-transfer efficiency between TiO2 and CNT in the sample with the assistance of DETA. Besides, a DETA-assisted formation mechanism of the TiO2/CNT composite has also been proposed in this study: DETA will work as a connecting bridge to facilitate the uniform adsorption of Ti4+ on the surface of CNT. With the increase of solvothermal temperature, the adsorbed Ti4+ gradually in situ crystallizes to form the TiO2/CNT composite. The DETA-assisted in situ synthesis could be expected to be a promising method for the preparation of metal oxides supported on carbon materials with well-defined structure and superior photocatalytic or photoelectrochemical properties.
1. Introduction
Water pollution derived from organic contaminants has recently become a prominent problem that endangers human health, and needs to be solved. Up to now, many approaches have been developed to effectively remove organic pollutions in water, including adsorption,1,2 biodegradation,3 and photodegradation.4–14 Particularly, photodegradation is considered as an ideal treating technology, due to the straightforward process, low energy consumption and high efficiency. Among exciting semiconductor photocatalysts, TiO2 not only features a low cost, rich resources, and nontoxicity, but also high photocatalytic activity and stability under the irradiation of UV-light, thus attracting tremendous research interest.15 Even so, the photocatalytic activity of TiO2 still fails to meet the industrial requirements, thus needing to be significantly improved.It is found that the hybridization of carbon materials can greatly enhance the photocatalytic activity of TiO2.16–20 On the one hand, as catalyst supports, the carbon materials can disperse and anchor TiO2 particles to exhibit more catalytic active sites. On the other hand, benefiting from the high electrical conductivity, the carbon materials can effectively capture the photogenerated electrons on the surface of TiO2, prolonging the lifetime of oxidative active species.21 Among the selectable carbon materials, carbon nanotube (CNT) is regarded as a promising carbon material to be combined with TiO2, due to the large surface area, strong adsorption effect, and high structural flexibility.22,23 Baiju K. Vijayan et al.24 utilized a simple hydration/dehydration method to prepare CNT/TiO2 composites, which show great photocatalytic activity for degradation of acetaldehyde. Taicheng An et al.25 reported that TiO2 spheres with controllable crystallite size and dominant crystal facets such as {001}, {101}, or polycrystalline were successful supported on CNT, exhibiting the significant synergistic effect and enhanced photocatalytic efficiency. Jing Di et al.26 successfully synthesized plant leaf-shape TiO2 supported on CNT, which features not only a large specific surface area, but also a high light absorption due to the great scattering ability. Although some efforts have been devoted in previous works, due to the hydrophobicity, CNT shows a poor dispersity in water27 and a bad combination with metal cations, making it very difficult to uniformly deposit metal oxide on the surface. Up to now, it is still a big challenge to fabricate the TiO2/CNT composite photocatalyst with the uniformly loaded and structural controllable TiO2 particles, as well as the high photocatalytic performance.
Herein, a simple and facile solvothermal method is utilized to prepare TiO2 nanoparticles supported on CNT with the assistance of DETA. By tuning different precursor (isopropyl titanate, CNT, DETA), a group of TiO2/CNT composites have been separately obtained, and their adsorption capacity and photodegrading activity for MB removal were evaluated in detail. It is found that DETA greatly affect the loading uniformity and dispersity of TiO2 particles supported, and the TiO2/CNT composite with 0.02 mL DETA added shows a strong interaction and the rapid electron-transfer efficiency between TiO2 and CNT. Moreover, a possible DETA-assisted formation mechanism of the TiO2/CNT composite has also been proposed in this work.
2. Experimental
2.1 Preparation of a series of the TiO2/CNT composites
2.2 Characterization of the TiO2/CNT composites
The morphology of different TiO2/CNT samples was observed using a scanning electron microscope (SEM, Hitachi S-4800), transmission electron microscope (TEM, Hitachi S-7650) and high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2100). The crystal structure was measured by X-ray diffraction (XRD, Bruker D8 Advance A25, Cu-Kα radiation, 40 kV, 40 mA). The N2 adsorption–desorption isotherm was tested on Autosorb-iQ-C. X-ray photoelectron spectroscopy (XPS) was carried out on a Thermo Fisher K-Aepra ESCA. The UV-vis diffuse reflectance spectra (DRS) were recorded in a range of 200–800 nm on a Shimadzu UV2700 instrument. Photoluminescence (PL) spectra were measured at room temperature on a fluorescence spectrophotometer (Hitachi F-7000), of which the excitation wavelength was 294 nm.2.3 Photocatalytic experiments
MB aqueous solution was adopted to evaluate the adsorption capacity and photocatalytic performance of different TiO2/CNT composites. All the experiments were carried out in a photochemical reactor at room temperature under the ambient situations. In the typical procedure, 0.01 g sample was dispersed in 40 mL MB (20 mg L−1) aqueous solution and magnetically stirred for 30 min in dark to achieve the adsorption/desorption equilibrium. Then, the reaction mixture was exposed to the UV-light, which was provided by a mercury lamp with an average intensity of 150–200 mW cm−3. During the UV-irradiated process, about 4 mL mixture was taken out at intervals of ten minutes, and centrifugated to separate the catalyst from the reaction solution. The resultant filtrate was measured by UV-vis spectrophotometer (Thermo evolution 300). Particularly, the catalytic activity of different samples was evaluated by C/C0, where C0 was the MB absorbance of initial solution at 664 nm, and C was the MB real-time absorbance of reaction solution.Three colorless antibiotics were also used as a target for photodegradation. In the reaction, 0.01 g sample was dispersed in three antibiotics solution (20 mg L−1 tetracycline (TC), 20 mg L−1 enrofloxacin hydrochloride (ENRH) and 10 mg L−1 ciprofloxacin (CIP)) and magnetically stirred for 30 min in dark to achieve the adsorption/desorption equilibrium. And then, the photocatalytic experiment began at the same condition. About 4 mL mixture was taken out per 20 minutes, and then centrifugal separation to obtain the reaction solution. The catalytic activity of different samples was evaluated by C/C0, where C0 was the three colorless antibiotics (TC, ENRH and CIP) absorbance of initial solution at 357, 273 and 273 nm, and C was the three colorless antibiotics real-time absorbance of reaction solution.
3. Results and discussions
3.1 Adsorption capacity and photocatalytic activity of different TiO2/CNT composites
In the present case, two important parameters are adopted to evaluate the structure of TiO2 particles loaded in the TiO2/CNT composites: adsorption capacity for MB molecules in dark and photocatalytic efficiency in the degradation of MB solution under the irradiation of UV-light. As shown in Fig. S1,† the pure CNT exhibits a remarkable MB adsorption, while the commercial TiO2 sample almost has no adsorption for MB under the same conditions. As for the TiO2/CNT composites, they should have the decreasing adsorption capacity compared with the pure CNT, due to the CNT surface has been covered by TiO2 particles. Meanwhile, CNT can rapidly transfer the surface photogenerated electrons to enhance its photocatalytic activity of TiO2.19 The detailed adsorption and photocatalysis processes of the TiO2/CNT composite have been shown in Scheme 1: in dark, the hydrophobicity of CNT will impel MB molecules in solution to be preferentially adsorbed on the bare CNT surface. After exposed to UV-light, TiO2 particles are excited to produce the electron/hole pairs (reaction a), most of which will rapidly recombine in a very short time (reaction b). Benefiting from the high conductivity, the hybridized CNT can provide a transferred channel to effectively separate the photogenerated electrons (e−) from the contacted TiO2 particles. The yielded photogenerated holes (h+) can oxidize MB molecules to organic intermediates, as shown in Scheme 1 (reaction c). Simultaneously, the photogenerated electrons react with the dissolved oxygen in H2O to form hydroxyl radicals (˙OH, reaction d), which has also been proved to be capable to decompose the MB molecules (reaction e). Therefore, in the TiO2/CNT composite, while the interaction with CNT and dispersity of TiO2 particles could be evaluated by the improvement of the photocatalytic activity. From Fig. S2,† it should be noted that the self-degradation of MB is too slight to be considered in this work.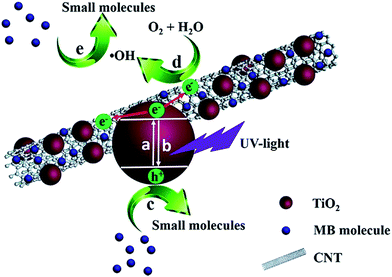 | ||
| Scheme 1 Adsorption and photocatalytic processes of TiO2/CNT composite for the MB removal under UV-light irradiation. | ||
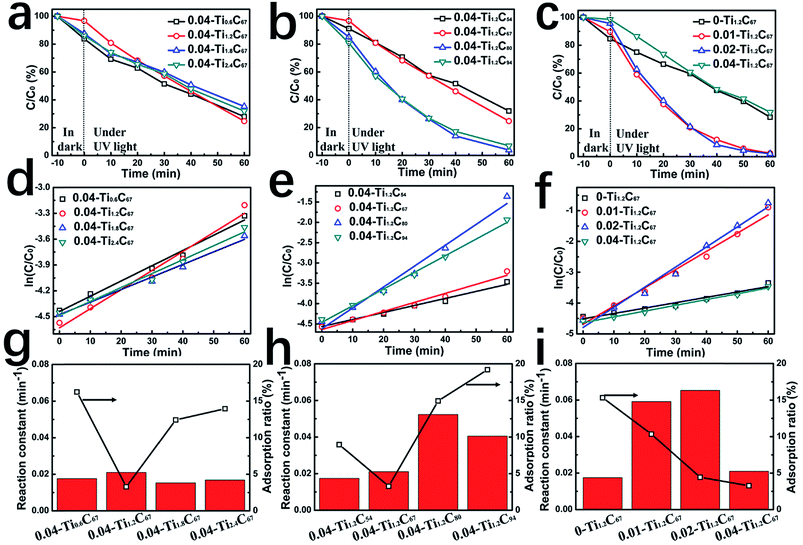
A series of TiO2/CNT composites have been prepared by tuning three precursor amounts, i.e. isopropyl titanate (IT), CNT, and DETA. Their theoretical chemical compositions have been summarized in Table S1,† and the adsorption capacity and photocatalytic activity have also been investigated systematically, as shown in Fig. 1.
By changing the weight ratio of TiO2 from 71% to 91% (Table S1†), the 0.04-TixC67 composites generally exhibit the similar MB-removed efficiency, as shown in Fig. 1a. By the plots of ln(C/C0) vs. illumination time (Fig. 1d), the first-order linear relationship could be found in all the four samples, of which the undistinguishable fitting-line slopes imply the similar photocatalytic activity of the 0.04-TixC67 composites. From the relationship between the adsorption and catalytic capability (Fig. 1g), it can be clearly seen that the 0.04-Ti1.2C67 shows slightly enhanced catalytic performance compared with others, as well as the remarkably lower adsorption capacity. Obviously, the TiO2 overloaded in TiO2/CNT composite may greatly affect the loading uniformity of TiO2 particles due to their aggregation, whereas exhibiting a negligible difference for their photocatalytic activity.
By increasing CNT amount, the adsorption capacity of corresponding samples firstly decreased and then increased, while their catalytic activity has the reverse trend (Fig. 1b, e and h). As shown in Fig. 1h, when the additive amount of CNT is 67 mg, the 0.04-Ti1.2C67 has the lowest adsorption for MB, indicating the highest coverage proportion among these samples. As the CNT content continuously increasing, more TiO2 active sites will be exposed, resulting in the increasing photocatalytic activity. Therefore, although improving the dispersity of TiO2 nanoparticles, the increase of CNT content seems not to improve their uniformity simultaneously, which fails to fabricate the TiO2/CNT composite with well-defined structure.
In addition, DETA in the system also plays an important role in controlling the structure of TiO2/CNT composite. As shown in Fig. 1c, the MB-removed efficiency of the 0.01-Ti1.2C67 and 0.02-Ti1.2C67 is much higher than that of the 0-Ti1.2C67, mainly owning to their greatly enhanced photocatalytic performance (Fig. 1f). Fig. 1i shows that the two TiO2/CNT composites have a gradually decreasing adsorption for MB with the DETA amount, indicating that the introduce of DETA can effectively promote the TiO2 loading uniformity. However, excess DETA, in the case of 0.04-Ti1.2C67, inversely leads to the obvious decrease in photocatalytic performance, meanwhile it exhibits the lowest adsorption capacity compared with other samples. Therefore, the appropriate DETA will play an important role in fabricating the TiO2/CNT composite with both the great TiO2 loading uniformity and the high dispersity.
Besides MB, the 0.02-Ti1.2C67 also exhibits a great photocatalytic performance for the degradation of colorless organics (TC, ENRH, CIP), the reaction processes of three antibiotics have shown in Fig. 2. Obviously, three antibiotics are found to be rapidly degradated over the 0.02-Ti1.2C67 under the UV irradiation, which removes almost 99% TC, 94% ENRH and 95% CIP after exposed in UV light for 120 min. It is indicated that the resultant 0.02-Ti1.2C67 has a good photocatalytic efficiency for many organic molecules.
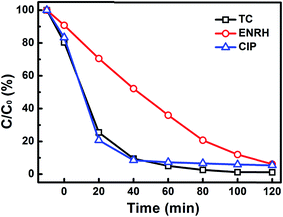 | ||
| Fig. 2 Photocatalytic degradation of three antibiotics (TC, ENRH, CIP) over 0.02-Ti1.2C67 under UV-light irradiation. | ||
3.2 Effect of DETA on the structure of the TiO2/CNT composite
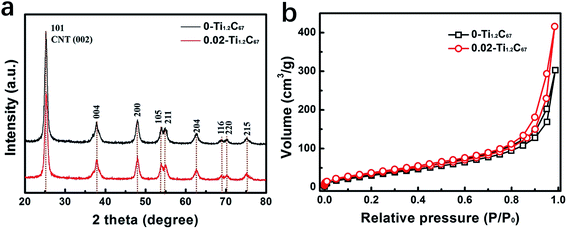
To figure out the role of DETA, in the present case, the 0-Ti1.2C67 and 0.02-Ti1.2C67 are chosen and explore the structural difference. Fig. 3a compares the XRD patterns of the 0-Ti1.2C67 and 0.02-Ti1.2C67, showing the identical diffraction peaks assigned to anatase phase of TiO2 (JCPDS no. 21-1272).21,28 It is indicated that the DETA has no influence on the crystal growth of TiO2 under the solvothermal situations. It should be noted that no feature peak of CNT can be found in two XRD patterns, which is due to that the diffraction peak of CNT at ca. 26° is overlapped with the TiO2 (101).21 The structural difference between the 0-Ti1.2C67 and 0.02-Ti1.2C67 will be further discussed in this paper below. Besides, other TiO2/CNT samples obtained also have the same crystal compositions with the 0-Ti1.2C67 and 0.02-Ti1.2C67, resulting from the same XRD patterns (Fig. S3†).From Fig. 3b, we can clearly see that the 0-Ti1.2C67 and 0.02-Ti1.2C67 show the coincident N2 adsorption–desorption isotherms, and their BET surface areas have been estimated to be 138.863 m3 g−1 and 147.217 m3 g−1, respectively.
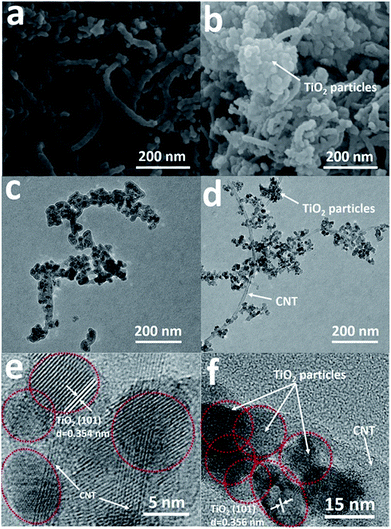
SEM images of the two samples loaded by TiO2 particles with/without the assistance of DETA (0.02-Ti1.2C67 and 0-Ti1.2C67) have been shown in Fig. 4a and b, both the two samples have partially maintained the initial wire-type morphology of CNT (Fig. S4†), illustrating that CNT works as the framework in the fabrication of the TiO2/CNT composite. Particularly, the 0.02-Ti1.2C67 displays a highly entangled one-dimensional parasitic architecture with a 40–45 nm diameter size, which is slightly larger than the pure CNT (30–35 nm). On the contrary, a cluster of TiO2 particles could be clearly observed in the 0-Ti1.2C67 (Fig. 4b), through a lot of coarse nanowires are also presented in the appearance. The detailed structure of the two products would be revealed by the corresponding TEM images (Fig. 4c and d). For the sample 0.02-Ti1.2C67, TiO2 particles are evenly grown along the CNT, whereas the 0-Ti1.2C67 has both the badly aggregated TiO2 particles and the bare CNT. From the SEM and TEM images, it is indicated that the TiO2 loading uniformity of the TiO2/CNT composite could be significantly improved, when DETA is introduced into the solvothermal system. This structural difference can also be seen from their HRTEM images (Fig. 4e and f). As shown, compared with the 0-Ti1.2C67, the 0.02-Ti1.2C67 possesses the well-dispersed TiO2 particles on the CNT. Moreover, the clear lattice fringes with interplanar spacing of 0.354 nm and 0.356 nm are found in two HRTEM images, which match well with the TiO2 (101) lattice plane. Obviously, in spite of the different uniformity and dispersity, TiO2 particles in two samples (Fig. 4e and f) show a high crystallinity and the same crystal structure.
XPS is conducted to further distinguish the structural difference between the 0.02-Ti1.2C67 and 0-Ti1.2C67, and the detailed results have been displayed in Fig. 5. From their XPS survey spectra (Fig. 5a), we can see the identical peaks in both XPS spectra, indicating the similar general structure. Besides, the 0.02-Ti1.2C67 and 0-Ti1.2C67 also exhibit the same high-resolution XPS spectra of Ti (Fig. 5b), in which two bands centered at 458.6 and 464.3 eV correspond to Ti(IV) 2p1/2 and Ti(IV) 2p3/2,29–31 respectively. However, as shown in Fig. 5c, the peak at 532.1 eV assigned to C–O–Ti could be clearly observed in XPS spectrum of O 1s of the 0.02-Ti1.2C67, which is much stronger than that of 0-Ti1.2C67. This result demonstrates that an interaction excits between CNT and TiO2 in the 0.02-Ti1.2C67. Meanwhile, due to the extensive coverage of TiO2 particles, there are a lot of oxygen-containing groups reserved on CNT, thus a peak from C–O or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (533.5 eV) is also presented in the 0.02-Ti1.2C67.31–33 Consistence with the XPS result of O 1s, the 0.02-Ti1.2C67 also has an additive peak at 288.9 eV in the XPS spectrum of C 1s (Fig. 5d), which can be attributed to the Ti–O–C,34–37 further indicating the strong interaction between TiO2 and CNT in the 0.02-Ti1.2C67. Moreover, the detailed chemical compositions of the 0.02-Ti1.2C67 and 0-Ti1.2C67 analyzed by XPS have been summarized in Tables S2 and S3,† respectively.
O (533.5 eV) is also presented in the 0.02-Ti1.2C67.31–33 Consistence with the XPS result of O 1s, the 0.02-Ti1.2C67 also has an additive peak at 288.9 eV in the XPS spectrum of C 1s (Fig. 5d), which can be attributed to the Ti–O–C,34–37 further indicating the strong interaction between TiO2 and CNT in the 0.02-Ti1.2C67. Moreover, the detailed chemical compositions of the 0.02-Ti1.2C67 and 0-Ti1.2C67 analyzed by XPS have been summarized in Tables S2 and S3,† respectively.
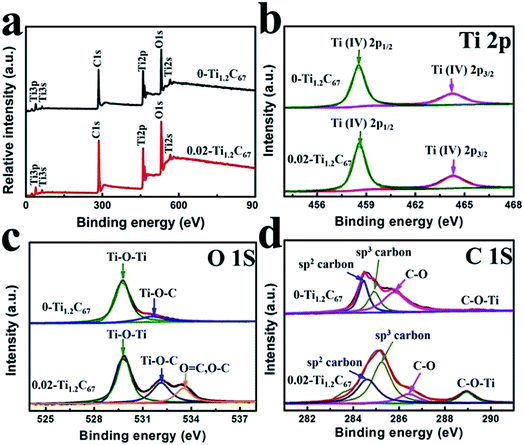 | ||
| Fig. 5 (a) XPS survey spectra of 0.02-Ti1.2C67 and 0-Ti1.2C67; high-resolution XPS spectra of (b) C 1s; (c) O 1s; (d) Ti 2p of 0.02-Ti1.2C67 and 0-Ti1.2C67. | ||
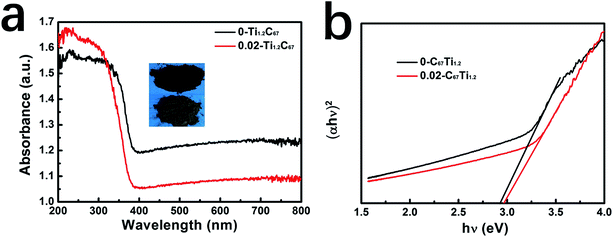
In UV-DRS of the 0-Ti1.2C67 and 0.02-Ti1.2C67 (Fig. 6a), both a strong absorption in UV region (below 400 nm) and visible absorption in 400–800 nm could be observed, while the 0.02-Ti1.2C67 shows a stronger visible absorption compared with the 0-Ti1.2C67, probably because of its high dispersity of TiO2 particles on CNT. However, from the plots of (αhν)2 versus (hν),38 we can find no obvious difference between the band gap energy (Eg) of 0-Ti1.2C67 and 0.02-Ti1.2C67, indicating no effect of DETA on the band gap of TiO2/CNT composites.
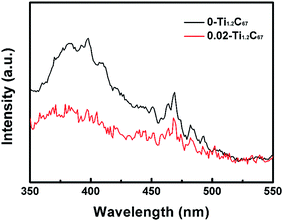
PL is a powerful tool to analyze the essential optical property of photocatalysts. Fig. 7 compares PL spectra of the 0-Ti1.2C67 and 0.02-Ti1.2C67 at an excitation wavelength of 294 nm, both of which include an emission peak at ca. 396 nm and series of peaks in the range of 440–500 nm. Generally, the strong peak at ca. 396 nm is attributed to the electron transition of the bandgap energy of anatase TiO2 (∼397 nm),39,40 while peaks ranging from 440 to 500 nm are ascribed to the electron migration resulted from the surface defects.41 For the 0.02-Ti1.2C67, the quenching fluorescence indicates a faster transfer of surface electrons than the 0-Ti1.2C67, resulting from the close contact between TiO2 and CNT. Therefore, the 0.02-Ti1.2C67 has exhibited the superior photocatalytic activity in the photodegradation of MB under the irradiation of UV-light. Meanwhile, the equivalent emission peaks from 440–500 nm can be clearly seen in two samples, indicating the similar TiO2 surface structure, which is very agreement with the XPS result discussed above. PL analysis of all samples obtained in this work (Fig. S5†), it is found the CNT and DETA play an important role on the electron transport efficiency of the TiO2/CNT composites, which well matches with the photocatalytic experimental results. However, the decreasing PL emission peak probably attribute to the increasing amount of CNT in samples. As for z-Ti1.2C67 samples, a declining PL peak has been observed in the 0.02-Ti1.2C67, indicating that when the additive amount of DETA is 0.02 mL, the corresponding product possesses the higher electron-transfer rate from TiO2 particles to CNT.
Furthermore, the photoelectrochemical property of two TiO2/CNT composites are investigated by their photocurrent responses and EIS, and the detailed results have been displayed in Fig. 8. From Fig. 8a, we can clearly see that both the 0-Ti1.2C67 and 0.02-Ti1.2C67 have transient photocurrent responses in three on–off cycles of UV-light irradiation in 0.1 M Na2SO4 solution, resulting from the separation of photogenerated electron/hole pairs in two samples. In comparison to the 0-Ti1.2C67, the photocurrent density of the 0.02-Ti1.2C67 is much higher, demonstrating an increasing electron-transfer efficiency. Consequently, in the 0.02-Ti1.2C67, the close contact between TiO2 and CNT successfully facilitates the migration efficiency of surface electrons. Meanwhile, both the 0.02-TixC67 and 0.02-Ti1.2Cy samples have the similar photocurrent intensity, as shown in Fig. S6.† It is demonstrated that the electron-migration efficiency of TiO2/CNT composites cannot be greatly affected by the amounts of CNT and TiO2 particles in system. On the contrary, DETA is the major influence factor to the materials which can be seen from the great difference photocurrent response.
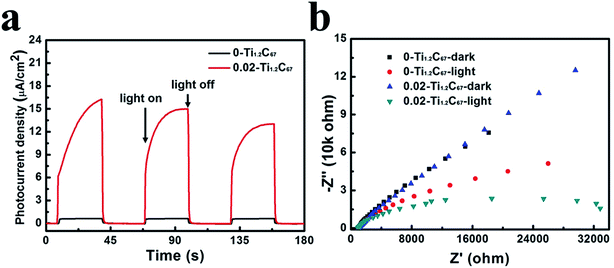 | ||
| Fig. 8 Photocurrent responses (a) and Nyquist plots (b) of 0-Ti1.2C67 and 0.02-Ti1.2C67 in 0.1 M Na2SO4 solution under UV-light irradiation. | ||
To further analyze the surface charge migration, EIS of the 0-Ti1.2C67 and 0.02-Ti1.2C67 were measured in 0.1 M Na2SO4 solution, both in dark and under the irradiation of UV-light. For photocatalysts, the Nyquist plot recorded in dark can represent their intrinsic charge-transfer resistance. From Fig. 8b, the identical Nyquist plot in dark indicates that the 0-Ti1.2C67 and 0.02-Ti1.2C67 have the similar CNT and TiO2 contents. After exposed to UV-light, the charge migration rate of the two samples have been dramatically accelerated, thus showing two depressed semicircles in the corresponding Nyquist plots. Particularly, the 0.02-Ti1.2C67 has the smaller radius than the 0-Ti1.2C67, verifying a higher charge migration efficiency. Consequently, the photoelectrochemical result agrees fairly well with the photocatalytic performance of the 0-Ti1.2C67 and 0.02-Ti1.2C67.
3.3 Growth mechanism of the CNT/TiO2 composite
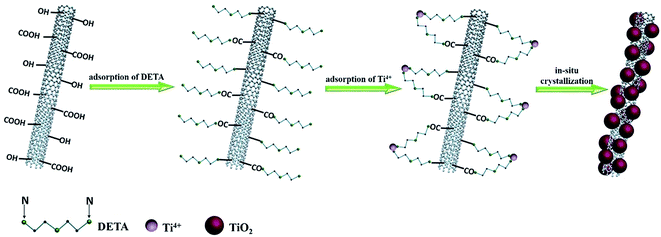
In terms of the available information, a possible mechanism has been proposed to figure out the role of DETA for the formation of the TiO2/CNT composite, as shown in Scheme 2. The whole preparation process of the TiO2/CNT composite can be probably divided into three steps: (a) first, due to the oxygen-containing functional groups, the acid-treated CNT can effectively combine with –NH2 in DETA via physisorption or electrostatic adsorption.42 (b) Afterwards, the unbinding –NH2 in the DETA-modified CNT further capture dissociated Ti4+ ions in solution to form the catalyst precursor, Ti4+-DETA-CNT.42,43 (c) As the hydrothermal temperature gradually increases, Ti4+ on the precursor will crystallize to yield TiO2 nanoparticles, which are in situ deposited on the surface of DETA-modified CNT. Finally, after thermally treating at the high temperature, DETA and functional groups on the CNT surface are removed, and the TiO2/CNT composite with the uniformly-loaded and well-dispersed TiO2 nanoparticles has been successfully prepared.4. Conclusions
In summary, series of TiO2/CNT composites have been synthesized via tuning the amount of various precursors (IT, CNT, and DETA) under the solvothermal situations. After investigated their adsorption capacity and photodegrading activity under the UV-light irradiation for MB removal, it is found that DETA in synthesis system can remarkably improve the photocatalytic activity of samples, meanwhile decreasing the adsorption amount for MB molecules. Particularly, the 0.02-Ti1.2C67 exhibits both the uniformly-loaded TiO2 nanoparticles on the surface, the high electrons-transfer efficiency and strong interaction between TiO2 and CNT. Furthermore, a possible DETA-assisted mechanism has been proposed to explain the in situ formation of TiO2/CNT composite, which could develop to be a general method for preparating of metal oxide/carbon (MO/C) composites with the well-defined structure and superior photocatalytic or photoelectrochemical properties.Conflicts of interest
No conflicts of interest.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (No. 21576211 and No. 21706190).References
- F. M. Machado, S. A. Carmalin, E. C. Lima, S. L. P. Dias, L. D. T. Prola, C. Saucier, I. M. Jauris, I. Zanella and S. B. Fagan, J. Phys. Chem. C, 2016, 120, 18296–18306 CAS.
- D. Li, Q. Li, N. Bai, H. Dong and D. Mao, ACS Sustainable Chem. Eng., 2017, 5, 5598–5607 CrossRef CAS.
- M. Ahmad, S. Liu, N. Mahmood, A. Mahmood, M. Ali, M. Zheng and J. Ni, ACS Appl. Mater. Interfaces, 2017, 9, 13188–13200 CAS.
- P. Zhang, M. Fujitsuka and T. Majima, Appl. Catal., B, 2016, 185, 181–188 CrossRef CAS.
- W. Jiang, Y. Liu, J. Wang, M. Zhang, W. Luo and Y. Zhu, Adv. Mater. Interfaces, 2016, 3, 1500502 CrossRef.
- K. Fujiwara, Y. Kuwahara, Y. Sumida and H. Yamashita, Langmuir, 2017, 33, 288–295 CrossRef CAS PubMed.
- Z. Chen, N. Zhang and Y.-J. Xu, CrystEngComm, 2013, 15, 3022 RSC.
- Z. Bian, T. Tachikawa, P. Zhang, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2014, 136, 458–465 CrossRef CAS PubMed.
- D. Yang, H. Liu, Z. Zheng, Y. Yuan, J.-cai Zhao, X. K. Eric, R. Waclawik and H. Zhu, J. Am. Chem. Soc., 2009, 131, 17885–17893 CrossRef CAS PubMed.
- J. Liu, J. Ke, D. Li, H. Sun, P. Liang, X. Duan, W. Tian, M. O. Tadé, S. Liu and S. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 11678–11688 CAS.
- C. Li, Y. Tang, B. Kang, B. Wang, F. Zhou, Q. Ma, J. Xiao, D. Wang and J. Liang, Sci. China, Ser. E: Technol. Sci., 2007, 50, 279–289 CrossRef CAS.
- Y. Dong, D. Tang and C. Li, Appl. Surf. Sci., 2014, 296, 1–7 CrossRef CAS.
- Z. Lu, X. Xiang, L. Zou and J. Xie, RSC Adv., 2015, 5, 42580–42586 RSC.
- Q. Zeng, H. Li, H. Duan, Y. Guo, X. Liu, Y. Zhang and H. Liu, RSC Adv., 2015, 5, 13430–13436 RSC.
- Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Chem. Rev., 2014, 114, 9987–10043 CrossRef CAS PubMed.
- N. Zhang and Y.-J. Xu, CrystEngComm, 2016, 18, 24–37 RSC.
- N. Zhang, M.-Q. Yang, S. Liu, Y. Sun and Y.-J. Xu, Chem. Rev., 2015, 115, 10307–10377 CrossRef CAS PubMed.
- P. Zhang, B. Li, Z. Zhao, C. Yu, C. Hu, S. Wu and J. Qiu, ACS Appl. Mater. Interfaces, 2014, 6, 8560–8566 CAS.
- P. Zhang, Y. Chen, X. Yang, J. Gui, Y. Li, H. Peng, D. Liu and J. Qiu, Langmuir, 2017, 33, 4452–4460 CrossRef CAS PubMed.
- K. Dai, X. Zhang, K. Fan, T. Peng and B. Wei, Appl. Surf. Sci., 2013, 270, 238–244 CrossRef CAS.
- J. Yu, T. Ma and S. Liu, Phys. Chem. Chem. Phys., 2011, 13, 3491–3501 RSC.
- A. Shayesteh Zeraati, S. A. Mirkhani and U. Sundararaj, J. Phys. Chem. C, 2017, 121, 8327–8334 CAS.
- V. H. Nguyen, C. Kang, C. Roh and J.-J. Shim, Ind. Eng. Chem. Res., 2016, 55, 7338–7343 CrossRef CAS.
- B. K. Vijayan, N. M. Dimitrijevic, D. Finkelstein-Shapiro, J. Wu and K. A. Gray, ACS Catal., 2012, 2, 223–229 CrossRef CAS.
- T. An, J. Chen, X. Nie, G. Li, H. Zhang, X. Liu and H. Zhao, ACS Appl. Mater. Interfaces, 2012, 4, 5988–5996 CAS.
- J. Di, S. Li, Z. Zhao, Y. Huang, Y. Jia and H. Zheng, Chem. Eng. J., 2015, 281, 60–68 CrossRef CAS.
- X. Li, H. Lu, Y. Zhang and F. He, Chem. Eng. J., 2017, 316, 893–902 CrossRef CAS.
- J. Zhang, M. Vasei, Y. Sang, H. Liu and J. P. Claverie, ACS Appl. Mater. Interfaces, 2016, 8, 1903–1912 CAS.
- Y. Y. Lu, Y. Y. Zhang, J. Zhang, Y. Shi, Z. Li, Z. C. Feng and C. Li, Appl. Surf. Sci., 2016, 370, 312–319 CrossRef CAS.
- H. Zhao, M. Wu, J. Liu, Z. Deng, Y. Li and B.-L. Su, Appl. Catal., B, 2016, 184, 182–190 CrossRef CAS.
- L. Zhao, X. Chen, X. Wang, Y. Zhang, W. Wei, Y. Sun, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 3317–3321 CrossRef CAS PubMed.
- B. Li, Z. Zhao, F. Gao, X. Wang and J. Qiu, Appl. Catal., B, 2014, 147, 958–964 CrossRef CAS.
- E. Bailón-García, A. Elmouwahidi, M. A. Álvarez, F. Carrasco-Marín, A. F. Pérez-Cadenas and F. J. Maldonado-Hódar, Appl. Catal., B, 2017, 201, 29–40 CrossRef.
- K. M. Cho, K. H. Kim, H. O. Choi and H.-T. Jung, Green Chem., 2015, 17, 3972–3978 RSC.
- X. Fan, C. Yu, J. Yang, Z. Ling and J. Qiu, Carbon, 2014, 70, 130–141 CrossRef CAS.
- X. Fan, C. Yu, Z. Ling, J. Yang and J. Qiu, ACS Appl. Mater. Interfaces, 2013, 5, 2104–2110 CAS.
- S. Liu, C. Liu, W. Wang, B. Cheng and J. Yu, Nanoscale, 2012, 4, 3193 RSC.
- S. Sadhu and P. Poddar, J. Phys. Chem. C, 2014, 118, 19363–19373 CAS.
- Y. T. Liang, B. K. Vijayan, O. Lyandres, K. A. Gray and M. C. Hersam, J. Phys. Chem. Lett., 2012, 3, 1760–1765 CrossRef CAS PubMed.
- M. Cao, P. Wang, Y. Ao, C. Wang, J. Hou and J. Qian, Chem. Eng. J., 2015, 264, 113–124 CrossRef CAS.
- Z. Bian, T. Tachikawa, W. Kim, W. Choi and T. Majima, J. Phys. Chem. C, 2012, 116, 25444–25453 CAS.
- Y.-X. Zhang, X. Guo, X. Zhai, Y.-M. Yan and K.-N. Sun, J. Mater. Chem. A., 2015, 3, 1761–1768 CAS.
- H. Jianfeng, W. Dan, Y. Lixiong, C. Liyun, O. Haibo, L. Jiayin and H. Wei, J. Alloys Compd., 2014, 612, 233–238 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra09324a |
| This journal is © The Royal Society of Chemistry 2017 |