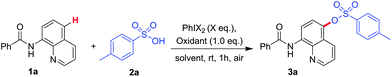Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iodobenzene-catalyzed synthesis of aryl sulfonate esters from aminoquinolines via remote radical C–O cross-coupling†
Chao Shen *a,
Ming Yangc,
Jun Xuc,
Chao Chenb,
Kai Zhenga,
Jiabing Shenc and
Pengfei Zhangc
*a,
Ming Yangc,
Jun Xuc,
Chao Chenb,
Kai Zhenga,
Jiabing Shenc and
Pengfei Zhangc
aCollege of Biology and Environmental Engineering, Zhejiang Shuren University, Hangzhou 310015, China. E-mail: shenchaozju@163.com; Fax: +86-571-28862867; Tel: +86-571-28862867
bCollege of Life Sciences, Huzhou Teachers College, Huzhou, 313000, China
cCollege of Material Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou 310036, China
First published on 24th October 2017
Abstract
A simple and efficient approach is established for the iodobenzene-catalyzed synthesis of aryl sulfonate esters from aminoquinolines via remote radical C–O cross-coupling in the absence of any transition metal catalysts. This unexpected reaction reveals superior reactivity, target products are obtained in good to excellent yields at room temperature.
Introduction
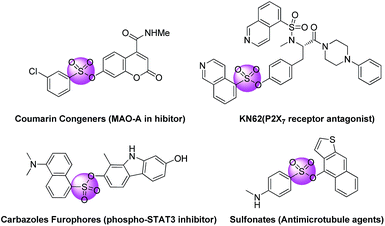
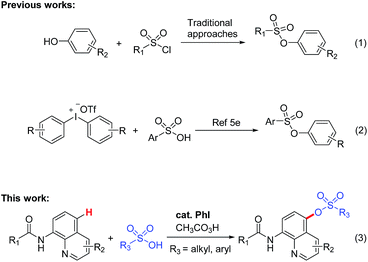
Aryl sulfonate esters are always deemed as attractive molecules for chemical researchers due to their special bioactivities.1 Additionally, sulfonate esters are often used as functional groups or substrates in photochemistry,2 materials3 and synthetic chemistry.4 Hence, valid approaches for the synthesis of these significant bioactive compounds containing sulfonate ester blocks are highly valuable (Fig. 1).Traditionally, aryl sulfonate esters are generally synthesized by reacting an appropriate sulfonyl chloride with the suitable alcohol or phenol in view of the direct transformation of sulfonic acid to the corresponding sulfonate esters is indeed difficult to fulfil (Scheme 1). These protocols, however, often suffer from a lot of limitations,5 such as harsh reaction conditions, dreary reaction routines, numbers of side reactions and low yields. Therefore, the conventional and available substrates direct convert into valuable aryl sulfonate esters products through C–H sulfonation is undoubtedly an efficient and energy saving method.6
Recently, the advance of C–O bond formation via C–H bond functionalization has attracted much attention.7 Majority of the researches on regioselective C–O bond formation are almost limited to hydroxylation.7a–f acetoxylation,7g–k benzoxylation7l–n and etherification.7o–s Despite these great advances, these methods generally undergo metal catalysts, high reaction temperatures, and acid or basic additives.
Quinolines are prevalent in many natural products and pharmaceuticals.8 For this purpose, plenty of investigations were proceeded employing quinolines as building blocks.9 Especially, the functionalization of quinolines on C5 position has also got a lot of attention lately.10 However, most of these kinds of reactions were relying on metal catalysts including Fe,10a Co,10b Ni,10c Cu,10d–n Ag10o and Pd.10p Although various reactions including carbon–carbon and carbon–heteroatom bonds cross-coupling have been reported, the methodology for the C-5 selective formation of C–O bond under transition-metal-free condition has never been established.
In consideration of the importance of aryl sulfonate esters and quinolines, a much more simple and efficient C–O bond formation method is required to access these worthy sulfonate esters. Importantly, no metal catalyst was required in the procedure.11 As we have seen, the direct sulfonation of quinolines on C5 position with aryl or alkyl sulfonic acids has not yet been achieved. Herein we report the first example of the synthesis of aryl sulfonate esters through iodobenzene-catalyzed direct sulfonylation of aromatic compounds with aryl or alkyl sulfonic acids in the presence of peracetic acid as a terminal oxidant at room temperature.
Results and discussion
Firstly, 8-aminoquinoline 1a and p-toluenesulfonic acid 2a were selected as a model compound to explore the optimized reaction conditions with iodine(III) at room temperature (Table 1). Interestingly, omission of metal catalyst and running the reaction in dioxane under air was successful when 2 equivalents of phenyliodine diacetate (PIDA) or phenyliodonium bis(trifluoro-acetate) (PIFA) were used as oxidant (entry 1 and entry 2, Table 1). Then we tried to promote the C–O coupling by in situ generated PIDA by using PhI (0.2 equiv.) as an iodine source with m-CPBA (1.0 equiv.) as an oxidant product 3a was obtained only in a 26% yield (entry 3, Table 1). To further improve the yield, various oxidants, including TBHP, H2O2, CH3CO3H and K2S2O8 were examined, and CH3CO3H increased the yield of 3a to 42% (entries 4–7, Table 1). We then studied the solvent effect on the reaction and found that the solvents such as toluene, DCM, DMSO, MeCN and HFIP have significant effects on the reaction (entries 8–11, Table 1). Delightedly, the yield was up to 93% by using HFIP as solvent (entry 11, Table 1). The reaction was moderately sensitive to temperature, with poorer results obtained at higher temperatures (entry 12, Table 1). When m-CPBA was used as oxidant, the sulfonated quinoline amide was obtained in a lower yield (entry 13, Table 1). Lastly, we reduced the amount of PhI from 20 mol% to 10 mol%, the yield of the isolated product dropped to 71% (entry 14, Table 1) and no product was detected in the absence of oxidant (entry 15, Table 1).| Entry | PhIX2 (equiv.) | Oxidant | Solvent | Yieldb/[%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (1.5 equiv.), PhIX2 (X equiv.), oxidant (1.0 equiv.), solvent (1.0 mL), stirred at rt, 1 h, under air, m-CPBA = m-chloroper benzoic acid, TBHP = tert-butyl hydroperoxide, HFIP = 1,1,1,3,3,3-hexafluoro-2-propanol.b Isolated yields.c Stirred at 50 °C. | ||||
| 1 | PhI(OAc)2 (2) | — | Dioxane | 68 |
| 2 | PhI(TFA)2 (2) | — | Dioxane | 75 |
| 3 | PhI (0.2) | m-CPBA | Dioxane | 26 |
| 4 | PhI (0.2) | TBHP | Dioxane | 0 |
| 5 | PhI (0.2) | H2O2 | Dioxane | 0 |
| 6 | PhI (0.2) | CH3CO3H | Dioxane | 42 |
| 7 | PhI (0.2) | K2S2O8 | Dioxane | Trace |
| 8 | PhI (0.2) | CH3CO3H | Toluene | 37 |
| 9 | PhI (0.2) | CH3CO3H | DCM | 0 |
| 10 | PhI (0.2) | CH3CO3H | MeCN | 15 |
| 11 | PhI (0.2) | CH3CO3H | HFIP | 93 |
| 12 | PhI (0.2) | CH3CO3H | HFIP | 81c |
| 13 | PhI (0.2) | m-CPBA | HFIP | 85 |
| 14 | PhI (0.1) | CH3CO3H | HFIP | 71 |
| 15 | PhI (0.2) | — | HFIP | 0 |
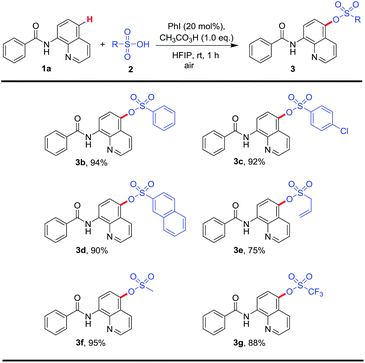
With the optimal condition in hand, then the scope of sulfonic acids was tested (Table 2). Numbers of sulfonic acids, including aliphatic and aromatic sulfonic acids revealed excellent reactivity, corresponding products were gained in good to excellent yields. Aryl sulfonic acids such as benzenesulfonic acid, p-chloro benzenesulfonic acid and β-naphthalenesulphonic acid converted to the corresponding products in 94%, 92% and 90% yields, respectively (3b–c). Allyl sulfonic acid and methanesulfonic acid are suitable substrates for this transformation (3e, 3f). Excitedly, trifluoro methanesulfonic acid also revealed good reactivity, and desired product was got in satisfactory yield (3g).
| a Reaction conditions: 1a (0.2 mmol), 2 (1.5 equiv.), PhI (20 mol%), CH3CO3H (1.0 equiv.), HFIP (1.0 mL), stirred at rt, 1 h, under air, isolated yields. |
|---|
 |
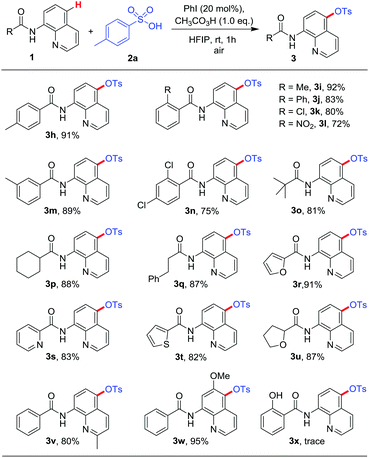
Subsequently, the reaction generality was investigated with various 8-amino quinolines. The results are summarized in Table 3 and this transformation demonstrated remarkable functional group tolerance. N-(Quinolin-8-yl)benzamide with multifarious substituted groups such as Me, Ph, Cl and NO2 on the benzene rings proved higher reactivity, and the desired products (3h–n) were received in good yields. The carboxamides with aliphatic group (t-butyl, cyclohexyl, phenylethyl) and heterocyclic ring (2-furyl, 2-pyridyl, 2-thienyl, tetrahydro-2-furyl) equipped C5-sulfonated quinolines amides (3o–u) in high yields. Moreover, N-(quinolin-8-yl)benzamide with Me and MeO on the quinoline rings also revealed reasonable reactivity (3v–w). Sadly, the substrate with hydroxyl group could not translate into relevant product due to the influence of reactive hydrogen (3x).
| a Reaction conditions: 1 (0.2 mmol), 2a (1.5 equiv.), PhI (20 mol%), CH3CO3H (1.0 equiv.), HFIP (1.0 mL), stirred at rt, 1 h, under air, isolated yields. |
|---|
 |
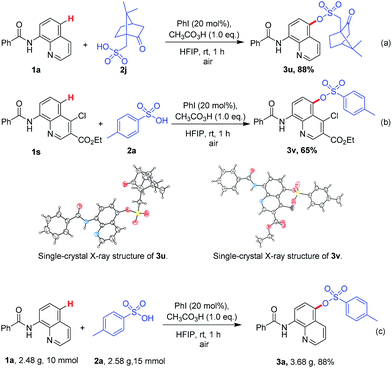
To prove the high reaction activity and selectivity of this method still further, the reactants with bigger steric hindrance such as camphorsulfonic acid (2j) and ethyl 8-benzamido-4-chloroquinoline-3-carboxylate (1s) were tested, respectively (Scheme 2a). The moderate to good yields of products 3y and 3z were obtained. And the molecular structures were further confirmed by X-ray crystallography. Critically, sulfonated quinolone derivate 3z was got in valuable yield (Scheme 2b), which with a potentially broader range of uses in biological and pharmacological fields. In addition, given the easy availability of the raw materials and the operational simplicity of this metal free method, we performed the reaction on a gramscale obtaining the sulfonic acid ester 3a in 88% yield (Scheme 2c).
Conclusions
We have developed an efficient protocol for the iodobenzene-catalyzed synthesis of aryl sulfonate esters from aminoquinolines via remote radical C–O cross-coupling in the presence of peracetic acid as a terminal oxidant at room temperature. This C–O coupling reaction proceeds under simple and mild conditions, reveals high efficiency and affords the sulfonated products in good to excellent yields with high selectivity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Zhejiang Provincial Natural Science Foundation of China (No. LY17B020005), Science and Technology Plan of Zhejiang Province (No. 2017C31054) and the National Natural Science Foundation of China (No. 21302171).Notes and references
- (a) L. Yan and C. E. Müller, J. Med. Chem., 2004, 47, 1031 CrossRef CAS PubMed; (b) A. Zuse, P. Schmidt, S. Baasner, K. J. Böhm, K. Müller, M. Gerlach, E. G. Günther, E. Unger and H. Prinz, J. Med. Chem., 2007, 50, 6059 CrossRef CAS PubMed; (c) L. Pisani, M. Barletta, R. Soto-Otero, O. Nicolotti, E. Mendez-Alvarez, M. Catto, A. Introcaso, A. Stefanachi, S. Cellamare, C. Altomare and A. Carotti, J. Med. Chem., 2013, 56, 2651 CrossRef CAS PubMed; (d) J.-H. Park, G.-E. Lee, S.-D. Lee, T. T. Hien, S. Kim, J. W. Yang, J.-H. Cho, H. Ko, S.-C. Lim, Y.-G. Kim, K.-W. Kang and Y.-C. Kim, J. Med. Chem., 2015, 58, 2114 CrossRef CAS PubMed; (e) S. Hou, Y. W. Yi, H. J. Kang, L. Zhang, H. J. Kim, Y. Kong, Y. Liu, K. Wang, H.-S. Kong, S. Grindrod, I. Bae and M. L. Brown, J. Med. Chem., 2014, 57, 6342 CrossRef CAS PubMed.
- (a) S. M. Pauff and S. C. Miller, Org. Lett., 2011, 13, 6196 CrossRef CAS PubMed; (b) S. M. Pauff and S. C. Miller, J. Org. Chem., 2013, 78, 711 CrossRef CAS PubMed.
- (a) M. Zhang, J. D. Moore, D. L. Flynn and P. R. Hanson, Org. Lett., 2004, 6, 2657 CrossRef CAS PubMed; (b) H. Mori, E. Kudo, Y. Saito, A. Onuma and M. Morishima, Macromolecules, 2010, 43, 7021 CrossRef CAS.
- (a) F. Li, T.-X. Liu and G.-W. Wang, Org. Lett., 2012, 14, 2176 CrossRef CAS PubMed; (b) J. R. DeBergh, N. Niljianskul and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 10638 CrossRef CAS PubMed; (c) B. G. Avitabile, C. A. Smith and D. B. Judd, Org. Lett., 2005, 7, 843 CrossRef CAS PubMed; (d) D. Elder, K. L. Facchine, J. N. Levy, R. Parsons, D. Ridge, L. Semo and A. Teasdale, Org. Process Res. Dev., 2012, 16, 1707 CrossRef CAS.
- (a) B. Das, S. Reddy and M. R. Reddy, Tetrahedron Lett., 2004, 45, 6717 CrossRef CAS; (b) B. Das and V. S. Reddy, Chem. Lett., 2004, 33, 1428 CrossRef CAS; (c) B. M. Choudary, N. S. Chowdari and M. L. Kantam, Tetrahedron, 2000, 56, 7291 CrossRef CAS; (d) S. Velusamy, J. S. K. Kumar and T. Punniyamurthy, Tetrahedron Lett., 2004, 45, 203 CrossRef CAS; (e) N. Jalalian, T. B. Petersen and B. Olofsson, Chem.–Eur. J., 2012, 18, 14140 CrossRef CAS PubMed.
- Y. Xu, G. Yan, Z. Ren and G. Dong, Nat. Chem., 2015, 7, 829 CrossRef CAS PubMed.
- (a) Y.-H. Zhang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 14654 CrossRef CAS PubMed; (b) Q. Lu, J. Zhang, F. Wei, Y. Qi, H. Wang, Z. Liu and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 7156 CrossRef CAS PubMed; (c) X. Yang, G. Shan and Y. Rao, Org. Lett., 2013, 15, 2334 CrossRef CAS PubMed; (d) W. Liu and L. Ackermann, Org. Lett., 2013, 15, 3484 CrossRef CAS PubMed; (e) X. Li, Y.-H. Liu, W.-J. Gu, B. Li, F.-J. Chen and B.-F. Shi, Org. Lett., 2014, 16, 3904 CrossRef CAS PubMed; (f) J. Dong, P. Liu and P. Sun, J. Org. Chem., 2015, 80, 2925 CrossRef CAS PubMed; (g) D.-H. Wang, X.-S. Hao, D.-F. Wu and J.-Q. Yu, Org. Lett., 2006, 8, 3387 CrossRef CAS PubMed; (h) R. Fan, Y. Sun and Y. Ye, Org. Lett., 2009, 11, 5174 CrossRef CAS PubMed; (i) R. K. Rit, M. R. Yadav and A. K. Sahoo, Org. Lett., 2014, 16, 968 CrossRef CAS PubMed; (j) L. Y. Chan, X. Meng and S. Kim, J. Org. Chem., 2013, 78, 8826 CrossRef CAS PubMed; (k) T. Cheng, W. Yin, Y. Zhang, Y. Zhang and Y. Huang, Org. Biomol. Chem., 2014, 12, 1405 RSC; (l) Z. Ye, W. Wang, F. Luo, S. Zhang and J. Cheng, Org. Lett., 2009, 11, 3974 CrossRef CAS PubMed; (m) Z. Wang and C. Kuang, Adv. Synth. Catal., 2014, 356, 1549 CrossRef CAS; (n) K. Raghuvanshi, K. Rauch and L. Ackermann, Chem.–Eur. J., 2015, 21, 1790 CrossRef CAS PubMed; (o) Z. Yin, X. Jiang and P. Sun, J. Org. Chem., 2013, 78, 10002 CrossRef CAS PubMed; (p) Q. Jiang, J.-Y. Wang and C. Guo, J. Org. Chem., 2014, 79, 8768 CrossRef CAS PubMed; (q) L.-B. Zhang, X.-Q. Hao, S.-K. Zhang, Z.-J. Liu, X.-X. Zheng, J.-F. Gong, J.-L. Niu and M.-P. Song, Angew. Chem., Int. Ed., 2015, 54, 272 CrossRef CAS PubMed; (r) J. Roane and O. Daugulis, Org. Lett., 2013, 15, 5842 CrossRef CAS PubMed; (s) S. K. Alla, P. Sadhu and T. Punniyamurthy, J. Org. Chem., 2014, 79, 7502 CrossRef CAS PubMed.
- (a) H. Jiang, J. E. Taggart, X. Zhang, D. M. Benbrook, S. E. Lind and W.-Q. Ding, Cancer Lett., 2011, 312, 11 CrossRef CAS PubMed; (b) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC; (c) Y.-C. Liu, J.-H. Wei, Z.-F. Chen, M. Liu, Y.-Q. Gu, K.-B. Huang, Z.-Q. Li and H. Liang, Eur. J. Med. Chem., 2013, 69, 554 CrossRef CAS PubMed; (d) E. Pan, N. W. Oswald, A. G. Legako, J. M. Life, B. A. Posner and J. B. MacMillan, Chem. Sci., 2013, 4, 482 RSC; (e) D. K. Heidary, B. S. Howerton and E. C. Glazer, J. Med. Chem., 2014, 57, 8936 CrossRef CAS PubMed.
- (a) D. E. Stephens, M. Valdes, M. Dovalina, H. D. Arman and O. V. Larionov, Org. Biomol. Chem., 2014, 12, 6190 RSC; (b) J. C. Fennewald and B. H. Lipshutz, Green Chem., 2014, 16, 1097 RSC; (c) T. Nishida, H. Ida, Y. Kuninobu and M. Kanai, Nat. Commun., 2014, 5, 3387 Search PubMed; (d) T. Iwai and M. Sawamura, ACS Catal., 2015, 5, 5031 CrossRef CAS; (e) D. E. Stephens and O. V. Larionov, Tetrahedron, 2015, 71, 8683 CrossRef CAS PubMed; (f) M. Nagase, Y. Kuninobu and M. Kanai, J. Am. Chem. Soc., 2016, 138, 6103 CrossRef CAS PubMed.
- (a) X. Cong and M. Zeng, Org. Lett., 2014, 16, 3716 CrossRef CAS PubMed; (b) C. Whiteoak, O. Planas, A. Company and X. Ribas, Adv. Synth. Catal., 2016, 358, 1679 CrossRef CAS; (c) H. Chen, P. Li, M. Wang and L. Wang, Org. Lett., 2016, 18, 4794 CrossRef CAS PubMed; (d) A. M. Suess, M. Z. Ertem, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 9797 CrossRef CAS PubMed; (e) C. Xia, K. Wang, J. Xu, C. Shen, D. Sun, H. Li, G. Wang and P. Zhang, Org. Biomol. Chem., 2017, 15, 531 RSC; (f) H. Qiao, S. Sun, F. Yang, Y. Zhu, W. Zhu, Y. Dong, Y. Wu, X. Kong, L. Jiang and Y. Wu, Org. Lett., 2015, 17, 6086 CrossRef CAS PubMed; (g) J. Xu, C. Shen, X. Zhu, P. Zhang, M. J. Ajitha, K.-W. Huang, Z. An and X. Liu, Chem.–Asian J., 2016, 11, 882 CrossRef CAS PubMed; (h) J. Xu, X. L. Zhu, G. B. Zhou, B. B. Ying, P. P. Ye, L. Y. Su, C. Shen and P. Zhang, Org. Biomol. Chem., 2016, 14, 3016 RSC; (i) X. Zhu, L. Qiao, P. Ye, B. Ying, J. Xu, C. Shen and P. Zhang, RSC Adv., 2016, 6, 89979 RSC; (j) L.-K. Jin, G.-P. Lua and C. Cai, Org. Chem. Front., 2016, 3, 1309 RSC; (k) C. Shen, J. Xu, B. Ying and P. Zhang, ChemCatChem, 2016, 8, 3560 CrossRef CAS; (l) J. Chen, T. Wang, T. Wang, A. Lin, H. Yao and J. Xu, Org. Chem. Front., 2017, 4, 130 RSC; (m) H. Sahoo, M. K. Reddy, I. Ramakrishna and M. Baidya, Chem.–Eur. J., 2016, 22, 1592 CrossRef CAS PubMed; (n) Y. Dou, Z. Xie, Z. Sun, H. Fang, C. Shen, P. Zhang and Q. Zhu, ChemCatChem, 2016, 8, 3570 CrossRef CAS; (o) M. Sun, S. Sun, H. Qiao, F. Yang, Y. Zhu, J. Kang, Y. Wu and Y. Wu, Org. Chem. Front., 2016, 3, 1646 RSC; (p) H. Guo, M. Chen, P. Jiang, J. Chen, L. Pan, M. Wang, C. Xie and Y. Zhang, Tetrahedron, 2015, 71, 70 CrossRef CAS; (q) D. Ji, X. He, Y. Xu, Z. Xu, Y. Bian, W. Liu, Q. Zhu and Y. Xu, Org. Lett., 2016, 18, 4478 CrossRef CAS PubMed; (r) Z. Wu, Y. He, C. Ma, X. Zhou, X. Liu, Y. Li, T. Hu, P. Wen and G. Huang, Asian J. Org. Chem., 2016, 5, 724 CrossRef CAS; (s) Y. Wang, Y. Wang, Q. Zhang and D. Li, Org. Chem. Front., 2017, 4, 514 RSC; (t) J. Chen, T. Wang, Y. Liu, T. Wang, A. Lin and H. Yao, Org. Chem. Front., 2017, 4, 622 RSC; (u) J. Xu, L. Qiao, B. Ying, X. Zhu, C. Shen and P. Zhang, Org. Chem. Front., 2017, 4, 1116 RSC; (v) Y. He, N. Zhao, L. Qiu, X. Zhang and X. Fan, Org. Lett., 2016, 18, 6054 CrossRef CAS PubMed; (w) H. Sahoo, I. Ramakrishna and M. Baidya, ChemistrySelect, 2016, 1, 1949 CrossRef CAS.
- (a) Y. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411 CrossRef CAS PubMed; (b) Q. Lu, C. Liu, Z. Huang, Y. Ma, J. Zhang and A. Lei, Chem. Commun., 2014, 50, 14101 RSC; (c) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed; (d) K. Yang and Q. Song, Green Chem., 2016, 18, 932 RSC; (e) D. Ma, W. Chen, G. Hu, Y. Zhang, Y. Gao, Y. Yin and Y. Zhao, Green Chem., 2016, 18, 3522 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra, 13CNMR spectrum, GC/MS profile, HRMS profile. CCDC 1515409 and 1515410. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra09053f |
| This journal is © The Royal Society of Chemistry 2017 |