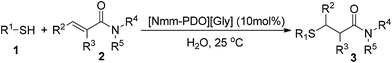Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thio-Michael addition of α,β-unsaturated amides catalyzed by Nmm-based ionic liquids†
Yawei Liu‡
a,
Zhenzhen Lai‡a,
Pengkun Yang‡a,
Yuanqing Xua,
Wenkai Zhang a,
Baoying Liua,
Minghua Lua,
Haibo Changa,
Tao Ding*a and
Hao Xu
a,
Baoying Liua,
Minghua Lua,
Haibo Changa,
Tao Ding*a and
Hao Xu *ab
*ab
aCollege of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, P. R. China. E-mail: xuhao@henu.edu.cn; dingtao@henu.edu.cn
bKey Laboratory of Natural Medicine and Immuno-Engineering, Henan University, Kaifeng 475004, P. R. China
First published on 5th September 2017
Abstract
A simple and practical thio-Michael addition of α,β-unsaturated amides catalyzed by Nmm-based ionic liquids with a 1,2-propanediol group has been developed. All the α,β-unsaturated amides without substituents at the carbon end could smoothly react with sulfur-nucleophiles in water. Meanwhile for thio-Michael addition of α,β-unsaturated amides with substituents at the carbon end, the relevant product could also be obtained successfully under solvent-free conditions at 55 °C. Furthermore, the IL-catalyst is recyclable and applicable for gram-scale synthesis.
Introduction
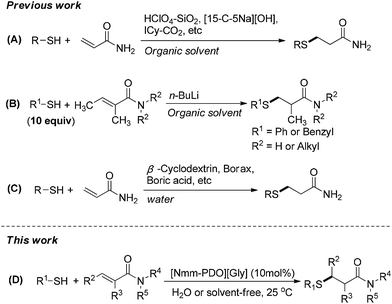
Ionic liquids (ILs) are acknowledged as eco-friendly alternatives to volatile organic solvents, because of their unique properties like negligible vapor pressure, high thermal stability, excellent dissolving capacity and ease of recyclability. In the past few years, many functional ILs have been also employed as catalysts for various reactions such as Michael addition,1 Knoevenagel condensation2 and Mannich reaction.3 Furthermore, well-designed ILs could significantly improve the reactivity and selectivity of reactions by modifying distinct cations and anions.4 Therefore, it is highly desirable to explore rationally-designed and readily available IL catalysts for different reactions.Thio-Michael reaction is one of the most important carbon–sulfur bond forming reactions, and plays a key role in the synthesis of many bioactive molecules.5 Traditionally, the reaction is performed in organic solvent under base-mediated or Lewis acid-mediated conditions.6 In recent years, some catalytic methods have been developed in the presence of catalysts including organic bases,7 Lewis or Brønsted acids,8 ionic liquids,9 enzymes10 and N-heterocyclic carbine.11 Although these methods above are efficient, the range of Michael acceptors was limited to relatively active substrates such as acrylonitrile, nitroalkenes, α,β-unsaturated ketones and esters. Several examples attempted to employ α,β-unsaturated amides as Michael acceptors (Scheme 1A and B),1c,8c,11,12 but only the acrylamide without substituents at carbon end could be transformed into the corresponding thio-Michael adduct easily because of less steric hindrance. Therefore, the systematical research on thio-Michael addition of α,β-unsaturated amides is very necessary.
“The best solvent is no solvent and if a solvent is needed it should preferably be water” stated by R. A. Sheldon.13 Thio-Michael addition in water is environmentally benign because water is cheap and safe. Recently, several examples conducted in water have been reported by employing catalysts including NiFeO4 nanoparticle,14 boric acid,15 borax16 and β-cyclodextrin (Scheme 1C).17 However, the poor tolerance for different α,β-unsaturated amides is still the main hurdle with thio-Michael addition in water, and only unsubstituted acrylamide was tolerated for its less steric hindrance. Furthermore, the reaction catalyzed by ionic liquid in water has not been found in the literature. In our previous work, a serial of novel ionic liquids based on N-methyl morpholine (Nmm) and 3-chloro-1,2-propanediol were synthesized and applied successfully in Knoevenagel condensation.2a In continuation of our endeavors to develop simple and practical methodology for organic synthesis,18 herein we report a thio-Michael addition of α,β-unsaturated amides catalyzed by Nmm-based ionic liquids in water (Scheme 1D), and most of the substrates could provide good yields in water. In addition, the catalysts are recyclable and applicable for the gram-scale synthesis.
Results and discussion
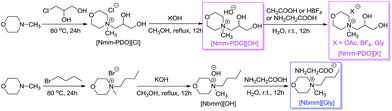
Five Nmm-based ionic liquids were synthesized by our previous methods (Scheme 2).2aInitially, the thio-Michael addition of propanethiol (1a) with N-phenylacrylamide (2c) was chosen as the model reaction to optimize conditions including catalysts, solvents and the amount of catalyst. As shown in Table 1, four Nmm-based IL catalysts were screened using water as the solvent at room temperature for 10 hours (Table 1, entries 1–4), and [Nmm-PDO][Gly] afforded the highest yield (entry 4). The reaction did not work in the absence of IL catalysts, even reaction time was extended to 24 h (entry 6). [Nbmm][Gly] was also used as the catalyst for comparison, but it was inferior to [Nmm-PDO][Gly] although the same glycine (Gly) anion existed in the two IL catalysts (compare entry 4 with entry 5); the results hinted the hydrogen bonding interaction between carbonyl group of N-phenylacrylamide (2c) and hydroxyl groups in the cation of [Nmm-PDO][Gly], which could increase the electrophilicity of the amide as Michael acceptor. The effect of solvents was also investigated (entries 4, 7, 8), and H2O was more suitable, perhaps because of its better dissolving ability for IL-catalysts. When the reaction was performed under solvent-free condition, an excellent yield could also be obtained (entry 9). Furthermore, the effect of catalyst loading was also investigated; when the amount of catalyst was decreased, the product (3n) could still be generated in moderate to good yield, but more reaction time was needed (entries 10, 11).
| Entry | Cat. | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: propanethiol (0.5 mmol, 45 μL), N-phenylacrylamide (0.5 mmol, 74 mg), catalyst (10 mol%), H2O (1 mL), room temperature.b Isolated yield.c Catalyst (5 mol%).d Catalyst (1 mol%). | ||||
| 1 | [Nmm-PDO][OH] | H2O | 10 | 95 |
| 2 | [Nmm-PDO][OAc] | H2O | 10 | 85 |
| 3 | [Nmm-PDO][BF4] | H2O | 10 | 65 |
| 4 | [Nmm-PDO][Gly] | H2O | 10 | 96 |
| 5 | [Nbmm][Gly] | H2O | 10 | 60 |
| 6 | Catalyst free | H2O | 24 | Trace |
| 7 | [Nmm-PDO][Gly] | CH2Cl2 | 10 | 83 |
| 8 | [Nmm-PDO][Gly] | CH3CN | 10 | 73 |
| 9 | [Nmm-PDO][Gly] | Neat | 10 | 90 |
| 10c | [Nmm-PDO][Gly] | H2O | 24 | 86 |
| 11d | [Nmm-PDO][Gly] | H2O | 48 | 52 |
With the optimized conditions in hand (10 mol% of [Nmm-PDO][OAc] as the catalyst, water as solvent, room temperature), the scope of thio-Michael addition of α,β-unsaturated amides catalyzed by Nmm-based ionic liquids in water was investigated; and most of the substrates provided good yields as shown in Table 2. For α,β-unsaturated amides, the steric hindrance had a great impact on their reactivity; the substrates with larger substituents showed lower reactivity (entries 1–5, 14–18). Especially for the substituted acrylamides with substituents at carbon end, only trace amount of products could be monitored by TLC; fortunately, the corresponding products could be obtained in good yields under solvent-free conditions at 55 °C (entries 26–30). Furthermore, substituted propynamide could also be transformed into relevant thio-Michael adduct in 42% yield under solvent-free conditions (entry 31), and the reaction is highly stereoselective for the Z-geometry. For the sulfur-nucleophile, the reactivity of thiols was also affected by the steric hindrance. Meanwhile the aromatic sulfur-nucleophile could react with α,β-unsaturated amides smoothly too (entry 6–9, 12–13, 19–21, 27), perhaps because the proton (H+) could be removed more easily than thiols although the steric hindrance of phenyl group was relatively larger. In addition, thiols with hydroxyl group or carbon–carbon double bond could be tolerated as well (entries 22, 23, 28).
| Entry | 1 | 2 | 3 | t/h | Yieldb/% |
|---|---|---|---|---|---|
| a Reaction conditions: sulfur-nucleophile 1 (0.5 mmol), α,β-unsaturated amides 2 (0.5 mmol), catalyst [Nmm-PDO][Gly] (10 mol%), water (1 mL), room temperature.b Isolated yield.c Solvent-free at 55 °C.d Sulfur-nucleophile 1a (1 mmol). | |||||
| 1 | 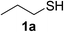 |
 |
 |
6 | 98 |
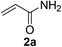
| 2 | 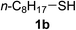 |
2a | 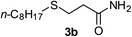 |
6 | 92 |
| 3 | 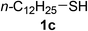 |
2a | 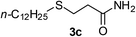 |
25 | 50(91c) |
| 4 | 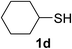 |
2a | 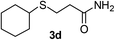 |
6 | 88 |
| 5 | 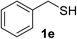 |
2a | 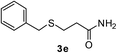 |
6 | 87 |
| 6 | 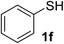 |
2a | 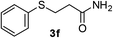 |
6 | 78 |
| 7 | 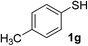 |
2a | 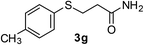 |
6 | 82 |
| 8 | 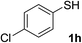 |
2a | 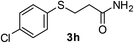 |
6 | 88 |
| 9 | 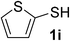 |
2a | 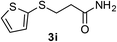 |
6 | 72 |
| 10 | 1a | 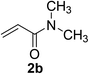 |
 |
6 | 90 |
| 11 | 1e | 2b | 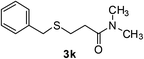 |
6 | 92 |
| 12 | 1f | 2b | 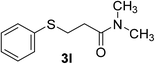 |
6 | 94 |
| 13 | 1g | 2b | 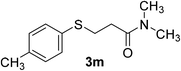 |
6 | 96 |
| 14 | 1a | 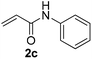 |
 |
10 | 96 |
| 15 | 1b | 2c | 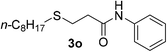 |
10 | 80 |
| 16 | 1c | 2c | 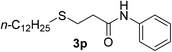 |
25 | 44(81c) |
| 17 | 1d | 2c | 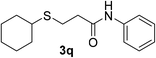 |
10 | 85 |
| 18 | 1e | 2c | 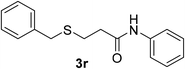 |
10 | 86 |
| 19 | 1f | 2c | 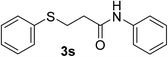 |
10 | 91 |
| 20 | 1g | 2c | 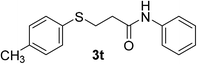 |
10 | 88 |
| 21 | 1h | 2c | 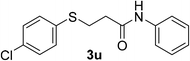 |
10 | 91 |
| 22 |  |
2c | 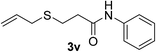 |
10 | 34(60c) |
| 23 |  |
2c | 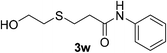 |
10 | 95 |
| 24 | 1a | 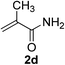 |
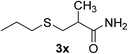 |
6 | 98 |
| 25 | 1f | 2d | 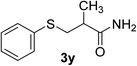 |
6 | 90 |
| 26 | 1a | 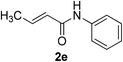 |
 |
10 | 78c |
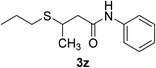
| 27 | 1f | 2e | 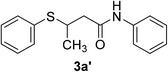 |
20 | 60c |
| 28 | 1j | 2e | 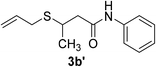 |
10 | 42c |
| 29 | 1a | 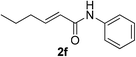 |
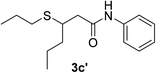 |
16 | 88c |
| 30 | 1a | 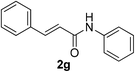 |
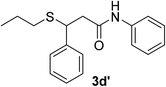 |
25 | 75c |
| 31 | 1a | 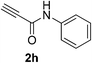 |
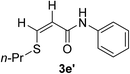 |
15 | 42 |
| 32 | 1a | 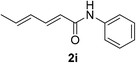 |
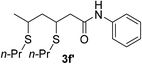 |
20 | 62d |
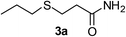
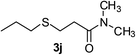
The reaction of propanethiol (1a) with N,N-dimethylacrylamide (2b) was chosen to examine the recyclability of IL-catalysts and applicability in the large-scale synthesis. The result showed that the catalyst still kept a high catalytic activity even in the third run (see Table S1 in the ESI†). Furthermore, the scaled-up experiment was performed on a gram-scale, and 2.90 g of 3j were prepared in 82% yield (2g of 2b was used); the result demonstrated that [Nmm-PDO][Gly] was also efficient catalyst for gram-scale synthesis.
A possible mechanism is proposed in Scheme 3 for the [Nmm-PDO][Gly] catalyzed thio-Michael addition (herein, reaction of propanethiol 1a with N,N-dimethylacrylamide 2b is chosen as the example). Firstly, the hydrogen-bonding interactions between the hydroxyl groups of [Nmm-PDO][Gly] and the carbonyl group of 2b can be formed, which making 2b more vulnerable by 1a. Then nucleophilic attack of thiol 1a on the β-carbon of N,N-dimethylacrylamide 2b lead to the intermediate A. Intermediate A take the proton from glycine (Gly), generating the thio-Michael product 3j.
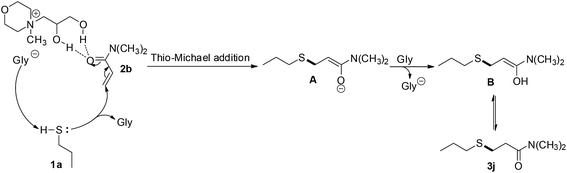 | ||
| Scheme 3 Possible mechanism for the thio-Michael addition catalyzed by [Nmm-PDO][Gly] between propanethiol (1a) and N,N-dimethylacrylamide (2b). | ||
Additionally, we compared the FTIR spectrum of the N,N-dimethylacrylamide with N,N-dimethylacrylamide-[Nmm-PDO][Gly] mixture, and the results were shown in Fig. 1. It was found that the absorption peak of carbonyl group in N,N-dimethylacrylamide was at 1648 cm−1, while it was red-shifted to 1568 cm−1 in the mixture; meanwhile we also compared the 13C NMR spectrum of N,N-dimethylacrylamide with N,N-dimethylacrylamide-[Nmm-PDO][Gly] mixture (Fig. S1 and S2 in the ESI†); the chemical shift of carbonyl group in N-phenylacrylamide was 165.23, while it was 165.47 in the mixture. All the results hinted the existence of the hydrogen bonding interactions between the carbonyl group of the N,N-dimethylacrylamide and the hydroxyl groups in [Nmm-PDO][Gly].
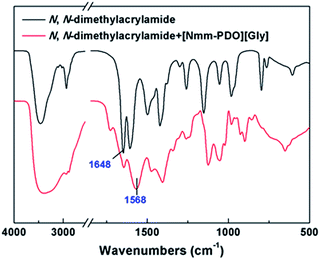 | ||
| Fig. 1 FTIR comparison of N,N-dimethylacrylamide with N,N-dimethylacryl-amide + [Nmm-PDO][Gly] mixture. | ||
Conclusion
Readily available and eco-friendly Nmm-based ILs are developed as the practical and recyclable catalysts for the thio-Michael addition of α,β-unsaturated amides. All the α,β-unsaturated amides without substituents at carbon end could smoothly react with sulfur-nucleophile in water, and only unsubstituted acrylamide was tolerated in water by the previous methods. Meanwhile for thio-Michael addition of α,β-unsaturated amides with substituents at carbon end, the relevant product could be obtained successfully under solvent-free condition at 55 °C, which was hard to be prepared via thio-Michael addition in the previous methods. Furthermore, the IL-catalyst is recyclable and applicable for the gram-scale synthesis. All the results above represent an efficient and eco-friendly IL-catalyst for the thio-Michael addition of α,β-unsaturated amides.General experimental procedures
The reaction is carried out at room temperature in water or solvent-free. NMR spectra were recorded on Bruker AVANCE III HD 400 MHz; proton and carbon magnetic resonance spectra (1H NMR and 13C NMR) were recorded using tetramethylsilane (TMS) in the solvent of CDCl3 as the internal standard (1H NMR: TMS at 0.00 ppm, CHCl3 at 7.26 ppm; 13C NMR: CDCl3 at 77.16 ppm) or were recorded using tetramethylsilane (TMS) in the solvent of DMSO-d6 as the internal standard (1H NMR: TMS at 0.00 ppm, DMSO at 2.50 ppm; 13C NMR: DMSO at 39.51 ppm).[Nmm-PDO][X] and [Nbmm][Gly]
Ionic liquids was synthesized by our previous method.2a[Nmm-PDO][Cl]
White solid; 1H NMR (D2O, 400 MHz, 25 °C): δ 4.44–4.22 (m, 1H), 4.03 (s, 4H), 3.72–3.46 (m, 8H), 3.30 (s, 3H). 13C NMR (D2O, 100 MHz, 25 °C): δ 66.4, 65.7, 63.7, 61.1, 60.9, 60.5, 48.4. ESI-MS [Nmm-PDO]+ 176.12.[Nmm-PDO][OH]
Colourless liquid; 1H NMR (D2O, 400 MHz, 25 °C): δ 4.43–4.23 (m, 1H), 4.04 (s, 4H), 3.73–3.46 (m, 8H), 3.30 (s, 3H). 13C NMR (D2O, 100 MHz, 25 °C) δ 66.5, 65.7, 63.7, 61.1, 60.7, 60.5, 48.4. ESI-MS [Nmm-PDO]+ 176.12.[Nmm-PDO][OAc]
Colourless liquid; 1H NMR (D2O, 400 MHz, 25 °C): δ 4.22–4.11 (m, 1H), 3.89 (s, 4H), 3.61–3.32 (m, 8H), 3.16 (s, 3H), 1.77 (s, 3H). 13C NMR (D2O, 100 MHz, 25 °C): δ 180.0, 66.4, 65.6, 63.6, 60.9, 60.6, 60.3, 48.3, 23.1. ESI-MS [Nmm-PDO]+ 176.12; ESI-MS [CH3COO]− m/z 59.06.[Nmm-PDO][BF4]
Colourless liquid; 1H NMR (D2O, 400 MHz, 25 °C): δ 4.47–4.32 (m, 1H), 4.12 (s, 4H), 3.81–3.51 (m, 8H), 3.37 (s, 3H). 13C NMR (D2O, 100 MHz, 25 °C) δ 66.6, 65.8, 63.8, 61.2, 60.7, 60.5, 48.3. ESI-MS [Nmm-PDO]+ 176.12; ESI-MS [BF4]− 87.03.[Nmm-PDO][Gly]
Colourless liquid; 1H NMR (D2O, 400 MHz, 25 °C): δ 4.30–4.20 (m, 1H), 3.97 (s, 4H), 3.66–3.39 (m, 8H), 3.23 (s, 3H), 1.81 (s, 2H). 13C NMR (D2O, 100 MHz, 25 °C) δ 181.1, 66.4, 65.6, 63.6, 61.0, 60.6, 60.4, 48.3, 23.4. ESI-HRMS [Nmm-PDO]+ m/z calcd for C8H18NO3 176.1281, found 176.1284; ESI-MS [Nmm-PDO]+ 176.12; ESI-MS [NH2CH2COO]− m/z 74.01.[Nbmm][Gly]
Colourless liquid; 1H NMR (D2O, 300 MHz, 25 °C): δ 3.95 (s, 4H), 3.50–3.31 (m, 6H), 3.10 (s, 3H), 1.82 (s, 2H), 1.76–1.61 (m, 2H), 1.41–1.24 (m, 2H), 0.88 (t, 3H). 13C NMR (D2O, 100 MHz, 25 °C) δ 180.5, 65.1, 60.4, 59.5, 46.7, 44.6, 23.6, 22.9, 19.1, 12.9. ESI-MS [Nbmm]+ m/z 158.25; ESI-MS [NH2CH2COO]− m/z 74.01.General procedure for synthesis of compounds 3a–w
Sulfur-nucleophile (0.5 mmol), α,β-unsaturated amides (0.5 mmol), IL catalyst (10 mol%) and water (1 mL) was added into 25 mL Schlenk tube and stirred at room temperature. Upon completion of the reaction (monitored by TLC), the reaction mixture was extracted with ethyl acetate (3 × 5 mL), the organic and aqueous layers were separated, and the aqueous layer was extracted with EtOAc (2 × 5 mL). The combined organic layers were dried over anhydrous Na2SO4, concentrated and purified by column chromatography on silica gel employing petroleum ether/ethyl acetate as eluent to afford target product 3 [the products of 3z–3d′, 3f′ were prepared at 55 °C under solvent-free condition; upon completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 × 5 mL). The combined organic layers were dried over anhydrous Na2SO4, they were purified by column chromatography on silica gel using petroleum ether/ethyl acetate as the eluent].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 72 mg (98%). White solid, mp 62–63 °C (lit.19 mp 59–61 °C). 1H NMR (d6-DMSO, 400 MHz, 25 °C) δ 7.33 (s, 1H), 6.83 (s, 1H), 2.64 (t, J = 7.4 Hz, 2H), 2.47 (t, J = 7.2 Hz, 2H), 2.31 (t, J = 7.4 Hz, 2H), 1.59–1.47 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 173.0, 36.1, 33.5, 27.3, 22.9, 13.7. ESI-MS [M + H]+ m/z 148.25; [M + Na]+ 170.19.
1). Yield 72 mg (98%). White solid, mp 62–63 °C (lit.19 mp 59–61 °C). 1H NMR (d6-DMSO, 400 MHz, 25 °C) δ 7.33 (s, 1H), 6.83 (s, 1H), 2.64 (t, J = 7.4 Hz, 2H), 2.47 (t, J = 7.2 Hz, 2H), 2.31 (t, J = 7.4 Hz, 2H), 1.59–1.47 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 173.0, 36.1, 33.5, 27.3, 22.9, 13.7. ESI-MS [M + H]+ m/z 148.25; [M + Na]+ 170.19.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 100 mg (92%). White solid, mp 90–91 °C (lit.19 mp 92–93 °C). 1H NMR (CDCl3, 400 MHz, 25 °C) δ 5.79 (s, 2H), 2.81 (t, J = 7.2 Hz, 2H), 2.60–2.45 (m, 4H), 1.64–1.53 (m, 2H), 1.42–1.23 (m, 10H), 0.88 (t, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.8, 36.0, 32.4, 31.8, 29.6, 29.2, 28.9, 27.5, 22.7, 14.1. ESI-MS [M + H]+ m/z 218.28; [M + Na]+ 240.29.
1). Yield 100 mg (92%). White solid, mp 90–91 °C (lit.19 mp 92–93 °C). 1H NMR (CDCl3, 400 MHz, 25 °C) δ 5.79 (s, 2H), 2.81 (t, J = 7.2 Hz, 2H), 2.60–2.45 (m, 4H), 1.64–1.53 (m, 2H), 1.42–1.23 (m, 10H), 0.88 (t, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.8, 36.0, 32.4, 31.8, 29.6, 29.2, 28.9, 27.5, 22.7, 14.1. ESI-MS [M + H]+ m/z 218.28; [M + Na]+ 240.29.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 68 mg (50%), solvent-free condition yield 124 mg (91%). White solid, mp 84–86 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 5.60 (d, J = 94.4 Hz, 2H), 2.81 (t, J = 7.2 Hz, 2H), 2.53 (m, J = 14.2, 7.3 Hz, 4H), 1.67–1.53 (m, 5H), 1.41–1.19 (m, 19H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 173.1, 36.0, 31.8, 31.5, 29.6, 29.5, 29.2, 29.1, 28.7, 27.4, 22.6. ESI-HRMS [M + H]+ m/z calcd for C15H32NOS 274.2199, found 274.2210.
1). Yield 68 mg (50%), solvent-free condition yield 124 mg (91%). White solid, mp 84–86 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 5.60 (d, J = 94.4 Hz, 2H), 2.81 (t, J = 7.2 Hz, 2H), 2.53 (m, J = 14.2, 7.3 Hz, 4H), 1.67–1.53 (m, 5H), 1.41–1.19 (m, 19H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 173.1, 36.0, 31.8, 31.5, 29.6, 29.5, 29.2, 29.1, 28.7, 27.4, 22.6. ESI-HRMS [M + H]+ m/z calcd for C15H32NOS 274.2199, found 274.2210.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 82 mg (88%). White solid, mp 74–75 °C. 1H NMR (d6-DMSO, 400 MHz, 25 °C) δ 7.35 (s, 1H), 6.85 (s, 1H), 2.66 (t, J = 7.4 Hz, 3H), 2.30 (t, J = 7.4 Hz, 2H), 1.89 (t, J = 10.2, 4.9 Hz, 2H), 1.76–1.62 (m, 2H), 1.62–1.50 (m, 1H), 1.24 (m, J = 14.3, 13.3, 7.1 Hz, 5H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 173.1, 43.0, 36.4, 33.7, 25.9, 25.7. ESI-MS [M + H]+ m/z 188.12; [M + Na]+ 210.11.
1). Yield 82 mg (88%). White solid, mp 74–75 °C. 1H NMR (d6-DMSO, 400 MHz, 25 °C) δ 7.35 (s, 1H), 6.85 (s, 1H), 2.66 (t, J = 7.4 Hz, 3H), 2.30 (t, J = 7.4 Hz, 2H), 1.89 (t, J = 10.2, 4.9 Hz, 2H), 1.76–1.62 (m, 2H), 1.62–1.50 (m, 1H), 1.24 (m, J = 14.3, 13.3, 7.1 Hz, 5H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 173.1, 43.0, 36.4, 33.7, 25.9, 25.7. ESI-MS [M + H]+ m/z 188.12; [M + Na]+ 210.11.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 85 mg (87%). White solid, mp 110–111 °C(lit.19 mp 104–105 °C). 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.35–7.22 (m, 5H), 5.73 (d, J = 69.6 Hz, 2H), 3.74 (s, 2H), 2.72 (t, J = 7.2 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.5, 138.2, 128.9, 128.6, 127.2, 36.7, 35.7, 27.0. ESI-MS [M + H]+ m/z 196.14; [M + Na]+ 218.12.
1). Yield 85 mg (87%). White solid, mp 110–111 °C(lit.19 mp 104–105 °C). 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.35–7.22 (m, 5H), 5.73 (d, J = 69.6 Hz, 2H), 3.74 (s, 2H), 2.72 (t, J = 7.2 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.5, 138.2, 128.9, 128.6, 127.2, 36.7, 35.7, 27.0. ESI-MS [M + H]+ m/z 196.14; [M + Na]+ 218.12.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 71 mg (78%). White solid, mp 116–118 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.40–7.34 (m, 2H), 7.33–7.27 (m, 2H), 7.24–7.18 (m, 1H), 5.71 (d, J = 68.2 Hz, 2H), 3.20 (t, J = 7.3 Hz, 2H), 2.52 (t, J = 7.3 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.3, 135.2, 129.8, 129.1, 126.5, 35.3, 29.1. ESI-MS [M + H]+ m/z 182.18; [M + Na]+ 204.15.
1). Yield 71 mg (78%). White solid, mp 116–118 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.40–7.34 (m, 2H), 7.33–7.27 (m, 2H), 7.24–7.18 (m, 1H), 5.71 (d, J = 68.2 Hz, 2H), 3.20 (t, J = 7.3 Hz, 2H), 2.52 (t, J = 7.3 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.3, 135.2, 129.8, 129.1, 126.5, 35.3, 29.1. ESI-MS [M + H]+ m/z 182.18; [M + Na]+ 204.15.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 80 mg (82%). White solid, mp 111–113 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.28 (d, 2H), 7.12 (d, J = 7.9 Hz, 2H), 5.69 (d, J = 53.8 Hz, 2H), 3.15 (t, J = 7.3 Hz, 2H), 2.49 (t, J = 7.3 Hz, 2H), 2.33 (s, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.38, 136.84, 131.30, 130.68, 129.87, 35.39, 29.89, 21.05. ESI-MS [M + H]+ m/z 196.14; [M + Na]+ 218.12.
1). Yield 80 mg (82%). White solid, mp 111–113 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.28 (d, 2H), 7.12 (d, J = 7.9 Hz, 2H), 5.69 (d, J = 53.8 Hz, 2H), 3.15 (t, J = 7.3 Hz, 2H), 2.49 (t, J = 7.3 Hz, 2H), 2.33 (s, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.38, 136.84, 131.30, 130.68, 129.87, 35.39, 29.89, 21.05. ESI-MS [M + H]+ m/z 196.14; [M + Na]+ 218.12.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 95 mg (88%). White solid, mp 116–117 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.34–7.23 (m, 4H), 5.63 (d, J = 60.2 Hz, 2H), 3.19 (t, J = 7.3 Hz, 2H), 2.51 (t, J = 7.3 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 172.9, 133.9, 132.5, 131.1, 129.2, 35.2, 29.3. ESI-MS [M + H]+ m/z 216.11; [M + Na]+ 238.09.
1). Yield 95 mg (88%). White solid, mp 116–117 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.34–7.23 (m, 4H), 5.63 (d, J = 60.2 Hz, 2H), 3.19 (t, J = 7.3 Hz, 2H), 2.51 (t, J = 7.3 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 172.9, 133.9, 132.5, 131.1, 129.2, 35.2, 29.3. ESI-MS [M + H]+ m/z 216.11; [M + Na]+ 238.09.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 67 mg (72%). Yellow solid, mp 88–90 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.30 (d, J = 5.4, 1.3 Hz, 1H), 7.08 (d, J = 3.5, 1.3 Hz, 1H), 6.92 (t, J = 5.4, 3.5 Hz, 1H), 5.75 (d, J = 120.5 Hz, 2H), 2.98 (t, J = 7.2 Hz, 2H), 2.44 (t, J = 7.2 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.3, 134.4, 133.6, 129.8, 127.7, 35.49, 33.98. ESI-HRMS [M + H]+ m/z calcd for C7H10NOS2 188.0198, found 188.0210.
1). Yield 67 mg (72%). Yellow solid, mp 88–90 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.30 (d, J = 5.4, 1.3 Hz, 1H), 7.08 (d, J = 3.5, 1.3 Hz, 1H), 6.92 (t, J = 5.4, 3.5 Hz, 1H), 5.75 (d, J = 120.5 Hz, 2H), 2.98 (t, J = 7.2 Hz, 2H), 2.44 (t, J = 7.2 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 173.3, 134.4, 133.6, 129.8, 127.7, 35.49, 33.98. ESI-HRMS [M + H]+ m/z calcd for C7H10NOS2 188.0198, found 188.0210.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
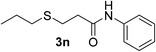
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 79 mg (90%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 2.79 (s, 3H), 2.70 (s, 3H), 2.55 (t, 2H), 2.36 (t, J = 7.6 Hz, 2H), 2.27 (t, J = 7.3, 1.5 Hz, 2H), 1.43–1.30 (m, 2H), 0.73 (t, J = 7.4, 1.4 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 171.0, 36.9, 35.1, 34.2, 33.6, 27.1, 22.7, 13.2. ESI-HRMS [M + H]+ m/z calcd for C8H18NOS 176.1104, found 176.1115.
1). Yield 79 mg (90%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 2.79 (s, 3H), 2.70 (s, 3H), 2.55 (t, 2H), 2.36 (t, J = 7.6 Hz, 2H), 2.27 (t, J = 7.3, 1.5 Hz, 2H), 1.43–1.30 (m, 2H), 0.73 (t, J = 7.4, 1.4 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 171.0, 36.9, 35.1, 34.2, 33.6, 27.1, 22.7, 13.2. ESI-HRMS [M + H]+ m/z calcd for C8H18NOS 176.1104, found 176.1115.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 103 mg (92%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.34–7.26 (m, 4H), 7.24–7.19 (m, 1H), 3.73 (s, 2H), 2.89 (d, J = 8.4 Hz, 6H), 2.74 (t, J = 8.2, 6.9 Hz, 2H), 2.48 (t, J = 7.5 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 171.1, 138.6, 128.8, 128.5, 127.0, 36.9, 35.4, 33.5, 27.0. ESI-HRMS [M + H]+ m/z calcd for C12H18NOS 224.1104, found 224.1109.
1). Yield 103 mg (92%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.34–7.26 (m, 4H), 7.24–7.19 (m, 1H), 3.73 (s, 2H), 2.89 (d, J = 8.4 Hz, 6H), 2.74 (t, J = 8.2, 6.9 Hz, 2H), 2.48 (t, J = 7.5 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 171.1, 138.6, 128.8, 128.5, 127.0, 36.9, 35.4, 33.5, 27.0. ESI-HRMS [M + H]+ m/z calcd for C12H18NOS 224.1104, found 224.1109.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 98 mg (94%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.26 (d, J = 7.4 Hz, 2H), 7.19 (t, J = 7.7 Hz, 2H), 7.08 (t, J = 7.3 Hz, 1H), 3.15 (t, J = 7.5 Hz, 2H), 2.83 (d, J = 6.8 Hz, 6H), 2.54 (t, J = 7.5 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 170.9, 136.0, 129.1, 129.0, 126.0, 37.0, 35.4, 33.1, 28.9. ESI-HRMS [M + H]+ m/z calcd for C11H16NOS 210.0947, found 210.0947.
1). Yield 98 mg (94%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.26 (d, J = 7.4 Hz, 2H), 7.19 (t, J = 7.7 Hz, 2H), 7.08 (t, J = 7.3 Hz, 1H), 3.15 (t, J = 7.5 Hz, 2H), 2.83 (d, J = 6.8 Hz, 6H), 2.54 (t, J = 7.5 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 170.9, 136.0, 129.1, 129.0, 126.0, 37.0, 35.4, 33.1, 28.9. ESI-HRMS [M + H]+ m/z calcd for C11H16NOS 210.0947, found 210.0947.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 107 mg (96%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.18 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 7.9 Hz, 2H), 3.14–3.05 (t, 2H), 2.83 (d, J = 5.2 Hz, 6H), 2.51 (t, J = 7.5 Hz, 2H), 2.22 (s, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 171.0, 136.2, 132.1, 130.0, 129.7, 37.0, 35.4, 33.3, 29.6, 21.0. ESI-HRMS [M + H]+ m/z calcd for C12H18NOS 224.1104, found 224.1109.
1). Yield 107 mg (96%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.18 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 7.9 Hz, 2H), 3.14–3.05 (t, 2H), 2.83 (d, J = 5.2 Hz, 6H), 2.51 (t, J = 7.5 Hz, 2H), 2.22 (s, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 171.0, 136.2, 132.1, 130.0, 129.7, 37.0, 35.4, 33.3, 29.6, 21.0. ESI-HRMS [M + H]+ m/z calcd for C12H18NOS 224.1104, found 224.1109.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 107 mg (96%). White solid, mp 42–44 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 8.53 (s, 1H), 7.43 (d, J = 7.5 Hz, 2H), 7.17 (t, J = 7.9 Hz, 2H), 6.98 (t, J = 7.4 Hz, 1H), 2.74 (t, J = 7.2 Hz, 2H), 2.53 (t, J = 7.3 Hz, 2H), 2.38 (t, J = 7.3 Hz, 2H), 1.55–1.40 (m, 2H), 0.85 (t, J = 7.4 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 170.4, 138.0, 128.9, 124.4, 120.4, 37.6, 34.4, 27.7, 22.9, 13.5. ESI-HRMS [M + H]+ m/z calcd for C12H18NOS 224.1104, found 224.1088.
1). Yield 107 mg (96%). White solid, mp 42–44 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 8.53 (s, 1H), 7.43 (d, J = 7.5 Hz, 2H), 7.17 (t, J = 7.9 Hz, 2H), 6.98 (t, J = 7.4 Hz, 1H), 2.74 (t, J = 7.2 Hz, 2H), 2.53 (t, J = 7.3 Hz, 2H), 2.38 (t, J = 7.3 Hz, 2H), 1.55–1.40 (m, 2H), 0.85 (t, J = 7.4 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 170.4, 138.0, 128.9, 124.4, 120.4, 37.6, 34.4, 27.7, 22.9, 13.5. ESI-HRMS [M + H]+ m/z calcd for C12H18NOS 224.1104, found 224.1088.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 117 mg (80%). White solid, mp 57–58 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.76 (s, 1H), 7.52 (d, 2H), 7.31 (t, J = 7.9 Hz, 2H), 7.10 (t, J = 7.4 Hz, 1H), 2.89 (t, J = 7.0 Hz, 2H), 2.63 (t, J = 7.0 Hz, 2H), 2.56 (t, J = 7.4 Hz, 2H), 1.64–1.54 (m, 2H), 1.41–1.20 (m, 10H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.7, 137.8, 129.0, 124.4, 119.9, 37.8, 32.5, 31.8, 29.6, 29.2, 28.9, 27.7, 22.7, 14.1. ESI-HRMS [M + H]+ m/z calcd for C17H28NOS 294.1886, found 294.1890.
1). Yield 117 mg (80%). White solid, mp 57–58 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.76 (s, 1H), 7.52 (d, 2H), 7.31 (t, J = 7.9 Hz, 2H), 7.10 (t, J = 7.4 Hz, 1H), 2.89 (t, J = 7.0 Hz, 2H), 2.63 (t, J = 7.0 Hz, 2H), 2.56 (t, J = 7.4 Hz, 2H), 1.64–1.54 (m, 2H), 1.41–1.20 (m, 10H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.7, 137.8, 129.0, 124.4, 119.9, 37.8, 32.5, 31.8, 29.6, 29.2, 28.9, 27.7, 22.7, 14.1. ESI-HRMS [M + H]+ m/z calcd for C17H28NOS 294.1886, found 294.1890.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 77 mg (44%), solvent-free condition yield 142 mg (81%). White solid, mp 75–77 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.66–7.42 (m, 3H), 7.33 (t, J = 7.9 Hz, 2H), 7.11 (t, J = 7.4 Hz, 1H), 2.90 (t, J = 7.0 Hz, 2H), 2.64 (t, J = 7.0 Hz, 2H), 2.57 (t, J = 7.4 Hz, 2H), 1.65–1.56 (m, 2H), 1.46–1.16 (m, 18H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.8, 169.6, 137.8, 129.0, 124.4, 120.0, 119.9, 50.9, 37.8, 37.7, 32.5, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.9, 27.7, 22.7, 14.2. ESI-HRMS [M + H]+ m/z calcd for C21H36NOS 350.2512, found 350.2517.
1). Yield 77 mg (44%), solvent-free condition yield 142 mg (81%). White solid, mp 75–77 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.66–7.42 (m, 3H), 7.33 (t, J = 7.9 Hz, 2H), 7.11 (t, J = 7.4 Hz, 1H), 2.90 (t, J = 7.0 Hz, 2H), 2.64 (t, J = 7.0 Hz, 2H), 2.57 (t, J = 7.4 Hz, 2H), 1.65–1.56 (m, 2H), 1.46–1.16 (m, 18H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.8, 169.6, 137.8, 129.0, 124.4, 120.0, 119.9, 50.9, 37.8, 37.7, 32.5, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.9, 27.7, 22.7, 14.2. ESI-HRMS [M + H]+ m/z calcd for C21H36NOS 350.2512, found 350.2517.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 112 mg (85%). White solid, mp 85–86 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.78 (s, 1H), 7.52 (d, 2H), 7.31 (t, J = 7.9 Hz, 2H), 7.11 (t, 1H), 2.91 (t, J = 7.1 Hz, 2H), 2.75–2.66 (m, 1H), 2.62 (t, J = 7.1 Hz, 2H), 2.03–1.94 (m, 2H), 1.80–1.73 (m, 2H), 1.65–1.57 (m, 1H), 1.38–1.22 (m, 5H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.7, 137.8, 129.0, 124.4, 119.9, 44.0, 38.0, 33.6, 26.1, 25.8, 25.7. ESI-HRMS [M + H]+ m/z calcd for C15H22NOS 264.1417, found 264.1416.
1). Yield 112 mg (85%). White solid, mp 85–86 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.78 (s, 1H), 7.52 (d, 2H), 7.31 (t, J = 7.9 Hz, 2H), 7.11 (t, 1H), 2.91 (t, J = 7.1 Hz, 2H), 2.75–2.66 (m, 1H), 2.62 (t, J = 7.1 Hz, 2H), 2.03–1.94 (m, 2H), 1.80–1.73 (m, 2H), 1.65–1.57 (m, 1H), 1.38–1.22 (m, 5H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.7, 137.8, 129.0, 124.4, 119.9, 44.0, 38.0, 33.6, 26.1, 25.8, 25.7. ESI-HRMS [M + H]+ m/z calcd for C15H22NOS 264.1417, found 264.1416.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 117 mg (86%). White solid, mp 80–82 °C. 1H NMR (d6-DMSO, 400 MHz, 25 °C) δ 9.98 (s, 1H), 7.59 (d, J = 8.0 Hz, 2H), 7.35–7.22 (m, 7H), 7.03 (t, J = 7.4 Hz, 1H), 3.78 (s, 2H), 2.66 (t, J = 6.3 Hz, 2H), 2.61 (t, J = 6.2 Hz, 2H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 167.0, 139.6, 139.0, 129.3, 129.2, 128.9, 127.3, 123.6, 119.5, 36.8, 35.4, 26.9. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 272.1104, found 272.1100.
1). Yield 117 mg (86%). White solid, mp 80–82 °C. 1H NMR (d6-DMSO, 400 MHz, 25 °C) δ 9.98 (s, 1H), 7.59 (d, J = 8.0 Hz, 2H), 7.35–7.22 (m, 7H), 7.03 (t, J = 7.4 Hz, 1H), 3.78 (s, 2H), 2.66 (t, J = 6.3 Hz, 2H), 2.61 (t, J = 6.2 Hz, 2H). 13C NMR (d6-DMSO, 100 MHz, 25 °C) δ 167.0, 139.6, 139.0, 129.3, 129.2, 128.9, 127.3, 123.6, 119.5, 36.8, 35.4, 26.9. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 272.1104, found 272.1100.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 117 mg (91%). White solid, mp 84–85 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.48 (d, 2H), 7.42 (s, 1H), 7.38 (d, J = 7.4 Hz, 2H), 7.34–7.27 (m, 4H), 7.22 (t, 1H), 7.10 (t, J = 7.4 Hz, 1H), 3.28 (t, J = 7.1 Hz, 2H), 2.64 (t, J = 7.2 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.4, 137.7, 135.2, 129.7, 129.2, 129.0, 126.6, 124.5, 120.1, 37.1, 29.3. ESI-MS [M + H]+ m/z 258.23; [M + Na]+ 280.21.
1). Yield 117 mg (91%). White solid, mp 84–85 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.48 (d, 2H), 7.42 (s, 1H), 7.38 (d, J = 7.4 Hz, 2H), 7.34–7.27 (m, 4H), 7.22 (t, 1H), 7.10 (t, J = 7.4 Hz, 1H), 3.28 (t, J = 7.1 Hz, 2H), 2.64 (t, J = 7.2 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.4, 137.7, 135.2, 129.7, 129.2, 129.0, 126.6, 124.5, 120.1, 37.1, 29.3. ESI-MS [M + H]+ m/z 258.23; [M + Na]+ 280.21.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 119 mg (88%). White solid, mp 94–96 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.64–7.41 (m, 3H), 7.34–7.25 (m, 4H), 7.10 (t, J = 7.9 Hz, 3H), 3.22 (t, J = 7.2 Hz, 2H), 2.59 (t, J = 7.1 Hz, 2H), 2.31 (s, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.5, 137.7, 136.9, 131.3, 130.7, 129.9, 129.0, 124.4, 120.1, 37.2, 30.1, 21.1. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 272.1104, found 272.1100.
1). Yield 119 mg (88%). White solid, mp 94–96 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.64–7.41 (m, 3H), 7.34–7.25 (m, 4H), 7.10 (t, J = 7.9 Hz, 3H), 3.22 (t, J = 7.2 Hz, 2H), 2.59 (t, J = 7.1 Hz, 2H), 2.31 (s, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.5, 137.7, 136.9, 131.3, 130.7, 129.9, 129.0, 124.4, 120.1, 37.2, 30.1, 21.1. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 272.1104, found 272.1100.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 133 mg (91%). White solid, mp 91–93 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.53–7.42 (m, 2H), 7.38–7.20 (m, 8H), 7.12 (t, J = 7.4 Hz, 1H), 3.28 (t, J = 7.1 Hz, 2H), 2.64 (t, J = 7.1 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 168.8, 137.5, 133.7, 132.6, 131.1, 129.3, 129.1, 124.6, 119.9, 37.1, 29.6. ESI-HRMS [M + H]+ m/z calcd for C15H15ClNOS 292.0557, found 292.0554.
1). Yield 133 mg (91%). White solid, mp 91–93 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.53–7.42 (m, 2H), 7.38–7.20 (m, 8H), 7.12 (t, J = 7.4 Hz, 1H), 3.28 (t, J = 7.1 Hz, 2H), 2.64 (t, J = 7.1 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 168.8, 137.5, 133.7, 132.6, 131.1, 129.3, 129.1, 124.6, 119.9, 37.1, 29.6. ESI-HRMS [M + H]+ m/z calcd for C15H15ClNOS 292.0557, found 292.0554.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 38 mg (34%), solvent-free condition yield 66 mg (60%). White solid, mp 38–40 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.91 (s, 1H), 7.44 (d, J = 7.9 Hz, 2H), 7.22 (t, J = 7.8 Hz, 2H), 7.02 (t, J = 7.4 Hz, 1H), 5.79–5.61 (m, 1H), 5.14–4.91 (m, 2H), 3.08 (d, J = 7.2 Hz, 2H), 2.74 (t, J = 7.1 Hz, 2H), 2.53 (t, J = 7.1 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.9, 137.8, 134.0, 129.0, 124.4, 120.1, 117.5, 37.4, 35.1, 26.4. ESI-HRMS [M + H]+ m/z calcd for C12H16NOS 222.0947, found 222.0952.
1). Yield 38 mg (34%), solvent-free condition yield 66 mg (60%). White solid, mp 38–40 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.91 (s, 1H), 7.44 (d, J = 7.9 Hz, 2H), 7.22 (t, J = 7.8 Hz, 2H), 7.02 (t, J = 7.4 Hz, 1H), 5.79–5.61 (m, 1H), 5.14–4.91 (m, 2H), 3.08 (d, J = 7.2 Hz, 2H), 2.74 (t, J = 7.1 Hz, 2H), 2.53 (t, J = 7.1 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.9, 137.8, 134.0, 129.0, 124.4, 120.1, 117.5, 37.4, 35.1, 26.4. ESI-HRMS [M + H]+ m/z calcd for C12H16NOS 222.0947, found 222.0952.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 107 mg (95%). White solid, mp 74–76 °C. 1H NMR (CDCl3, 300 MHz, 25 °C) δ 7.92 (s, 1H), 7.54 (d, J = 7.9 Hz, 2H), 7.33 (t, J = 7.6 Hz, 2H), 7.13 (t, J = 7.4 Hz, 1H), 3.80 (t, J = 5.4 Hz, 2H), 2.94 (t, J = 6.9 Hz, 2H), 2.85 (s, 1H), 2.77 (t, J = 5.8 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.7, 137.7, 129.0, 124.5, 120.0, 61.0, 37.8, 35.6, 27.5. ESI-HRMS [M + H]+ m/z calcd for C11H16NO2S 226.0896, found 226.0897.
1). Yield 107 mg (95%). White solid, mp 74–76 °C. 1H NMR (CDCl3, 300 MHz, 25 °C) δ 7.92 (s, 1H), 7.54 (d, J = 7.9 Hz, 2H), 7.33 (t, J = 7.6 Hz, 2H), 7.13 (t, J = 7.4 Hz, 1H), 3.80 (t, J = 5.4 Hz, 2H), 2.94 (t, J = 6.9 Hz, 2H), 2.85 (s, 1H), 2.77 (t, J = 5.8 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.7, 137.7, 129.0, 124.5, 120.0, 61.0, 37.8, 35.6, 27.5. ESI-HRMS [M + H]+ m/z calcd for C11H16NO2S 226.0896, found 226.0897.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 79 mg (98%). Yellow solid, mp 46–47 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 6.46–5.84 (d, 2H), 2.87–2.77 (m, 1H), 2.62–2.43 (m, 4H), 1.67–1.53 (m, 2H), 1.29–1.21 (d, 3H), 1.04–0.91 (t, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 177.7, 41.3, 35.9, 35.0, 23.0, 17.6, 13.5. ESI-HRMS [M + H]+ m/z calcd for C7H16NOS 162.0947, found 162.0947.
1). Yield 79 mg (98%). Yellow solid, mp 46–47 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 6.46–5.84 (d, 2H), 2.87–2.77 (m, 1H), 2.62–2.43 (m, 4H), 1.67–1.53 (m, 2H), 1.29–1.21 (d, 3H), 1.04–0.91 (t, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 177.7, 41.3, 35.9, 35.0, 23.0, 17.6, 13.5. ESI-HRMS [M + H]+ m/z calcd for C7H16NOS 162.0947, found 162.0947.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 88 mg (90%). Yellow solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.32–7.26 (m, 2H), 7.26–7.18 (m, 2H), 7.17–7.09 (m, 1H), 5.62 (d, J = 76.1 Hz, 2H), 3.25–3.10 (m, 1H), 2.94–2.84 (m, 1H), 2.47–2.36 (m, 1H), 1.20 (d, J = 6.9 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 177.0, 135.8, 129.6, 129.1, 126.4, 40.4, 37.3, 17.6. ESI-MS [M + H]+ m/z 196.16; [M + Na]+ 218.14.
1). Yield 88 mg (90%). Yellow solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.32–7.26 (m, 2H), 7.26–7.18 (m, 2H), 7.17–7.09 (m, 1H), 5.62 (d, J = 76.1 Hz, 2H), 3.25–3.10 (m, 1H), 2.94–2.84 (m, 1H), 2.47–2.36 (m, 1H), 1.20 (d, J = 6.9 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 177.0, 135.8, 129.6, 129.1, 126.4, 40.4, 37.3, 17.6. ESI-MS [M + H]+ m/z 196.16; [M + Na]+ 218.14.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 93 mg (78%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 8.26 (s, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.21 (t, J = 7.8 Hz, 2H), 7.01 (t, J = 7.4 Hz, 1H), 3.28–3.11 (m, 1H), 2.57–2.39 (m, 4H), 1.59–1.46 (m, 2H), 1.27 (d, J = 6.8 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.6, 137.9, 129.0, 124.4, 120.2, 45.2, 36.9, 33.1, 23.1, 21.8, 13.6. ESI-HRMS [M + H]+ m/z calcd for C13H20NOS 238.1260, found 238.1268.
1). Yield 93 mg (78%). Yellow liquid. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 8.26 (s, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.21 (t, J = 7.8 Hz, 2H), 7.01 (t, J = 7.4 Hz, 1H), 3.28–3.11 (m, 1H), 2.57–2.39 (m, 4H), 1.59–1.46 (m, 2H), 1.27 (d, J = 6.8 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.6, 137.9, 129.0, 124.4, 120.2, 45.2, 36.9, 33.1, 23.1, 21.8, 13.6. ESI-HRMS [M + H]+ m/z calcd for C13H20NOS 238.1260, found 238.1268.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 81 mg (60%). White solid, mp 60–61 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.80 (d, J = 25.2 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 7.1 Hz, 2H), 7.26–7.10 (m, 6H), 7.01 (t, J = 7.4 Hz, 1H), 3.76–3.59 (m, 1H), 2.67–2.50 (m, 1H), 2.49–2.27 (m, 1H), 1.29 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.1, 137.7, 133.9, 132.4, 129.1, 129.0, 127.5, 124.5, 120.1, 44.5, 40.0, 21.0. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 272.1104, found 272.1110.
1). Yield 81 mg (60%). White solid, mp 60–61 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.80 (d, J = 25.2 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 7.1 Hz, 2H), 7.26–7.10 (m, 6H), 7.01 (t, J = 7.4 Hz, 1H), 3.76–3.59 (m, 1H), 2.67–2.50 (m, 1H), 2.49–2.27 (m, 1H), 1.29 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.1, 137.7, 133.9, 132.4, 129.1, 129.0, 127.5, 124.5, 120.1, 44.5, 40.0, 21.0. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 272.1104, found 272.1110.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 49 mg (42%). Yellow solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.81 (s, 1H), 7.52 (d, J = 7.2 Hz, 1H), 7.32 (t, J = 7.9 Hz, 2H), 7.11 (t, J = 7.4 Hz, 1H), 5.94–5.72 (m, 1H), 5.16 (d, J = 16.9, 1.6 Hz, 1H), 5.11 (d, J = 10.0 Hz, 1H), 3.33–3.17 (m, 3H), 2.67–2.57 (m, 1H), 2.57–2.46 (m, 1H), 1.37 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.0, 137.8, 134.3, 129.0, 124.4, 120.0, 117.4, 45.1, 36.0, 34.3, 21.6. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 236.1104, found 236.1110.
1). Yield 49 mg (42%). Yellow solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.81 (s, 1H), 7.52 (d, J = 7.2 Hz, 1H), 7.32 (t, J = 7.9 Hz, 2H), 7.11 (t, J = 7.4 Hz, 1H), 5.94–5.72 (m, 1H), 5.16 (d, J = 16.9, 1.6 Hz, 1H), 5.11 (d, J = 10.0 Hz, 1H), 3.33–3.17 (m, 3H), 2.67–2.57 (m, 1H), 2.57–2.46 (m, 1H), 1.37 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.0, 137.8, 134.3, 129.0, 124.4, 120.0, 117.4, 45.1, 36.0, 34.3, 21.6. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 236.1104, found 236.1110.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 117 mg (88%). White solid, mp 55–57 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 8.33 (s, 1H), 7.46 (d, 2H), 7.21 (t, J = 7.7 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H), 3.11–2.96 (m, 1H), 2.58–2.37 (m, 4H), 1.60–1.28 (m, 6H), 0.88 (t, J = 7.3 Hz, 3H), 0.82 (t, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.9, 138.0, 128.9, 124.3, 120.1, 44.0, 42.5, 37.7, 33.3, 23.2, 20.1, 13.9, 13.6. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 266.1573, found 266.1574.
1). Yield 117 mg (88%). White solid, mp 55–57 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 8.33 (s, 1H), 7.46 (d, 2H), 7.21 (t, J = 7.7 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H), 3.11–2.96 (m, 1H), 2.58–2.37 (m, 4H), 1.60–1.28 (m, 6H), 0.88 (t, J = 7.3 Hz, 3H), 0.82 (t, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 169.9, 138.0, 128.9, 124.3, 120.1, 44.0, 42.5, 37.7, 33.3, 23.2, 20.1, 13.9, 13.6. ESI-HRMS [M + H]+ m/z calcd for C16H18NOS 266.1573, found 266.1574.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 112 mg (75%). White solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.36–7.09 (m, 9H), 7.02–6.93 (m, 1H), 4.27 (t, J = 7.5 Hz, 1H), 2.89–2.67 (m, 2H), 2.32–2.13 (m, 2H), 1.53–1.30 (m, 2H), 0.79 (t, J = 7.3 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 167.6, 140.8, 136.6, 127.8, 127.6, 126.6, 126.4, 123.4, 119.2, 44.9, 44.0, 32.5, 21.5, 12.4. ESI-HRMS [M + H]+ m/z calcd for C18H22NOS 300.1417, found 300.1425.
1). Yield 112 mg (75%). White solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.36–7.09 (m, 9H), 7.02–6.93 (m, 1H), 4.27 (t, J = 7.5 Hz, 1H), 2.89–2.67 (m, 2H), 2.32–2.13 (m, 2H), 1.53–1.30 (m, 2H), 0.79 (t, J = 7.3 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 167.6, 140.8, 136.6, 127.8, 127.6, 126.6, 126.4, 123.4, 119.2, 44.9, 44.0, 32.5, 21.5, 12.4. ESI-HRMS [M + H]+ m/z calcd for C18H22NOS 300.1417, found 300.1425.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 46 mg (42%). Yellow solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.51 (d, J = 8.0 Hz, 2H), 7.29 (s, 1H), 7.21 (t, 2H), 7.00 (t, J = 7.4 Hz, 1H), 6.91 (d, J = 9.9 Hz, 1H), 5.86 (d, J = 10.0 Hz, 1H), 2.65 (t, J = 7.3 Hz, 2H), 1.67–1.58 (m, 2H), 0.95 (t, J = 7.3 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 163.5, 146.8, 137.1, 127.9, 122.9, 118.5, 114.3, 37.5, 22.6, 12.1. ESI-HRMS [M + H]+ m/z calcd for C12H16NOS 222.0947, found 222.0952.
1). Yield 46 mg (42%). Yellow solid, mp 55–56 °C. 1H NMR (CDCl3, 400 MHz, 25 °C) δ 7.51 (d, J = 8.0 Hz, 2H), 7.29 (s, 1H), 7.21 (t, 2H), 7.00 (t, J = 7.4 Hz, 1H), 6.91 (d, J = 9.9 Hz, 1H), 5.86 (d, J = 10.0 Hz, 1H), 2.65 (t, J = 7.3 Hz, 2H), 1.67–1.58 (m, 2H), 0.95 (t, J = 7.3 Hz, 3H). 13C NMR (CDCl3, 100 MHz, 25 °C) δ 163.5, 146.8, 137.1, 127.9, 122.9, 118.5, 114.3, 37.5, 22.6, 12.1. ESI-HRMS [M + H]+ m/z calcd for C12H16NOS 222.0947, found 222.0952.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 105 mg (62%). Yellow liquid. 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 6.4 Hz, 1H), 7.46 (t, J = 8.0, 4.9 Hz, 2H), 7.24 (t, J = 7.7 Hz, 2H), 7.03 (t, J = 7.4 Hz, 1H), 3.42–3.15 (m, 1H), 3.07–2.86 (m, 1H), 2.62–2.39 (m, 6H), 1.81–1.44 (m, 6H), 1.23 (t, J = 6.7, 5.4 Hz, 3H), 0.96–0.85 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 169.1, 137.9, 137.8, 129.0, 128.9, 124.3, 124.2, 120.0, 119.9, 99.9, 44.4, 43.8, 42.6, 42.5, 40.8, 40.3, 37.8, 37.1, 33.5, 33.1, 32.3, 31.9, 23.3, 23.2, 23.1, 22.6, 21.4, 13.7, 13.6. ESI-HRMS [M + H]+ m/z calcd for C18H30NOS2 340.1763, found 340.1773.
1). Yield 105 mg (62%). Yellow liquid. 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 6.4 Hz, 1H), 7.46 (t, J = 8.0, 4.9 Hz, 2H), 7.24 (t, J = 7.7 Hz, 2H), 7.03 (t, J = 7.4 Hz, 1H), 3.42–3.15 (m, 1H), 3.07–2.86 (m, 1H), 2.62–2.39 (m, 6H), 1.81–1.44 (m, 6H), 1.23 (t, J = 6.7, 5.4 Hz, 3H), 0.96–0.85 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 169.1, 137.9, 137.8, 129.0, 128.9, 124.3, 124.2, 120.0, 119.9, 99.9, 44.4, 43.8, 42.6, 42.5, 40.8, 40.3, 37.8, 37.1, 33.5, 33.1, 32.3, 31.9, 23.3, 23.2, 23.1, 22.6, 21.4, 13.7, 13.6. ESI-HRMS [M + H]+ m/z calcd for C18H30NOS2 340.1763, found 340.1773.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 21502042, 51403051, 21477033, 21204018), Scientific Research Key Project Fund of Henan Provincial Education Department (Grant No. 15A150041, 16A150003), Scientific Research Fund of Henan University (No. 2013YBZR008).Notes and references
- (a) R. S. Tukhvatshin, A. S. Kucherenko, Y. V. Nelyubina and S. G. Zlotin, ACS Catal., 2017, 7, 2981 CrossRef CAS; (b) C. Yang, W.-Q. Su and D.-Z. Xu, RSC Adv., 2016, 6, 99656 RSC; (c) Y.-Y. Song, H.-W. Jing, B. Li and D.-S. Bai, Chem.–Eur. J., 2011, 17, 8731 CrossRef CAS PubMed.
- (a) H. Xu, L.-Y. Pan, X.-M. Fang, B.-Y. Liu, W.-K. Zhang, M.-H. Lu, Y.-Q. Xu, T. Ding and H.-B. Chang, Tetrahedron Lett., 2017, 58, 2360 CrossRef CAS; (b) A.-G. Ying, Y.-X. Ni, S.-L. Xu, S. Liu, J.-G. Yang and R.-R. Li, Ind. Eng. Chem. Res., 2014, 53, 5678 CrossRef CAS; (c) D.-Z. Xu, Y.-J. Liu, S. Shi and Y.-M. Wang, Green Chem., 2010, 12, 514 RSC.
- (a) W. Senapak, R. Saeeng and U. Sirion, RSC Adv., 2017, 7, 30380 RSC; (b) L.-C. Feng, Y.-W. Sun, W.-J. Tang, L.-J. Xu, K.-L. Lam, Z.-Y. Zhou and A. S. C. Chan, Green Chem., 2010, 12, 949 RSC.
- R. L. J. Vekariya, J. Mol. Liq., 2017, 227, 44 CrossRef CAS.
- (a) Z.-C. Geng, N. Li, J. Chen, X.-F. Huang, B. Wu, G.-G. Liu and X.-W. Wang, Chem. Commun., 2012, 48, 4713 RSC; (b) A. Kumar, V. D. Tripathi, P. Kumar, L. P. Gupta, R. Trivedi, H. Bid, V. L. Nayak, J. A. Siddiqui, B. Chakravarti, R. Saxena, A. Dwivedi, M. I. Siddiquee, U. Siddiqui, R. Konwar and N. Chattopadhyay, Bioorg. Med. Chem., 2011, 19, 5409 CrossRef CAS PubMed; (c) J. P. Cherkauskas and T. Cohen, J. Org. Chem., 1992, 57, 6 CrossRef CAS.
- (a) G.-L. Huang and X. Li, Curr. Org. Chem., 2017, 14, 568 CAS; (b) C. F. H. Allen, W. J. Humphlett and J. O. Fournier, Can. J. Chem., 1964, 42, 2616 CrossRef CAS; (c) L. A. Gorthey, M. Vairamani and C. J. Djerassi, J. Org. Chem., 1985, 50, 4173 CrossRef CAS; (d) F. M. Moghaddam, G. R. Bardajee and R. O. C. Veranlou, Synth. Commun., 2005, 35, 2427 CrossRef CAS.
- (a) A. C. Breman, S. E. M. Telderman, R. P. M. van Santen, J. I. Scott, J. H. van Maarseveen, S. Ingemann and H. Hiemstra, J. Org. Chem., 2015, 80, 10561 CrossRef CAS PubMed; (b) A. Mohammad, Tetrahedron Lett., 2012, 53, 3683 CrossRef; (c) P. McDaid, Y. Chen and L. Deng, Angew. Chem., Int. Ed., 2002, 41, 338 CrossRef CAS.
- (a) M. P. D. Rocha, A. R. Oliveira, T. B. Albuquerque, C. D. G. da Silva, R. Katla and N. L. C. Domingues, RSC Adv., 2016, 6, 4979 RSC; (b) M. P. Darbem, A. R. Oliveira, C. R. Winck, A. W. Rinaldi and N. L. C. Domingues, Tetrahedron Lett., 2014, 55, 5179 CrossRef CAS; (c) A. T. Khan, S. Ghosh and L. H. Choudhury, Eur. J. Org. Chem., 2006, 2226 CrossRef CAS.
- (a) A. Kumar, S. Srivastava, G. Gupta, P. Kumar and J. Sarkar, RSC Adv., 2013, 3, 3548 RSC; (b) F. Han, L. Yang, Z. Li and C. Xia, Org. Biomol. Chem., 2012, 10, 346 RSC.
- P. V. S. Rizzo, L. A. Boarin, I. O. M. Freitas, R. S. Gomes, A. Beatriz, A. W. Rinaldi and N. L. C. Domingues, Tetrahedron Lett., 2014, 55, 430 CrossRef CAS.
- M. Hans, L. Delaude, J. Rodriguez and Y. Coquerel, J. Org. Chem., 2014, 79, 2758 CrossRef CAS PubMed.
- O. Miyata, T. Shinada, I. Ninomiya, T. Naito, T. Date, K. Okamura and S. Inagaki, J. Org. Chem., 1991, 56, 6556 CrossRef CAS.
- U. M. Lindstorm, Chem. Rev., 2006, 35, 68 RSC.
- S. Payra, A. Saha and S. Banerjee, RSC Adv., 2016, 6, 95951 RSC.
- M. K. Chaudhuri and S. Hussain, J. Mol. Catal. A: Chem., 2007, 269, 214 CrossRef CAS.
- S. Hussain, S. K. Bharadwaj, M. K. Chaudhuri and H. Kalita, Eur. J. Org. Chem., 2007, 374 CrossRef CAS.
- N. S. Krishnaveni, K. Surendra and K. R. Rao, Chem. Commun., 2005, 669 RSC.
- (a) H. Xu, S. Ma, Y.-Q. Xu, L.-X. Bian, T. Ding, X.-X. Fang, W.-K. Zhang and Y.-R. Ren, J. Org. Chem., 2015, 80, 1789 CrossRef CAS PubMed; (b) H.-Y. Li, J.-Y. Jie, S.-X. Wu, X.-B. Yang and H. Xu, Org. Chem. Front., 2017, 4, 250 RSC; (c) H.-M. Su, L.-Y. Wang, H.-H. Rao and H. Xu, Org. Lett., 2017, 19, 2226 CrossRef CAS PubMed.
- H. Firouzabadi, N. Iranpoor and M. Abbasi, Adv. Synth. Catal., 2010, 351, 755 CrossRef.
- Y.-M. Lin, G.-P. Lu, C. Cai and W.-B. Yi, RSC Adv., 2015, 46, 27107 RSC.
- J. Toda, M. Sakagami, Y. Goan, M. Simakata, T. Saitoh, Y. Horiguchi and T. Sano, Chem. Pharm. Bull., 2000, 48, 1854 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR spectra of these synthesized compounds. See DOI: 10.1039/c7ra08956b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |