Open Access Article
Open Access ArticleSynthesis and unimolecular micellar behavior of amphiphilic star-shaped block copolymers obtained via the Passerini three component reaction†
S. Oelmann and
M. A. R. Meier *
*
Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry (IOC), Materialwissenschaftliches Zentrum MZE, Straße am Forum 7, 76131 Karlsruhe, Germany. E-mail: m.a.r.meier@kit.edu
First published on 21st September 2017
Abstract
A series of new amphiphilic star-shaped block copolymers with hydrophobic cores and hydrophilic shells was synthesized, using the Passerini three component step-growth polymerization (Passerini-3CP). The degree of polymerization of the Passerini hydrophobic cores (20, 10 and 5 repeating units) was controlled and the chain ends were quantitatively functionalized with different sized PEG-aldehydes and/or PEG-isocyanides via another Passerini reaction. The encapsulation and phase transfer properties of the star-shaped block copolymers were followed visually and by using UV/VIS-spectroscopy, using Orange II and Para Red dyes as guest molecules. The investigated polymers showed a unimolecular micellar behavior, as shown by dynamic light scattering (DLS) and the mentioned encapsulation experiments.
Unimolecular micelles show interesting properties for a wide range of applications, including catalysis or the encapsulation of low molecular weight organic guest molecules.1 These covalently connected core–shell architectures can be based on amphiphilic hyperbranched polymers, dendrimers or star-shaped block copolymers. Unimolecular micelles are characterized by an increased stability in solution, due to their covalently linked structure, thus preventing the usually observed dynamic equilibrium between the individual amphiphilic molecules and the micelle.2 The first described systems behaving like unimolecular micelles were based on dendrimers.3–5 However, dendrimers often require difficult multi-step organic syntheses. Polymer-based systems with core–shell architecture often show similar properties, but are easier to synthesize and thus constitute suitable alternatives. For example, amphiphilic star-shaped polymers having a defined number of arms and, accordingly, end groups, can be used in different applications, due to their behavior as unimolecular micelles.6–10 Most commonly, these amphiphilic star-shaped polymers are synthesized by atom transfer radical polymerization (ATRP) or other controlled/living polymerization techniques (e.g. nitroxide-mediated radical polymerization (NMP), reversible addition–fragmentation chain transfer (RAFT) or anionic polymerization).11–13 Additionally, ring-opening polymerization of lactides and lactones is often used to synthesize star-shaped polymers.14 In previous work, we have demonstrated that star-shaped block copolymers can be synthesized via the Passerini three component reaction (Passerini-3CR) in a step-growth process.15 The Passerini-3CR is an efficient reaction using a carboxylic acid, an oxo-component (ketone or aldehyde) and an isocyanide in a one-pot procedure under very mild conditions.16
We recently summarized the manifold and straightforward synthesis possibilities in polymer chemistry using the Passerini reaction, demonstrating a variety of application possibilities and achievable molecular architectures.17,18 By using bifunctional components (AA- + BB-type monomers or AB-type monomers), a polymerization process is induced and substituted polyesters, polyamides or poly(ester amide)s can be obtained.18–20
In the case of the star-shaped polymer, advantageously, this approach enables to easily tune the side chains of the polymer cores, thus adjusting its properties. Additionally, this step-growth process led to a control of the molecular weight with 100% end-group fidelity.
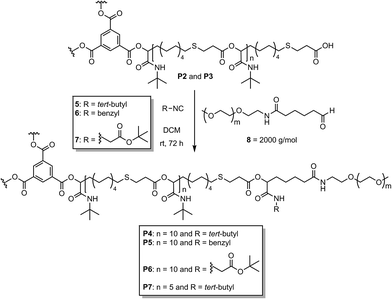
Following our previous study, this work describes the synthesis of well-defined amphiphilic star-shaped block copolymers using the Passerini-3CR and the subsequent encapsulation of two different dyes (Scheme 1). This approach is based on the use of a trifunctional carboxylic acid as an irreversible chain transfer agent (ICTA) in combination with the AB-type monomer 3. We have recently shown that by using an ICTA, an AB-type monomer (combination of aldehyde and carboxylic acid), and an excess of isocyanide, the molecular weights obtained in a Passerini-3CP are determined by the ratio of monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ICTA (in analogy to the monomer
ICTA (in analogy to the monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator ratio in living/controlled polymerizations). To investigate potential applications of these star-shaped homopolymers, we now focused on their functionalization with PEG chains, to produce amphiphilic polymers and to study their encapsulation behavior. The synthesis of AB-type monomer 3 and star-shaped homopolymer P1 has been described in our previous publication (Scheme 2), also demonstrating very high end-group fidelity, which is essential for this investigation.15 Additionally, two star-shaped homopolymers with shorter arm lengths (P2 and P3, 10 and 5 repeating units) were synthesized using ratios of 30
initiator ratio in living/controlled polymerizations). To investigate potential applications of these star-shaped homopolymers, we now focused on their functionalization with PEG chains, to produce amphiphilic polymers and to study their encapsulation behavior. The synthesis of AB-type monomer 3 and star-shaped homopolymer P1 has been described in our previous publication (Scheme 2), also demonstrating very high end-group fidelity, which is essential for this investigation.15 Additionally, two star-shaped homopolymers with shorter arm lengths (P2 and P3, 10 and 5 repeating units) were synthesized using ratios of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 15
1 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of [3]
1 of [3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4] (10
[4] (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 per arm respectively). The molecular weights (Mn) of the star-shaped polymers determined by 1H NMR end group integration were consistent with the expected molecular weights (Formula S1†) and confirmed a degree of polymerization of 10 and 5, respectively (Table S1†). The observed molecular weight distribution (Fig. S17†) was symmetric and confirmed full monomer conversion. The obtained dispersities (1.23–1.40) are lower than for a classic step-growth polymerization, indicating the presence of both step-growth and chain-growth mechanisms in this system, as expected. The star-shaped homopolymers P2 and P3 were subsequently functionalized with a water-soluble PEG-based (polyethylene glycol) shell to obtain water-soluble star-shaped block copolymers with the goal to achieve unimolecular micellar behavior. Two strategies based on the Passerini-3CR were used to produce linear or branched star-shaped block copolymers. For this purpose, the COOH end groups of the star-shaped polymers reacted with a PEG aldehyde and an isocyanide or with a PEG aldehyde and a PEG isocyanide in a Passerini-3CR to form a water-soluble shell with either one or two PEG chains per arm. In first tests, P2 was converted with a commercially available PEG-aldehyde 8 (2000 g mol−1) in a ratio of 1
1 per arm respectively). The molecular weights (Mn) of the star-shaped polymers determined by 1H NMR end group integration were consistent with the expected molecular weights (Formula S1†) and confirmed a degree of polymerization of 10 and 5, respectively (Table S1†). The observed molecular weight distribution (Fig. S17†) was symmetric and confirmed full monomer conversion. The obtained dispersities (1.23–1.40) are lower than for a classic step-growth polymerization, indicating the presence of both step-growth and chain-growth mechanisms in this system, as expected. The star-shaped homopolymers P2 and P3 were subsequently functionalized with a water-soluble PEG-based (polyethylene glycol) shell to obtain water-soluble star-shaped block copolymers with the goal to achieve unimolecular micellar behavior. Two strategies based on the Passerini-3CR were used to produce linear or branched star-shaped block copolymers. For this purpose, the COOH end groups of the star-shaped polymers reacted with a PEG aldehyde and an isocyanide or with a PEG aldehyde and a PEG isocyanide in a Passerini-3CR to form a water-soluble shell with either one or two PEG chains per arm. In first tests, P2 was converted with a commercially available PEG-aldehyde 8 (2000 g mol−1) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (per arm) and an excess of tert-butyl isocyanide 5, benzyl isocyanide 6 or tert-butyl-2-isocyanoacetate 7 (Scheme 3). After precipitation in diethyl ether, the functionalized polymers P4, P5 and P6 were obtained in good yields. Complete conversion of the end groups was confirmed by 1H NMR and SEC measurements (Fig. S10–S12 and S18†). Although the polymer end groups were fully functionalized, the water solubility of the polymers P4, P5 and P6 were rather poor and insufficient to obtain unimolecular micelles soluble in water. Thus, we used the smaller cores (P2 and P3) and did not continue the investigations with P1.
1 (per arm) and an excess of tert-butyl isocyanide 5, benzyl isocyanide 6 or tert-butyl-2-isocyanoacetate 7 (Scheme 3). After precipitation in diethyl ether, the functionalized polymers P4, P5 and P6 were obtained in good yields. Complete conversion of the end groups was confirmed by 1H NMR and SEC measurements (Fig. S10–S12 and S18†). Although the polymer end groups were fully functionalized, the water solubility of the polymers P4, P5 and P6 were rather poor and insufficient to obtain unimolecular micelles soluble in water. Thus, we used the smaller cores (P2 and P3) and did not continue the investigations with P1.
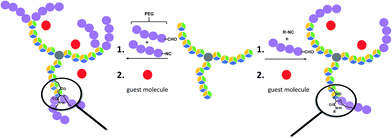 | ||
| Scheme 1 Schematic representation of the applied synthesis strategy to obtain the described different polymer architectures (including an indication of their potential for guest encapsulation). | ||
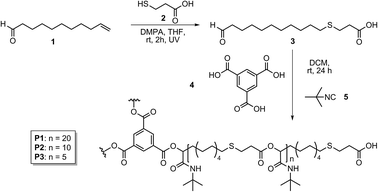 | ||
| Scheme 2 Synthesis of AB-type monomer 3 and subsequent polymerization to obtain homopolymers P1, P2 and P3 with different number of repeating units.15 | ||
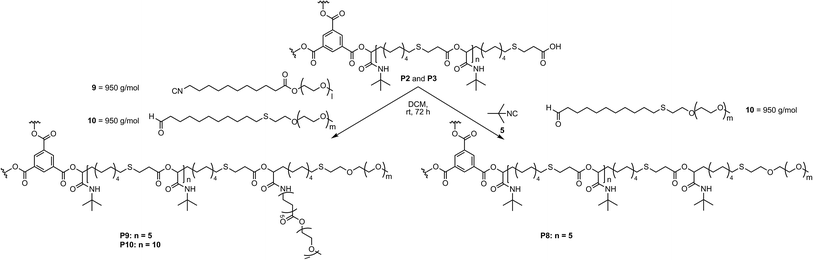
Therefore, P7 was synthesized using the smaller hydrophobic core P3 with tert-butyl isocyanide 5 and PEG aldehyde 8 in order to increase the hydrophilic fraction of the copolymers. Moreover, in order to investigate the influence of the molecular weight of the PEG chains as well as the effect of branching at the core–shell connection on the aggregation and encapsulation behavior of the star-shaped block copolymers in water, lower molecular weight functionalized PEGs were used. For the preparation of PEG isocyanide 9 (950 g mol−1), first, isocyanide 17 was prepared from amine 11, which was subsequently transformed to PEG isocyanide 9 via a transesterification with methoxy-PEG 18 (Scheme S1†).21 PEG aldehyde 10 (950 g mol−1) was synthesized by end group modification of PEG thiol 19 via a thiol–ene reaction with 10-undecenal 1 (Scheme S2†). Having the functionalized PEGs in hand, the star-shaped block copolymers P8, P9 and P10 were synthesized via the Passerini-3CR, using tert-butyl isocyanide 5 and PEG aldehyde 10, or PEG isocyanide 9 and PEG aldehyde 10 to obtain polymers with either one or two PEGs (950 g mol−1) and either 5 or 10 repeating units of the core (Scheme 4, compare also Scheme 1). Thus, a small library of five different amphiphilic star-shaped block copolymers was produced and further investigated, allowing the correlation of the different polymer architecture to the performance as unimolecular micelles. The full conversion of the end groups was determined by 1H NMR measurements (Fig. S14–S16†). Due to an increased hydrophilic ratio, polymers P7, P8, P9 and P10 showed improved water solubility. All following evaluations were performed only on these four water soluble polymers. Due to the differences in solubility of the two blocks in water, the formation of aggregates might be expected, contradicting a unimolecular micellar behavior. Nevertheless, dynamic light scattering (DLS) measurements confirmed unimolecularity for the four star-shaped block copolymers in a concentration range from 0.1 mg mL−1 and 1.0 mg mL−1 (Fig. S20–S23†). The measurements were carried out in water and dichloromethane (DCM). Two different preparation protocols, typical for the preparation of micellar solutions, were used for the analysis in water.22
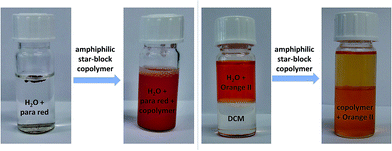
On the one hand, the sample was directly dissolved in water (without acetone: woA) and on the other hand, the sample was dissolved in 2–3 drops of acetone and then water was added dropwise (with acetone: wA). The values of all four polymers measured woA were slightly higher (13.5–21.0 nm) compared to the values wA (8.7–15.7 nm), which may indicate some aggregation in water. To confirm this and possibly break these aggregates, P7 wA and woA was ultrasonicated for 5 minutes each (P7 showed the largest size difference for the two preparation modes). Afterwards, all samples showed the same value (value of the sample wA: Fig. S24†), indeed indicating that small aggregates were present for the woA samples and also indicating that the wA samples indeed represent unimers that cannot be further dissociated. The values measured in DCM were slightly lower (4.2–6.5 nm), which can be attributed to a different swelling behavior of these macromolecules in an aqueous solution. Most interestingly, the guest encapsulation of the star-shaped block copolymers and a phase transfer phenomenum was observed and could be visualized in the following tests, also unambiguously confirming unimolecularity. First, amphiphilic star-shaped block copolymers were added to the water insoluble dye Para Red, rendering it water-soluble due to encapsulation (Fig. 1 and S26†). Polymers with shorter hydrophobic cores and higher hydrophilic parts were better soluble in water and exhibited the best encapsulation behavior of the dye.
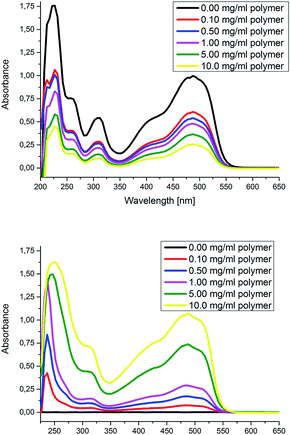
In a second test, the water soluble dye Orange II was added to a two-phase water/DCM system (Fig. 1 and S27†). After addition of the amphiphilic star-shaped block copolymers and shaking for a few seconds, most of the dye was transferred to the DCM phase. The color change from orange to yellow of Orange II is due to a change of the microenvironment of the dye and confirms that the polymers can encapsulate and phase transfer the investigated guest molecule. This results is a clear proof of the unimolecular behavior of the polymer, since a self-assembled micelle would dissociate in DCM, not being able to phase transfer the dye. Using these initial visual inspections, the best results for both tests were obtained for polymer P9, which shows the highest ratio of hydrophilic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydrophobic parts (Fig. 1). Therefore, we investigated the phase transfer behavior (water/DCM) of P9 in more detail by a UV/VIS titration experiment (the polymer was titrated to the biphasic mixture of dye and the solvents, Fig. 2). The absorption of the dye in both phases was separately measured and the concentration of P9 was increased after each measurement by addition of further aliquots. While the absorption of the dye in water decreased with increasing concentration of polymer P9, the absorption in DCM increased. This behavior can be explained by an encapsulation and phase transfer of Orange II from the water to the organic phase by the amphiphilic star-shaped block copolymer P9. After recording a calibration curve for Orange II in water (Fig. S19†), the concentrations in water could be determined as well as the number of encapsulated dye molecules per polymer (Table 1). An average up to 2.5 dye molecules per polymer was able to be encapsulated when 0.10 mg mL−1 of P9 was added, starting with 0.0225 mg mL−1 of dye in water. However, at this concentration, less than half of the introduced dye phase transfers to the DCM phase. A concentration of P9 of 10 mg mL−1 was required to encapsulate around 75% of the dye. Finally, the hydrodynamic diameter of polymer P9 with and without guest molecule was compared via DLS (Fig. S25†). The slight increase of around 25% and the absence of larger aggregates also indicate that the guest molecules are encapsulated in the polymers.
hydrophobic parts (Fig. 1). Therefore, we investigated the phase transfer behavior (water/DCM) of P9 in more detail by a UV/VIS titration experiment (the polymer was titrated to the biphasic mixture of dye and the solvents, Fig. 2). The absorption of the dye in both phases was separately measured and the concentration of P9 was increased after each measurement by addition of further aliquots. While the absorption of the dye in water decreased with increasing concentration of polymer P9, the absorption in DCM increased. This behavior can be explained by an encapsulation and phase transfer of Orange II from the water to the organic phase by the amphiphilic star-shaped block copolymer P9. After recording a calibration curve for Orange II in water (Fig. S19†), the concentrations in water could be determined as well as the number of encapsulated dye molecules per polymer (Table 1). An average up to 2.5 dye molecules per polymer was able to be encapsulated when 0.10 mg mL−1 of P9 was added, starting with 0.0225 mg mL−1 of dye in water. However, at this concentration, less than half of the introduced dye phase transfers to the DCM phase. A concentration of P9 of 10 mg mL−1 was required to encapsulate around 75% of the dye. Finally, the hydrodynamic diameter of polymer P9 with and without guest molecule was compared via DLS (Fig. S25†). The slight increase of around 25% and the absence of larger aggregates also indicate that the guest molecules are encapsulated in the polymers.
| Conc. polymer [mg mL−1] | Conc. dye in water [mg mL−1] | Conc. dye in DCM [mg mL−1] | Ratio polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dye in DCM dye in DCM |
|---|---|---|---|
| 0.00 | 0.0225 | 0.00 | — |
| 0.10 | 0.0144 | 0.0081 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50 2.50 |
| 0.50 | 0.0128 | 0.0097 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
| 1.00 | 0.0114 | 0.0111 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.38 0.38 |
| 5.00 | 0.0086 | 0.0139 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10 0.10 |
| 10.00 | 0.0061 | 0.0164 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 0.06 |
In conclusion, new unimolecular core–shell architectures were successfully synthesized via Passerini-3CR. Controlling the polymerization conditions, amphiphilic three-arm star-shaped block copolymers with Passerini derived cores and different PEG shells were obtained. The unimolecular behavior, the encapsulation of water-soluble and water-insoluble guest molecules as well as the transport abilities from water to an organic phase were demonstrated by UV/VIS spectroscopy and DLS measurements. Thus, these star-shaped block copolymers are multifunctional materials with a potential for applications in drug delivery systems or as phase-transfer catalysts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof. Podlech and his group for access to the UV/VIS equipment.Notes and references
- A. Heise, J. L. Hedrick, C. W. Frank and R. D. Miller, J. Am. Chem. Soc., 1999, 121, 8647 CrossRef CAS.
- G. Chen and Z. Guan, J. Am. Chem. Soc., 2004, 126, 2662 CrossRef CAS PubMed.
- M. E. Piotti, F. Rivera, R. Bond, C. J. Hawker and J. M. J. Fréchet, J. Am. Chem. Soc., 1999, 121, 9471 CrossRef CAS.
- S. Stevelmans, J. C. M. van Hest, J. F. G. A. Jansen, D. A. F. J. van Boxtel, E. M. M. de Brabander-van den Berg and E. W. Meijer, J. Am. Chem. Soc., 1996, 118, 7398 CrossRef CAS.
- J. F. G. Jansen, E. M. M. de Brabander-van den Berg and E. W. Meijer, Science, 1994, 266, 1226 CAS.
- O. G. Schramm, G. M. Pavlov, H. P. van Erp, M. A. R. Meier, R. Hoogenboom and U. S. Schubert, Macromolecules, 2009, 42, 1808 CrossRef CAS.
- M. A. R. Meier and U. S. Schubert, J. Comb. Chem., 2005, 7, 356 CrossRef CAS PubMed.
- M. A. R. Meier, J.-F. Gohy, C.-A. Fustin and U. S. Schubert, J. Am. Chem. Soc., 2004, 126, 11517 CrossRef CAS PubMed.
- D. Kul, L. M. van Rentergheim, M. A. R. Meier, S. Strandmann, H. Tenhu, S. S. Yilmaz, U. S. Schubert and F. E. Du Prez, J. Polym. Sci., Part A: Polym. Chem., 2007, 46, 650 CrossRef.
- M. A. R. Meier and U. S. Schubert, Chem. Commun., 2005, 4610 RSC.
- W. Yuan, J. Zhang, J. Wei, C. Zhang and J. Ren, Eur. Polym. J., 2011, 47, 949 CrossRef CAS.
- J.-L. Zhu, K. L. Liu, Z. Zhang, X.-Z. Zhang and J. Li, Chem. Commun., 2011, 47, 12849 RSC.
- A. Vazaios, D. J. Lohse and N. Hadjichristidis, Macromolecules, 2005, 38, 5468 CrossRef CAS.
- W.-P. Zhu, A. Nese and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1942 CrossRef CAS.
- S. Oelmann, S. C. Solleder and M. A. R. Meier, Polym. Chem., 2016, 7, 1857 RSC.
- M. Passerini and L. Simone, Gazz. Chim. Ital., 1921, 51, 126 CAS.
- A. Sehlinger and M. A. R. Meier, Adv. Polym. Sci., 2015, 269, 61 CrossRef CAS.
- A. Llevot, A. C. Boukis, S. Oelmann, K. Wetzel and M. A. R. Meier, Top. Curr. Chem., 2017, 375, 66 CrossRef PubMed.
- X.-X. Deng, L. Li, Z.-L. Li, A. Lv, F.-S. Du and Z.-C. Li, ACS Macro Lett., 2012, 1, 1300 CrossRef CAS.
- Y.-Z. Wang, X.-X. Deng, L. Li, Z.-L. Li, F.-S. Du and Z.-C. Li, Polym. Chem., 2013, 3, 444 RSC.
- S. C. Solleder, D. Zengel, K. S. Wetzel and M. A. R. Meier, Angew. Chem., Int. Ed., 2016, 55, 1204 CrossRef CAS PubMed.
- H. M. Aliabadi, S. Elhasi, A. Mahmud, R. Gulamhusein, P. Mahdipoor and A. Lavasanifar, Int. J. Pharm., 2017, 329, 158 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08982a |
| This journal is © The Royal Society of Chemistry 2017 |